Guard Cell and Tropomyosin Inspired Chemical Sensor
Abstract
:1. Introduction
2. Related Work and Background
2.1. Sensor Design
2.2. Biomimetic Chemical Sensors
2.3. Biological Sensing Mechanisms
3. Design Approach
| Step | Description |
|---|---|
| 1. Needs or Curiosity | Problem-driven: The traditional route, which involves gathering a set of needs, requirements and mapping them to important flows. Biology-driven: identify an interesting biological system to explore. |
| 2. Decompose | The needs or interesting biological system are decomposed into, first, a black box model and, second, a functional model. All models created with this method use the Functional Basis modeling lexicon [52]. When following the solution-driven route, the designer can refer to the general biological modeling methodology presented in [53] for assistance with creating a biological functional model. |
| 3. Query | Knowledge bases are queried to identify solutions to each function/flow pair of the functional model. Two knowledge bases are required: one containing engineered systems and the other containing biological systems. Both are indexed by engineering function and flow. |
| 4. Make Connections | Connections are made through analogies, metaphors and first principles to assist with making the leap between the biology and engineering domains. |
| 5. Concept Generation | Concept generation is performed to create biologically-inspired conceptual designs. Concept synthesis involves analysis, reflection and synthesis. Analysis is on the returned engineered and biological solutions from Step 3. Reflection is on the connections to the engineering domain formulated in Step 4. Synthesis is of the existing engineering solutions, engineering solutions inspired by biology and inventive solutions inspired by biology to derive a new design. |

- Physiology: concerned with the vital functions and activities of organisms, as opposed to their structure. (e.g., Detect stimuli , parallel sampling)
- Morphology: the form and structure of an organism, and the associations amongst the structures of an organism. (e.g., Porous surface, helical shape)
- Behavior: the sum of the responses of an organism to internal or external stimuli. (e.g., Actuate muscles, change orientation)
