Challenges and Opportunities of Centrifugal Microfluidics for Extreme Point-of-Care Testing
Abstract
:1. Introduction
2. Evolving Diagnostic Needs in Resource Limited Settings
2.1. Healthcare Infrastructure in Developing Countries
2.2. IVD in Developing Countries
3. Centrifugal Microfluidic-Based IVD
3.1. Instrumentation and Operation of Centrifugal Microfluidic Systems
3.2. Clinical Applications
3.2.1. Clinical Chemistry
3.2.2. Immunoassays
3.2.3. Nucleic Acid Tests
4. Centrifugal Microfluidic Systems for EPOCT
4.1. Opportunities of Cenfrifugal Microfluidics for EPOCT
4.2. Challenges of Cenfrifugal Microfluidics for EPOCT
4.2.1. Disc Requirements
4.2.2. Supporting Instruments
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ducrée, J.; Haeberle, S.; Brenner, T.; Glatzel, T.; Zengerle, R. Patterning of flow and mixing in rotating radial microchannels. Microfluid. Nanofluid. 2006, 2, 97–105. [Google Scholar] [CrossRef]
- Lai, S.; Wang, S.; Luo, J.; Lee, L.J.; Yang, S.-T.; Madou, M.J. Design of a compact disk-like microfluidic platform for enzyme-linked immunosorbent assay. Anal. Chem. 2004, 76, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- Madou, M.J.; Kellogg, G.J. Labcd: A centrifuge-based microfluidic platform for diagnostics. In SPIE Proceedings of the Systems and Technologies for Clinical Diagnostics and Drug Discovery, San Jose, CA, USA, 27 January 1998; Volume 3259, pp. 80–93.
- Gorkin, R.; Park, J.; Siegrist, J.; Amasia, M.; Lee, B.S.; Park, J.-M.; Kim, J.; Kim, H.; Madou, M.; Cho, Y.-K. Centrifugal microfluidics for biomedical applications. Lab Chip 2010, 10, 1758–1773. [Google Scholar] [CrossRef] [PubMed]
- Strohmeier, O.; Keller, M.; Schwemmer, F.; Zehnle, S.; Mark, D.; von Stetten, F.; Zengerle, R.; Paust, N. Centrifugal microfluidic platforms: Advanced unit operations and applications. Chem. Soc. Rev. 2015, 44, 6187–6229. [Google Scholar] [CrossRef] [PubMed]
- Urdea, M.; Penny, L.A.; Olmsted, S.S.; Giovanni, M.Y.; Kaspar, P.; Shepherd, A.; Wilson, P.; Dahl, C.A.; Buchsbaum, S.; Moeller, G.; et al. Requirements for high impact diagnostics in the developing world. Nature 2006, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Millipore. Rapid Lateral Flow Test Strips: Consideration for Product Development; Millipore: Darmstadt, Germany, 2009. [Google Scholar]
- St John, A.; Price, C.P. Existing and emerging technologies for point-of-care testing. Clin. Biochem. Rev. 2014, 35, 155–167. [Google Scholar] [PubMed]
- Sajid, M.; Kawde, A.-N.; Daud, M. Designs, formats and applications of lateral flow assay: A literature review. J. Saudi Chem. Soc. 2015, 19, 689–705. [Google Scholar] [CrossRef]
- Broadhurst, M.J.; Kelly, J.D.; Miller, A.; Semper, A.; Bailey, D.; Groppelli, E.; Simpson, A.; Brooks, T.; Hula, S.; Nyoni, W.; et al. Reebov antigen rapid test kit for point-of-care and laboratory-based testing for ebola virus disease: A field validation study. Lancet 2015, 386, 867–874. [Google Scholar] [CrossRef]