- Strategy: a generic behavior that is exhibited among multiple biological ranks to achieve different goals. (e.g., Shape change, up-front processing)
4. Results and Discussion
4.1. Step One
| Customer Need/Constraint | Functional Basis Flow |
|---|---|
| Selectivity | Status signal |
| Response time | Electrical energy |
| Reusable | Sensing layer (material) |
| Indirect sensing mechanism | Sensing layer (material)/Chemical stimulus (energy) |
4.2. Step Two
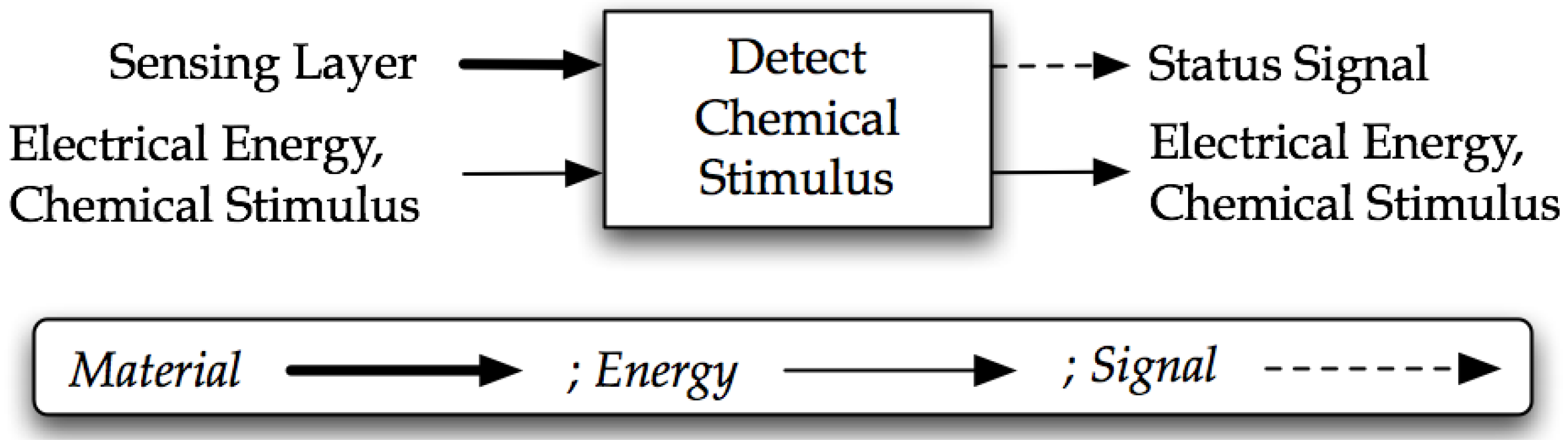
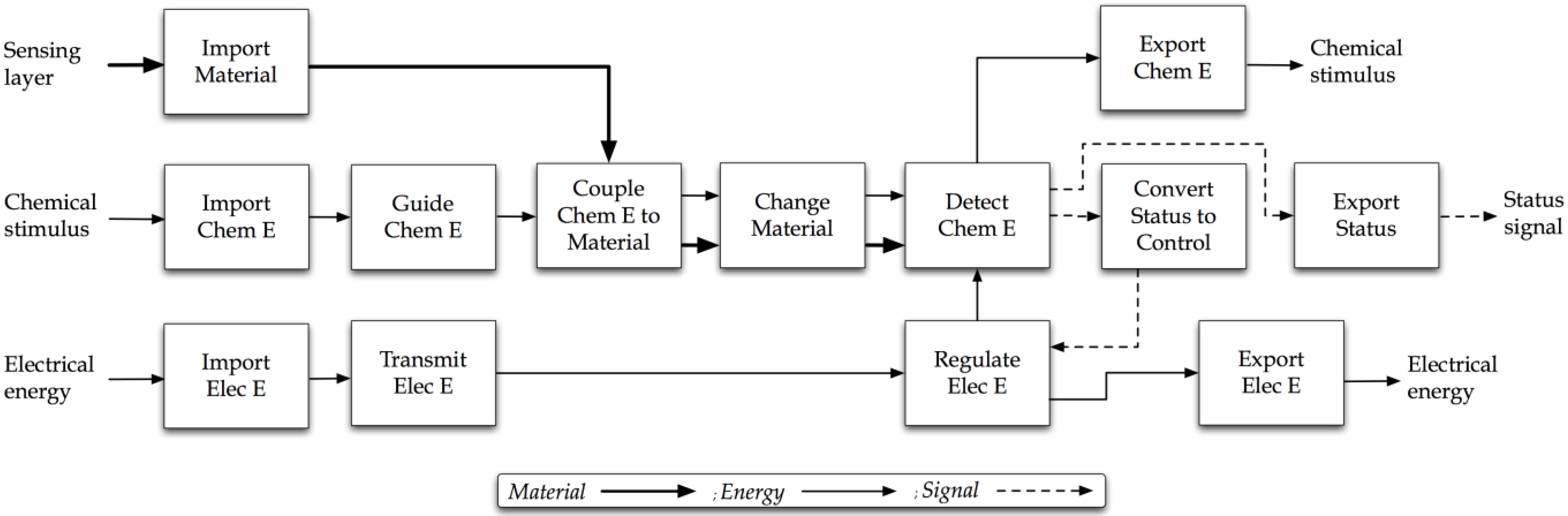
4.3. Step Three
| Function/Flow | Engineering Solutions |
|---|---|
| Import/ Material | Housing, reservoir, spring |
| Import/ Chemical Energy | Container, nozzle |
| Guide/ Chemical Energy | Tube |
| Couple/Chemical Energy to Material | Basket, container, iron, nozzle, carburetor, burner, housing |
| Import/ Electrical Energy | Battery, wire, circuit board, motor, cord, switch |
| Transmit/Electrical Energy | Wire, battery contacts, circuit board, compound eye |
| Change/ Material | Blade, impeller, heating element, punch, filter, staple plate, popcorn popper |
| Detect/ Chemical Energy | Fly chemoreceptor, protein, animalia chemoreception, plantae chemoreception |
| Regulate/Electrical Energy | Circuit board, actuator, heating element, switch, resistor, diode |
| Convert/Status to Control Signal | Circuit board |
| Export/ Chemical Energy | Nozzle, bowl, tube, exhaust, bucket |
| Export/ Electrical Energy | Wire, circuit board, cord, switch |
| Export/ Status Signal | LCD screen, circuit board, wire, cord, level, speaker |
| Function/Flow | Biological Corpus Results |
|---|---|
| Change/Material | The resulting change in membrane potential causes the sensory cell either to fire action potentials itself or to change its secretion of neurotransmitter onto an associated cell that fires action potentials. Photosensitivity depends on the ability of rhodopsins to absorb photons of light and to undergo a change in conformation. Dynein is a enzyme that catalyzes the hydrolysis of ATP and uses the released energy to change its shape, thereby generating mechanical force. A gated channel opens when something happens to change the shape of the protein. Microtubules change the shapes of cells and move cells by polymerizing