- Nema, V. Tuberculosis diagnostics: Challenges and opportunities. Lung India 2012, 29, 259–266. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). The Use of Malarial Rapid Diagnostic Test, 2nd ed.; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Perkins, M.; Bell, D. Working without a blindfold: The critical role of diagnostics in malaria control. Malar. J. 2008, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, P.J. How do we best diagnose malaria in africa? Am. J. Trop. Med. Hyg. 2012, 86, 192–193. [Google Scholar] [CrossRef] [PubMed]
- Derda, R.; Gitaka, J.; Klapperich, C.M.; Mace, C.R.; Kumar, A.A.; Lieberman, M.; Linnes, J.C.; Jores, J.; Nasimolo, J.; Ndung’u, J.; et al. Enabling the development and deployment of next generation point-of-care diagnostics. PLoS Negl. Trop. Dis. 2015, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the developing world: Microfluidic paper-based analytical devices. Anal. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Fu, E.; Liang, T.; Spicar-Mihalic, P.; Houghtaling, J.; Ramachandran, S.; Yager, P. Two-dimensional paper network format that enables simple multistep assays for use in low-resource settings in the context of malaria antigen detection. Anal. Chem. 2012, 84, 4574–4579. [Google Scholar] [CrossRef] [PubMed]
- Pirnstill, C.W.; Coté, G.L. Malaria diagnosis using a mobile phone polarized microscope. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.; Mudanyali, O.; Schneider, E.M.; Zengerle, R.; Ozcan, A. Cellphone-based devices for bioanalytical sciences. Anal. Bioanal. Chem. 2014, 406, 3263–3277. [Google Scholar] [CrossRef] [PubMed]
- Ramachandraiah, H.; Amasia, M.; Cole, J.; Sheard, P.; Pickhaver, S.; Walker, C.; Wirta, V.; Lexow, P.; Lione, R.; Russom, A. Lab-on-dvd: Standard dvd drives as a novel laser scanning microscope for image based point of care diagnostics. Lab Chip 2013, 13, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Land, K.; Madou, M.; Kido, H. Rapid, low-cost prototyping of centrifugal microfluidic devices for effective implementation of various microfluidic components. S. Afr. J. Ind. Eng. 2015, 26, 179–190. [Google Scholar] [CrossRef]
- Antunes, P.; Watterson, D.; Parmvi, M.; Burger, R.; Boisen, A.; Young, P.; Cooper, M.A.; Hansen, M.F.; Ranzoni, A.; Donolato, M. Quantification of NS1 dengue biomarker in serum via optomagnetic nanocluster detection. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphreys, M.; Varshney, A. Violent conflict and the millennium development goals: Diagnosis and recommendations. Available online: http://www.columbia.edu/~mh2245/papers1/HV.pdf (accessed on 17 February 2016).
- Requejo, J.H.; Bryce, J.; Barros, A.J.D.; Berman, P.; Bhutta, Z.; Chopra, M.; Daelmans, B.; de Francisco, A.; Lawn, J.; Maliqi, B.; et al. Countdown to 2015 and beyond: Fulfilling the health agenda for women and children. Lancet 2015, 385, 466–476. [Google Scholar] [CrossRef]
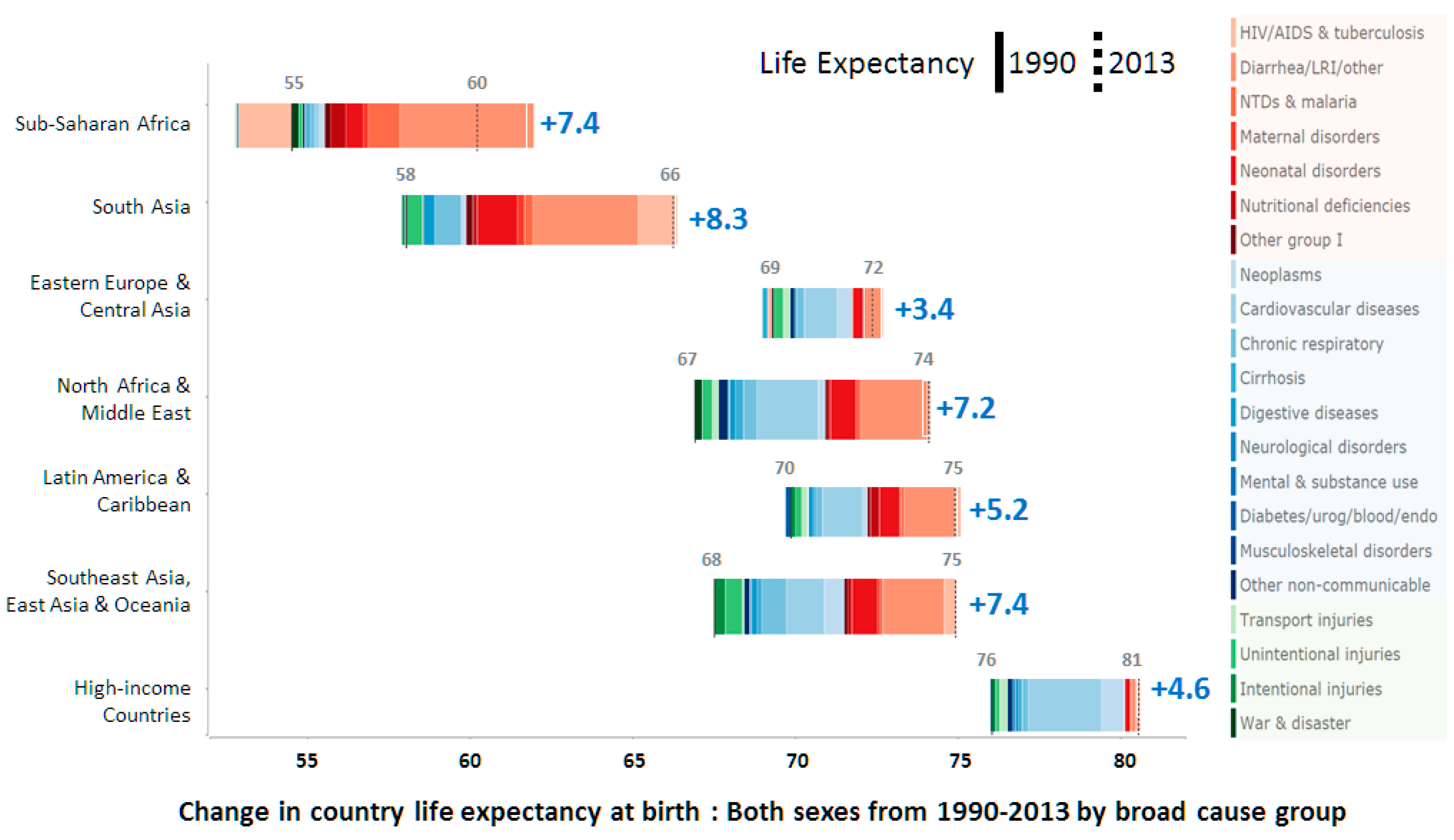
- Murray, C.J.L.; Ortblad, K.F.; Guinovart, C.; Lim, S.S.; Wolock, T.M.; Roberts, D.A.; Dansereau, E.A.; Graetz, N.; Barber, R.M.; Brown, J.C.; et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: A systematic analysis for the global burden of disease study. Lancet 2013, 384, 1005–1070. [Google Scholar] [CrossRef]
- Institute for Health Metrics and Evaluation (IHME). Life Expectancy & Probability of Death; IHME: Seattle, WA, USA, 2014. [Google Scholar]
- Mbonye, A.K.; Magnussen, P.; Lal, S.; Hansen, K.S.; Cundill, B.; Chandler, C.; Clarke, S.E. A cluster randomised trial introducing rapid diagnostic tests into registered drug shops in Uganda: Impact on appropriate treatment of malaria. PLoS ONE 2015, 10, e0129545. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.; Dheda, K.; Pant Pai, N.; Cogill, D.; Pai, M.; Engel, N. A survey on use of rapid tests and tuberculosis diagnostic practices by primary health care providers in south Africa: Implications for the development of new point-of-care tests. PLoS ONE 2015, 10, e0141453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuon, F.F.; Rocha, J.L.; Merlini, A.B. Combined therapy for multi-drug-resistant acinetobacter baumannii infection—Is there evidence outside the laboratory? J. Med. Microbiol. 2015, 64, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R. Bringing diagnostics to developing countries: An interview with Rosanna Peeling. Expert Rev. Mol. Diagn. 2015, 15, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- United Nations (UN). Goal 3: Ensure Healthy Lives and Promote Well-Being for All at All Ages; UN: New York, NY, USA, 2015. [Google Scholar]
- Girosi, F.; Olmsted, S.S.; Keeler, E.; Hay Burgess, D.C.; Lim, Y.-W.; Aledort, J.E.; Rafael, M.E.; Ricci, K.A.; Boer, R.; Hilborne, L.; et al. Developing and interpreting models to improve diagnostics in developing countries. Nature 2006, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Drain, P.K.; Hyle, E.P.; Noubary, F.; Freedberg, K.A.; Wilson, D.; Bishai, W.R.; Rodriguez, W.; Bassett, I.V. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect. Dis. 2014, 14, 239–249. [Google Scholar] [CrossRef]
- Chin, C.D.; Linder, V.; Sia, S.K. Lab-on-a-chip devices for global health: Past studies and future opportunities. Lab Chip 2007, 7, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Mutocheluh, M.; Owusu, M.; Kwofie, T.; Akadigo, T.; Appau, E.; Narkwa, P. Risk factors associated with hepatitis B exposure and the reliability of five rapid kits commonly used for screening blood donors in Ghana. BMC Res. Notes 2014, 7, 873. [Google Scholar] [CrossRef] [PubMed]