and depolymerizing the protein tubulin. Cell movement is generated by two structures, microtubules and microfilaments, both of which consist of long protein molecules that can change their length or shape. Actin microfilaments can change the shape of a cell simply by polymerizing and depolymerizing. Nets of actin and myosin beneath the cell membrane change a cell’s shape during endocytosis. Chromatophores are pigment-containing cells in the skin that can change the color and pattern of the animal. Because the troponin is bound to the tropomyosin, this conformational change of the troponin twists the tropomyosin enough to ex-pose the actin-myosin binding sites. The Ca2+ ions bind to troponin and change its conformation, pulling the tropomyosin strands away from the myosin binding sites on the actin filament. Because the viruses are too large to go through these channels, special proteins bind to them and help change their shape so that they can squeeze through the pores. Guard cells are modified epidermal cells that change their shape, thereby opening or closing pores called stomata, which serve as passageways between the environment and the leaf's interior. The silk protein that stretches contains amino acids that allow it to curl into a spiral, and when these spirals associate into silk fibers, they can slip along each other to change the fiber’s length. Ionizing radiation (X rays) produces highly reactive chemical species called free radicals, which can change bases in DNA to unrecognizable (by DNA polymerase) forms or break the sugar-phosphate backbone, causing chromosomal abnormalities. |
| Function/Flow | Biological Corpus Results |
|---|---|
| Detect/Chemical Energy | Smell and taste receptors, for example, are epithelial cells that detect specific chemicals. Most sensory cells possess a membrane receptor protein that detects the stimulus and responds by altering the flow of ions across the plasma membrane. Eukaryotic cells carry out cellular respiration in their mitochondria, which are located in the cytoplasm-an aqueous medium. Chemoreceptors are responsible for smell, taste, and the monitoring of aspects of the internal environment such as the level of carbon dioxide in the blood. Crabs and flies, for example, have chemoreceptor hairs