- Maeseneer, J.D.; van Weel, C.; Egilman, D.; Mfenyana, K.; Kaufman, A.; Sewankambo, N.; Flinkenflögel, M. Funding for primary health care in developing countries. BMJ Br. Med. J. 2008, 336, 518–519. [Google Scholar] [CrossRef] [PubMed]
- Mabey, D.; Peeling, R.W.; Ustianowski, A.; Perkins, M.D. Tropical infectious diseases: Diagnostics for the developing world. Nat. Rev. Microbiol. 2004, 2, 231–240. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Who List of Prequalified in Vitro Diagnostic Products; World Health Organization: Geneva, Switzerland, 2015; pp. 1–8. [Google Scholar]
- Ritchie, A.V.; Ushiro-Lumb, I.; Edemaga, D.; Joshi, H.A.; De Ruiter, A.; Szumilin, E.; Jendrulek, I.; McGuire, M.; Goel, N.; Sharma, P.I.; et al. SAMBA HIV semiquantitative test, a new point-of-care viral-load-monitoring assay for resource-limited settings. J. Clin. Microbiol. 2014, 52, 3377–3383. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Smith, P.G.; Bossuyt, P.M.M. A guide for diagnostic evaluations. Nat. Rev. Microbiol. 2006, 12, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. In Vitro Diagnostics and Laboratory Technology; World Health Organization: Geneva, Switzerland, 2015; pp. 1–8. [Google Scholar]
- Kettler, H.; White, K.; Hawkes, S. Mapping the Landscape of Diagnostics for Sexually Transmitted Infections: Key Findings and Recommendations; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Fransisca, L.; Kusnanto, J.H.; Satoto, T.B.T.; Sebayang, B.; Supriyanto; Andriyan, E.; Bangs, M.J. Comparison of rapid diagnostic test Plasmotec Malaria-3, microscopy, and quantitative real-time PCR for diagnoses of Plasmodium falciparum and Plasmodium vivax infections in Mimika Regency, Papua, Indonesia. Malar. J. 2015, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Bassett, I.V.; Regan, S.; Chetty, S.; Giddy, J.; Uhler, L.M.; Holst, H.; Ross, D.; Katz, J.N.; Walensky, R.P.; Freedberg, K.A.; et al. Who starts antiretroviral therapy in Durban, South Africa?… not everyone who should. AIDS 2010, 24, S37–S44. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.L.; Ouyang, Y.; Duarte, G.R.M.; Carrilho, E.; Krauss, S.T.; Landers, J.P. Inexpensive, rapid prototyping of microfluidic devices using overhead transparencies and a laser print, cut and laminate fabrication method. Nat. Protoc. 2015, 10, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Focke, M.; Kosse, D.; Muller, C.; Reinecke, H.; Zengerle, R.; von Stetten, F. Lab-on-a-foil: Microfluidics on thin and flexible films. Lab Chip 2010, 10, 1365–1386. [Google Scholar] [CrossRef] [PubMed]
- Espira Inc. Available online: https://www.espirainc.com (accessed on 17 February 2016).
- GenePOC. Available online: http://www.genepoc-diagnostics.com (accessed on 17 February 2016).
- Van Oordt, T.; Stevens, G.B.; Vashist, S.K.; Zengerle, R.; von Stetten, F. Rapid and highly sensitive luciferase reporter assay for the automated detection of botulinum toxin in the centrifugal microfluidic labdisk platform. RSC Adv. 2013, 3, 22046–22052. [Google Scholar] [CrossRef]
- Lee, B.S.; Lee, Y.U.; Kim, H.-S.; Kim, T.-H.; Park, J.; Lee, J.-G.; Kim, J.; Kim, H.; Lee, W.G.; Cho, Y.-K. Fully integrated lab-on-a-disc for simultaneous analysis of biochemistry and immunoassay from whole blood. Lab Chip 2011, 11, 70–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, H.; Kim, Y.; Cho, J.; Lee, J.-Y.; Choi, M.-S.; Cho, Y.-K. Lab-on-a-disc for simultaneous determination of nutrients in water. Anal. Chem. 2013, 85, 2954–2960. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Sunkara, V.; Kim, T.-H.; Hwang, H.; Cho, Y.-K. Lab-on-a-disc for fully integrated multiplex immunoassays. Anal. Chem. 2012, 84, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Czugala, M.; Gorkin, R., III; Phelan, T.; Gaughran, J.; Curto, V.F.; Ducree, J.; Diamond, D.; Benito-Lopez, F. Optical sensing system based on wireless paired emitter detector diode device and ionogels for lab-on-a-disc water quality analysis. Lab Chip 2012, 12, 5069–5078. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.D.; Linder, V.; Sia, S.K. Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip 2012, 12, 2118–2134. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zaman, M.H. Low-cost tools for diagnosing and monitoring HIV infection in low-resource settings. Bull. World Health Organ. 2012, 90, 914–920. [Google Scholar] [CrossRef] [PubMed]
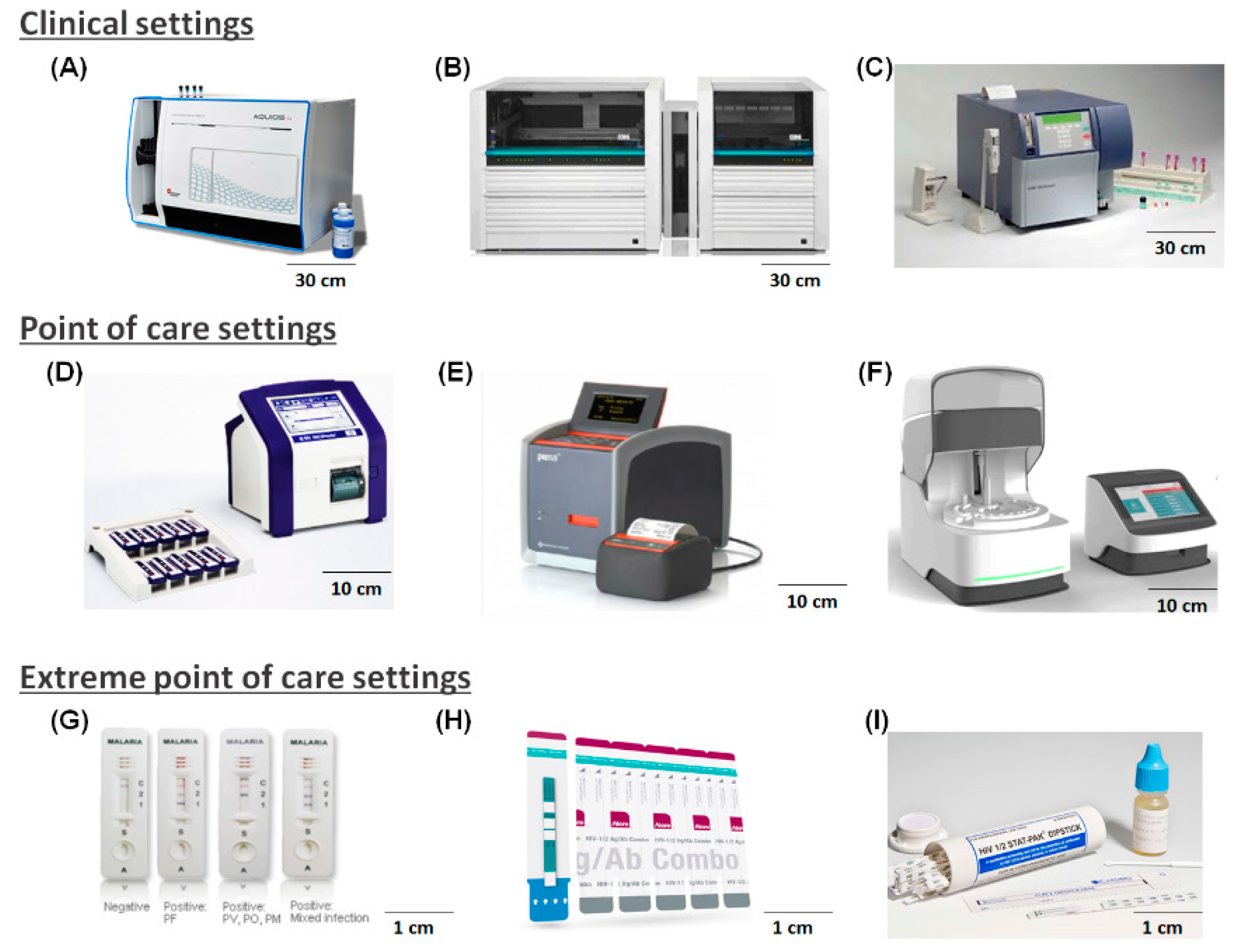

| Infrastructure Availability | Urban Health Centers or Hospitals (59%) | Primary Healthcare Centers (27%) | Non-Clinical Settings (14%) |
|---|---|---|---|
| Clean water | O | × | × |
| Electricity | O | Δ | × |
| Dust free environment | O | Δ | × |
| Cold storage | O | × | × |
| Stable temperature | O | × | × |
| Trained professional | O | O | × |