on their feet; they taste potential food by stepping in it. After a fly tastes a drop of sugar water by stepping in it, its proboscis (a tubular feeding structure) extends to feed. Since both AT and GC pairs obey the base-pairing rules, how does the repair mechanism “know” whether the AC pair should be repaired by removing the C and replace it with T, for instance, or by removing the A and replacing it with G? The repair mechanism can detect the "wrong" base because a newly synthesized DNA strand is chemically modified some time after replication. Whether the receptor protrudes from the plasma membrane surface or is located in the cytoplasm, the result of ligand binding is the same: the receptor protein changes its three-dimensional structure and initiates a cellular response. So the unique drug resistance phenotype of the cells with recombinant DNA (tetracycline-sensitive and ampicillin-resistant) marks them in a way that can be detected by simply adding ampicillin and/or tetracycline to the medium surrounding the cells. This receptor is located at the plasma membranes of vertebrate skeletal muscle cells and binds the ligand acetylcholine, which is released from nerve cells. |
4.4. Step Four

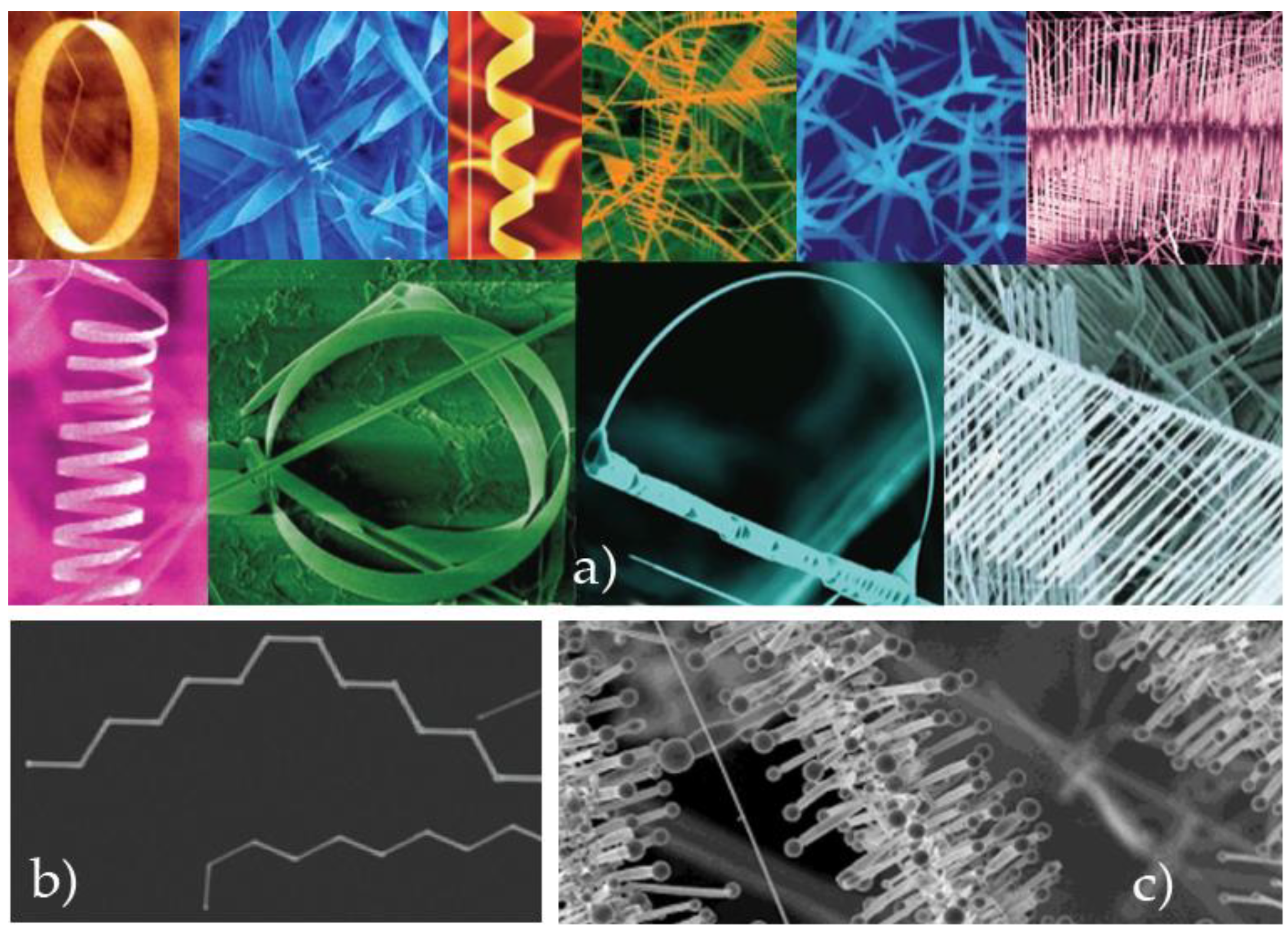
4.5. Step Five
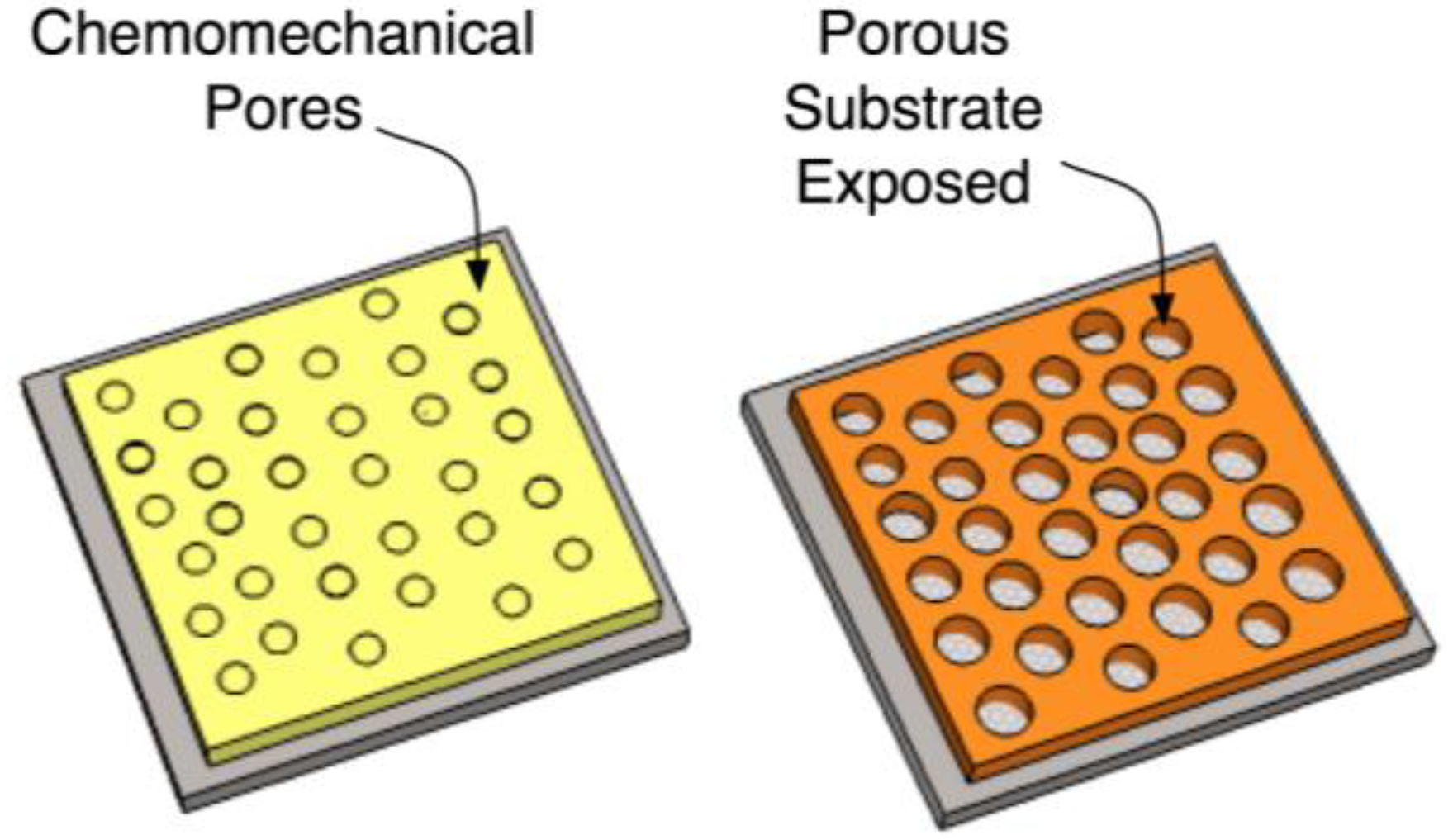
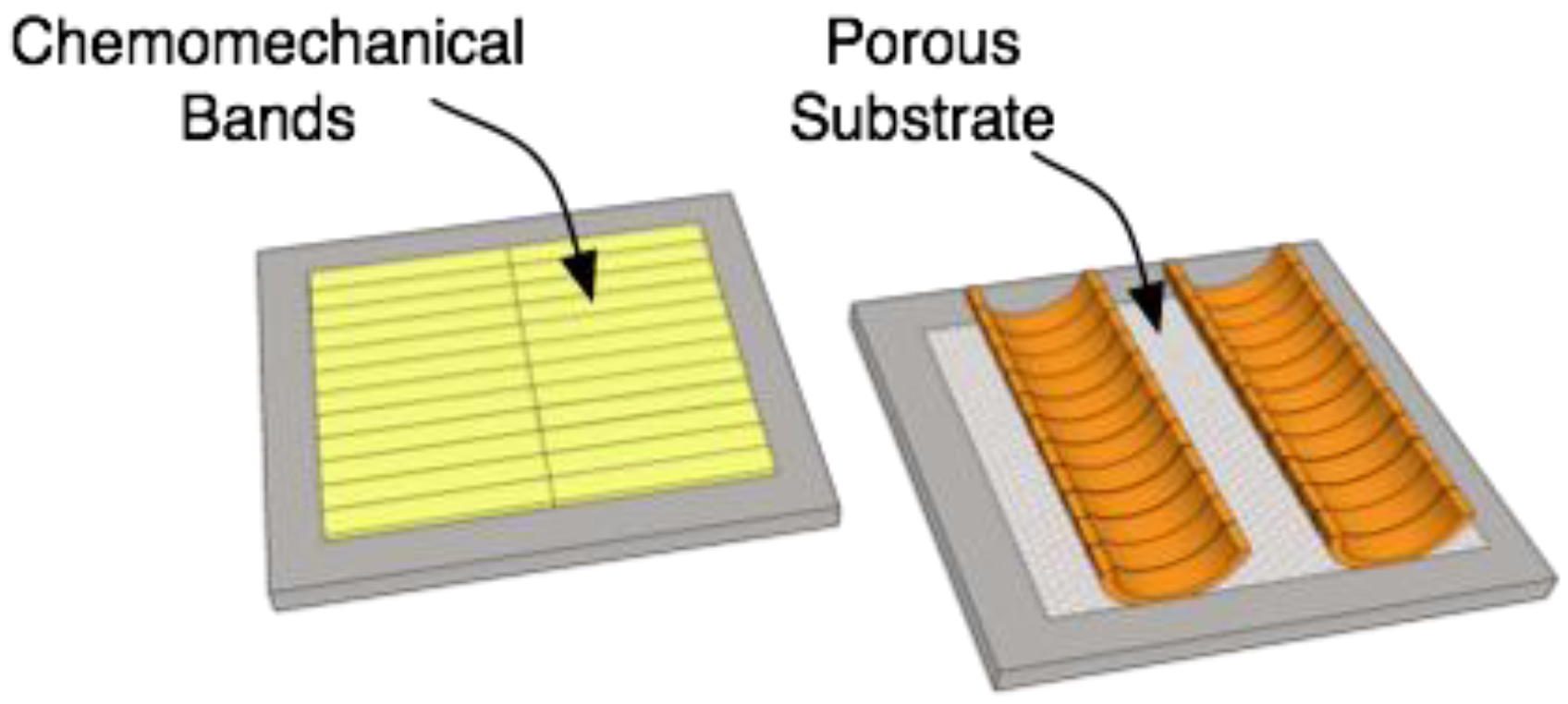

4.6. Final Concept

4.7. Applications of Biologically-Inspired Chemical Sensor
4.8. Evolution of Biologically-Inspired Chemical Sensor Design
5. Conclusions and Future Work
Conflicts of Interest
References
- Rolfe, P. Sensors and systems that mimic nature. Eng. Sci. Educ. J. 1997, 6, 155–166. [Google Scholar] [CrossRef]
- Stroble, J.K.; Stone, R.B.; Watkins, S.E. An overview of biomimetic sensor technology. Sens. Rev. 2009, 28, 112–119. [Google Scholar] [CrossRef]
- Del Valle, M. Bioinspired sensor systems. Sensors 2011, 11, 10180–10186. [Google Scholar] [CrossRef]
- Hussain, M.; Wackerlig, J.; Lieberzeit, P. Biomimetic strategies for sensing biological species. Biosensors 2013, 3, 89–107. [Google Scholar] [CrossRef]
- Ciosek, P.; Wróblewski, W. Potentiometric electronic tongues for foodstuff and biosample recognition—An overview. Sensors 2011, 11, 4688–4701. [Google Scholar] [CrossRef]
- Biggins, P.H.J.K.A. Bio-Inspired Materials and Sensing Systems; RSC Publishing: Cambridge, UK, 2011. [Google Scholar]
- Bar-Cohen, Y. Biomimetics Biologically Inspired Technologies; CRC/Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Brebbia, C.A. Design and Nature III: Comparing Design in Nature with Science and Engineering; WIT: Boston, MA, USA, 2006. [Google Scholar]
- Brebbia, C.A.; Collins, M.W. Design and Nature II: Comparing Design in Nature with Science and Engineering; WIT: Boston, MA, USA, 2004. [Google Scholar]
- Brebbia, C.A.; Sucharov, L.J.; Pascolo, P. Design and Nature: Comparing Design in Nature with Science and Engineering; WIT: Boston, MA, USA, 2002. [Google Scholar]
- Bleckmann, H.; Schmitz, H.; von der Emde, G. Nature as a model for technical sensors. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2004, 190, 971–981. [Google Scholar] [CrossRef]
- Barth, F.G.; Humphrey, J.A.C.; Secomb, T.W. Sensors and Sensing in Biology and Engineering; Springer: Wien, NY, USA, 2003. [Google Scholar]
- Toko, K. Biomimetic Sensor Technology; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Martin-Pereda, J.A.; Gonzalez-Marcos, A.P. A New Approach to Optical Fibre Sensing Techniques Based on the Sensory Systems of Living Bodies. In Handbook of Optical Fibre Sensing Technology; López-Higuera, J.M., Ed.; Wiley: New York, NY, USA, 2002. [Google Scholar]
- McGruer, N.E.; Ayers, J.; Davis, J.L.; Rudolph, A. Biomimetic Flow and Contact/Bending Mems Sensors. In Neurotechnology for Biomimetic Robots; The MIT Press: Cambridge, MA, USA, 2002; pp. 13–30. [Google Scholar]
- Krijnen, G.J.M.; Lammerink, T.S.J.; Wiegerink, R.J.; Casas, J. Cricket Inspired Flow-Sensor Arrays. In Proceedings of IEEE Sensors, Atlanta, GA, USA, 28–31 October 2007.
- Wu, W.-C.; Schenato, L.; Wood, R.J.; Fearing, R.S. Biomimetic Sensor Suite for Flight Control of a Micromechanical Flying Insect: Design and Experimental Results. In Proceedings of the IEEE International Conference on Robotics and Automation (ICRA), Pasadena, CA, USA, 14–19 September 2003.
- Van der Spiegel, J.; Nishimura, M. Biologically Inspired Vision Sensor for the Detection of Higher-Level Image Features. In Proceedings of the 2003 IEEE Conference on Electron Devices and Solid-State Circuits, HongKong, 2003; pp. 11–16.