| Internet access | O | Δ | × |
| Healthcare Setting | Urban Health Centers or Hospitals | Primary Healthcare Centers | Non-Clinical Settings |
|---|---|---|---|
| Device used | Non-POCT | POCT | EPOCT |
| Sample type | All kinds of samples (Venous blood, saliva, sputum, urine, nasal fluid, etc.) | Minimally invasive samples (Finger prick blood, urine, saliva, etc.) | Minimally invasive samples (Finger prick blood, urine, saliva, etc.) |
| Sample volume | High (>1 mL Blood) | Low (<10 µL Blood) | Low (<10 µL Blood) |
| Sample preparation | Manual/Automatic | Semi-automatic | Automatic |
| Sampling Size | Many | One ~ Few | One |
| Existing laboratory equipment | Advanced | Simple (Centrifuge, light microscope, etc.) | None |
| Device footprint | Large | Medium | Hand-held |
| Power supply | Normal | Battery/normal | Battery |
| Operation difficulty | Low ~ High | Medium | Low |
| Processing time | Fast/moderate | Fast | Fast |
| Usage | Heavily used | Minimally used | Used on demand |
| Durability | Low | Medium | High |
| Device cost | High | Medium | Low |
| Consumable cost | Low ~ High | Low | Low |
| Diagnostics | Screening, Quantification | Screening, Quantification | Screening, Quantification |
| Medical data storage | Local, Cloud | Local, Cloud | Cloud |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michael, I.J.; Kim, T.-H.; Sunkara, V.; Cho, Y.-K. Challenges and Opportunities of Centrifugal Microfluidics for Extreme Point-of-Care Testing. Micromachines 2016, 7, 32. https://doi.org/10.3390/mi7020032
Michael IJ, Kim T-H, Sunkara V, Cho Y-K. Challenges and Opportunities of Centrifugal Microfluidics for Extreme Point-of-Care Testing. Micromachines. 2016; 7(2):32. https://doi.org/10.3390/mi7020032
Chicago/Turabian StyleMichael, Issac J., Tae-Hyeong Kim, Vijaya Sunkara, and Yoon-Kyoung Cho. 2016. "Challenges and Opportunities of Centrifugal Microfluidics for Extreme Point-of-Care Testing" Micromachines 7, no. 2: 32. https://doi.org/10.3390/mi7020032