- Jaxx, K.N.; Hannaford, B. Mechatronic design of an actuated biomimetic length and velocity sensor. IEEE Trans. Rob. Autom. 2004, 20, 390–398. [Google Scholar] [CrossRef]
- Kuc, R. Biomimetic sonar and neuromorphic processing eliminate reverberation artifacts. IEEE Sens. J. 2007, 7, 361–369. [Google Scholar] [CrossRef]
- Skordos, A.; Chan, P.H.; Vincent, J.F.V.; Jeronimidis, G. A novel strain sensor based on the campaniform sensillum of insects. Phys. Trans. R. Soc. Lond. A 2002, 360, 239–253. [Google Scholar] [CrossRef] [Green Version]
- Fraden, J. Handbook of Modern Sensors: Physics, Designs, and Applications; Springer: New York, NY, USA, 2004. [Google Scholar]
- Wilson, J.S. Sensor Technology Handbook; Elsevier: Boston, MA, USA, 2005. [Google Scholar]
- Frank, R. Understanding Smart Sensors; Artech House: Norwood, MA, USA, 1996. [Google Scholar]
- Webster, J.G. The Measurement, Instrumentation, and Sensors Handbook; CRC Press in Cooperation with IEEE Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Stillwell, H.R. Electronic Product Design for Automated Manufacturing; Marcel Dekker: New York, NY, USA, 1989. [Google Scholar]
- Ward, A.E.; Angus, J.A.S. Electronic Product Design; Chapman & Hall: London, UK, 1996. [Google Scholar]
- Haskell, B. Portable Electronics Product Design & Development: For Cellular Phones, Pdas, Digital Cameras, Personal Electronics and More; McGraw-Hill Professional: New York, NY, USA, 2009. [Google Scholar]
- Ulrich, K.T.; Eppinger, S.D. Product Design and Development; McGraw-Hill/Irwin: Boston, MA, USA, 2004. [Google Scholar]
- Ullman, D.G. The Mechanical Design Process, 4th ed.; McGraw-Hill, Inc.: New York, NY, USA, 2009. [Google Scholar]
- Pahl, G.; Beitz, W.; Feldhusen, J.; Grote, K.H. Engineering Design: A Systematic Approach, 3rd ed.; Springer Verlag: Berlin, Germany, 2007. [Google Scholar]
- Otto, K.N.; Wood, K.L. Product Design: Techniques in Reverse Engineering and New Product Development; Prentice-Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Doebelin, E.O. Measurement Systems: Application and Design; McGraw-Hill: Boston, MA, USA, 2004. [Google Scholar]
- Toko, K. Measurement of Taste and Smell Using Biomimetic Sensor. In Proceedings of the 17th IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Maastricht, The Netherlands, 25–29 January 2004; pp. 201–207.
- Ghasemi-Varnamkhasti, M.; Mohtasebi, S.S.; Siadat, M. Biomimetic-based odor and taste sensing systems to food quality and safety characterization: An overview on basic principles and recent achievements. J. Food Eng. 2010, 100, 377–387. [Google Scholar] [CrossRef]
- Tan, Y.; Nie, L.; Yao, S. A piezoelectric biomimetic sensor for aminopyrine with a molecularly imprinted polymer coating. Analyst 2001, 126, 664–668. [Google Scholar] [CrossRef]
- Nagle, H.T.; Gutierrez-Osuna, R.; Schiffman, S.S. The how and why of electronic noses. IEEE Spectr. 1998, 35, 22–31. [Google Scholar]
- Che Harun, F.K.; Taylor, J.E.; Covington, J.A.; Gardner, J.W. An electronic nose employing dual-channel odour separation columns with large chemosensor arrays for advanced odour discrimination. Sens. Actuat. B Chem. 2009, 141, 134–140. [Google Scholar] [CrossRef]
- Bar-Cohen, Y. Biomimetics—Using nature to inspire human innovation. J. Bioinspir. Biomim. 2006, 1, P1–P12. [Google Scholar] [CrossRef]
- Brudzewski, K.; Osowski, S.; Ulaczyk, J. Differential electronic nose of two chemo sensor arrays for odor discrimination. Sens. Actuat. B Chem. 2010, 145, 246–249. [Google Scholar] [CrossRef]
- Chen, P.C.; Ishikawa, F.N.; Chang, H.K.; Ryu, K.; Zhou, C. A nanoelectronic nose: A hybrid nanowire/carbon nanotube sensor array with integrated micromachined hotplates for sensitive gas discrimination. Nanotechnology 2009, 20, 1–8. [Google Scholar]
- Lee, S.H.; Park, T.H. Recent advances in the development of bioelectronic nose. Biotechnol. Bioprocess. Eng. 2010, 15, 22–29. [Google Scholar] [CrossRef]
- Williamson, M.M. Biologically Inspired Approaches to Computer Security; Information Infrastructure Laboratory, HP Laboratories: Bristol, UK, 2002. [Google Scholar]
- Smith, C.U.M. Biology of Sensory Systems; John Wiley: Chichester, NY, USA, 2000. [Google Scholar]
- Møller, A.R. Sensory Systems: Anatomy and Physiology; Academic Press: Boston, MA, USA, 2003. [Google Scholar]
- Spudich, J.L.; Satir, B.H. Sensory Receptors and Signal Transduction; Wiley-Liss: New York, NY, USA, 1991; Volume 10. [Google Scholar]
- Chamovitz, D. What a Plant Knows: A Field Guide to the Senses; Scientific American/Farrar, Straus and Giroux: New York, NY, USA, 2012. [Google Scholar]
- Nagel, J.K.S.; Stone, R.B.; McAdams, D.A. Exploring the Use of Category and Scale to Scope a Biological Functional Model. In Proceedings of the ASME International Design Engineering Technical Conference & Computers and Information in Engineering Conference, IDETC/CIE 2010, Montreal, Quebec, Canada, 15–18 August 2010.
- Nagel, J.K.S.; Stone, R.B. A Systematic Approach to Biologically-Inspired Engineering Design. In Proceedings of the ASME International Design Engineering Technical Conference & Computers and Information in Engineering Conference, IDETC/CIE 2011, Washington, DC, USA, 29–31 August 2011.
- Goel, A.; McAdams, D.A.; Stone, R.B. Biologically Inspired Design: Computational Methods and Tools; Springer: London, UK, 2013. [Google Scholar]
- Gebeshuber, I.C.; Drack, M. An attempt to reveal synergies between biology and mechanical engineering. Proc. Inst. Mech. Eng. C 2008, 222, 1281–1287. [Google Scholar] [CrossRef]
- Hirtz, J.; Stone, R.; McAdams, D.; Szykman, S.; Wood, K. A functional basis for engineering design: Reconciling and evolving previous efforts. Res. Eng. Design 2002, 13, 65–82. [Google Scholar]
- Nagel, J.K.S.; Nagel, R.L.; Stone, R.B.; McAdams, D.A. Function-based, biologically inspired concept generation. Aitif. Intell. Eng. Des. Anal. Manuf. 2010, 24, 521–535. [Google Scholar] [CrossRef]
- Campbell, N.A.; Reece, J.B. Biology; Pearson Benjamin Cummings: San Francisco, FL, USA, 2003. [Google Scholar]
- Raven, P.H.; Johnson, G.B. Biology; McGraw-Hill: Boston, MA, USA, 2002. [Google Scholar]
- Martin, E.; Hine, R.S. Oxford Dictionary of Biology; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Henderson, I.F.; Lawrence, E. Henderson’s Dictionary of Biology; Pearson Education: Harlow, Essex, England, 2005. [Google Scholar]
- Farabee, M.J. Plants and Their Structure. Available online: http://www.biologie.uni-hamburg.de/b-online/library/onlinebio/BioBookPLANTANAT.html (accessed on 1 May 2010).
- Shahinpoor, M.; Schneider, H.-J. Intelligent Materials; RSC Publication: Cambridge, UK, 2008. [Google Scholar]
- Schneider, H.-J.R.; Kato, K.; Strongin, R.M. Chemomechanical polymers as sensors and actuators for biological and medicinal applications. Sensors 2007, 7, 1578–1611. [Google Scholar] [CrossRef]
- Wang, Z.L. Self-assembled nanoarchitectures of polar nanobelts/nanowires. J. Mater. Chem. 2005, 15, 1021–1024. [Google Scholar] [CrossRef]
- Hart, A.; Sengupta, P. Sensory Transduction Mechanisms. In Encyclopedia of Life Sciences; John Wiley & Sons: London, UK, 2005; Volume 17, pp. 107–114. [Google Scholar]
- Mitchell, B.K. Chemoreception. In Encyclopedia of Insects; Academic Press: Amsterdam, The Netherlands, 2003; pp. 169–174. [Google Scholar]
- Lu, W.; Lieber, C.M. Semiconductor nanowires. J. Phys. D Appl. Phys. 2006, 39, R387–R406. [Google Scholar] [CrossRef]
- Kalantar-zadeh, K.; Fry, B.N. Nanotechnology-Enabled Sensors; Springer: New York, NY, USA, 2008. [Google Scholar]
- Harnett, C. Nanotechnology in environmental sensors. IEEE Instrum. Measur. Mag. 2010, 13, 8–12. [Google Scholar] [CrossRef]
- Wang, Z.L. Piezoelectric nanostructures: From growth phenomena to electric nanogenerators. MRS Bull. 2007, 32, 109–116. [Google Scholar] [CrossRef]
- Tian, B.; Xie, P.; Kempa, T.J.; Bell, D.C.; Lieber, C.M. Single crystalline kinked semiconductor nanowire superstructures. Nat. Nanotechnol. 2009, 4, 824–829. [Google Scholar] [CrossRef]
- Gao, P.; Wang, Z.L. Self-assembled nanowire-nanoribbon junction arrays of ZnO. J. Phys. Chem. B 2002, 106, 12653–12658. [Google Scholar] [CrossRef]
- Bhushan, B. Springer Handbook of Nanotechnology; Springer: New York, NY, USA, 2004. [Google Scholar]
- Lavrik, N.V.; Sepaniak, M.J.; Datskos, P.G. Cantilever transducers as a platform for chemcial and biological sensors. Rev. Sci. Instrum. 2004, 75, 2229–2253. [Google Scholar] [CrossRef]
- Law, M.; Goldberger, J.; Yang, P. Semiconductor nanowires and nanotubes. Annu. Rev. Mater. Res. 2004, 34, 83–122. [Google Scholar] [CrossRef]
- Menon, M.; Srivastava, D. Nanomechanics of silicon nanowires. Phys. Rev. B 2004, 70, 125313. [Google Scholar] [CrossRef]
- Postma, H.W.C.; Kozinsky, I.; Husain, A.; Roukes, M.L. Dynamic range of nanotube- and nanowire-based electromechanical systems. Appl. Phys. Lett. 2005, 86, 223105. [Google Scholar] [CrossRef]
- Tonisch, K.; Cimalla, V.; Will, F.; Weise, F.; Stubenrauch, M.; Albrecht, A.; Hoffmann, M.; Ambacher, O. Nanowire-based electromechanical biomimetic sensor. Phys. E 2007, 37, 208–211. [Google Scholar] [CrossRef]
- Lübbers, B.; Kittler, G.; Ort, P.; Linkohr, S.; Wegener, D.; Baur, B.; Gebinoga, M.; Weise, F.; Eickhoff, M.; Maroldt, S.; et al. A novel gan-based multiparameter sensor system for biochemical analysis. Phys. Status Solidi C 2008, 5, 2361–2362. [Google Scholar] [CrossRef]
- Niebelschütz, F.; Cimalla, V.; Tonisch, K.; Haupt, C.; Brückner, K.; Stephan, R.; Hein, M.; Ambacher, O. Algan/gan-based mems with two-dimensional electron gas for novel sensor applications. Phys. Status Solidi C 2008, 5, 1914–1916. [Google Scholar] [CrossRef]
- Brueckner, K.; Niebelschuetz, F.; Tonisch, K.; Stephan, R.; Cimalla, V.; Ambacher, O.; Hein, M.A. Resonant Piezoelectric Algan/Gan Mems Sensors in Longitudinal Mode Operation. In Proceedings of the Micro Electro Mechanical Systems (MEMS 2009), Sorrento, Italy, 25–29 January 2009; pp. 927–930.
- Lauhon, L.J.; Gudiksen, M.S.; Wang, D.; Lieber, C.M. Epitaxial core-shell and core-multi-shell nanowire heterostructures. Nature 2002, 420, 57–61. [Google Scholar] [CrossRef]
- Lu, W.; Xie, P.; Lieber, C.M. Nanowire transistor performance limits and applications. IEEE Trans. Electron. Devices 2008, 55, 2859–2876. [Google Scholar] [CrossRef]
- Tian, B.; Kempa, T.J.; Lieber, C.M. Single nanowire photovoltaics. Chem. Soc. Rev. 2009, 38, 16–24. [Google Scholar] [CrossRef]
- Kong, X.Y.; Wang, Z.L. Spontaneous polarization-induced nanohelixes, nanosprings, and nanorings of piezoelectric nanobelts. Nano Lett. 2003, 3, 1625–1631. [Google Scholar] [CrossRef]
- Richters, J.-P.; Voss, T.; Wischmeier, L.; Ruckmann, I.; Gutowski, J. Influence of polymer coating on the low-temperature photoluminescence properties of ZnO nanowires. Appl. Phys. Lett. 2008, 92, 011103. [Google Scholar] [CrossRef]
- Tenhaeff, W.E.; Gleason, K.K. Initiated and oxidative chemical vapor deposition of polymeric thin films: iCVD and oCVD. Adv. Funct. Mater. 2008, 18, 979–992. [Google Scholar] [CrossRef]
- Gao, P.-X.; Liu, J.; Buchine, B.A.; Weintraub, B.; Wang, Z.L.; Lee, J.L. Bridged ZnO nanowires across trenched electrodes. Appl. Phys. Lett. 2007, 91, 142108. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, D.; Chang, P.-C.; Tseng, W.-Y.; Lu, J.G. ZnO nanowire field-effect transistor and oxygen sensing property. Appl. Phys. Lett. 2004, 85, 5923–5925. [Google Scholar] [CrossRef]
- Fan, Z.; Lu, J.G. Chemical sensing with ZnO nanowire field-effect transistor. IEEE Trans. Nanotechnol. 2006, 5, 393–396. [Google Scholar] [CrossRef]
- Mulchandani, A.; Sadik, O.A. Chemical and Biological Sensors for Environmental Monitoring; American Chemical Society: Washington, DC, USA, 2000. [Google Scholar]
- Fryxell, G.E.; Cao, G. Environmental Applications of Nanomaterials: Synthesis, Sorbents and Sensors; Imperial College Press: London, UK, 2007. [Google Scholar]
- Amann, A.; Smith, D. Breath Analysis for Clinical Diagnosis and Therapeutic Monitoring; World Scientific: Hackensack, NJ, USA, 2005. [Google Scholar]
- Cao, W.; Duan, Y. Breath analysis: Potential for clinical diagnosis and exposure assessment. Clin. Chem. 2006, 52, 800–811. [Google Scholar] [CrossRef]
- Spichiger-Keller, U.E. Chemical Sensors and Biosensors for Medical and Biological Applications; Wiley-VCH: Weinheim, Germany, 1998. [Google Scholar]
- Zhang, X.; Ju, H.; Wang, J. Electrochemical Sensors, Biosensors and Their Biomedical Applications; Elsevier/Academic Press: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Wynn, D.; Clarkson, J. Models of Designing. In Design Process Improvement; Clarkson, J., Eckert, C., Eds.; Springer: London, UK, 2005; pp. 34–59. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nagel, J.K.S. Guard Cell and Tropomyosin Inspired Chemical Sensor. Micromachines 2013, 4, 378-401. https://doi.org/10.3390/mi4040378
Nagel JKS. Guard Cell and Tropomyosin Inspired Chemical Sensor. Micromachines. 2013; 4(4):378-401. https://doi.org/10.3390/mi4040378
Chicago/Turabian StyleNagel, Jacquelyn K.S. 2013. "Guard Cell and Tropomyosin Inspired Chemical Sensor" Micromachines 4, no. 4: 378-401. https://doi.org/10.3390/mi4040378
APA StyleNagel, J. K. S. (2013). Guard Cell and Tropomyosin Inspired Chemical Sensor. Micromachines, 4(4), 378-401. https://doi.org/10.3390/mi4040378




