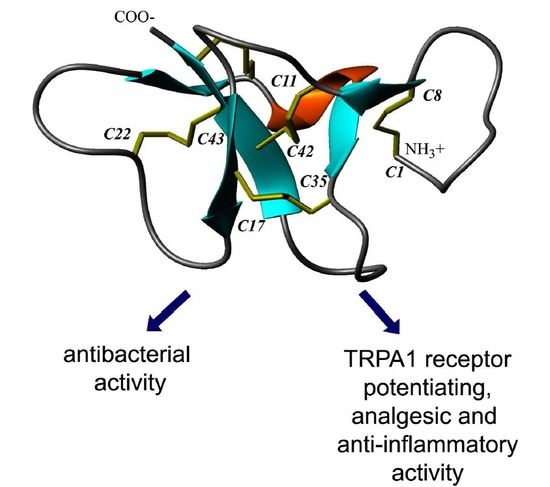
New Disulfide-Stabilized Fold Provides Sea Anemone Peptide to Exhibit Both Antimicrobial and TRPA1 Potentiating Properties
Abstract
:1. Introduction
2. Results
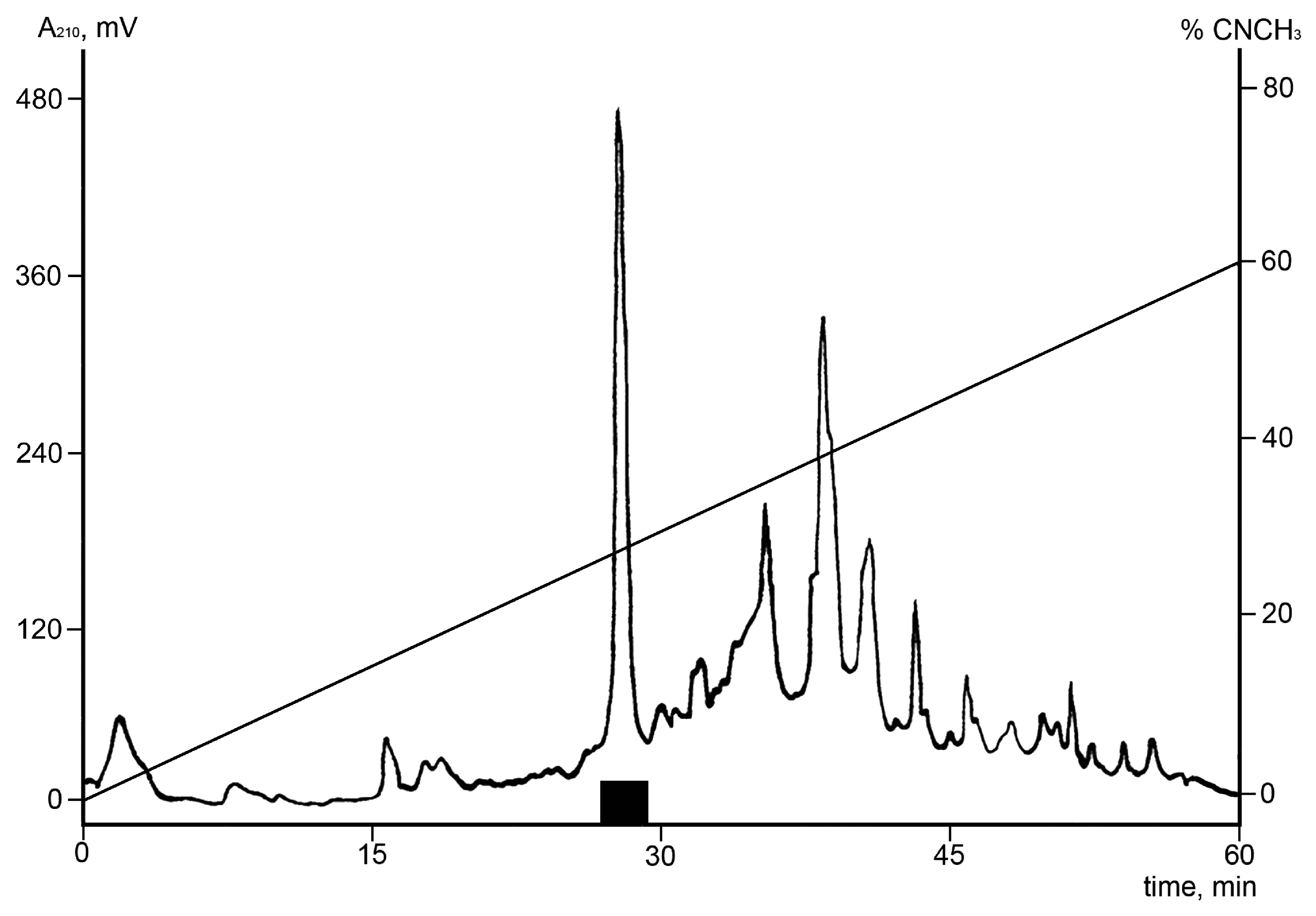
2.1. Isolation of Ueq 12-1
2.2. Ueq 12-1 Amino Acid Sequence Determination
2.3. Production of Recombinant Ueq 12-1
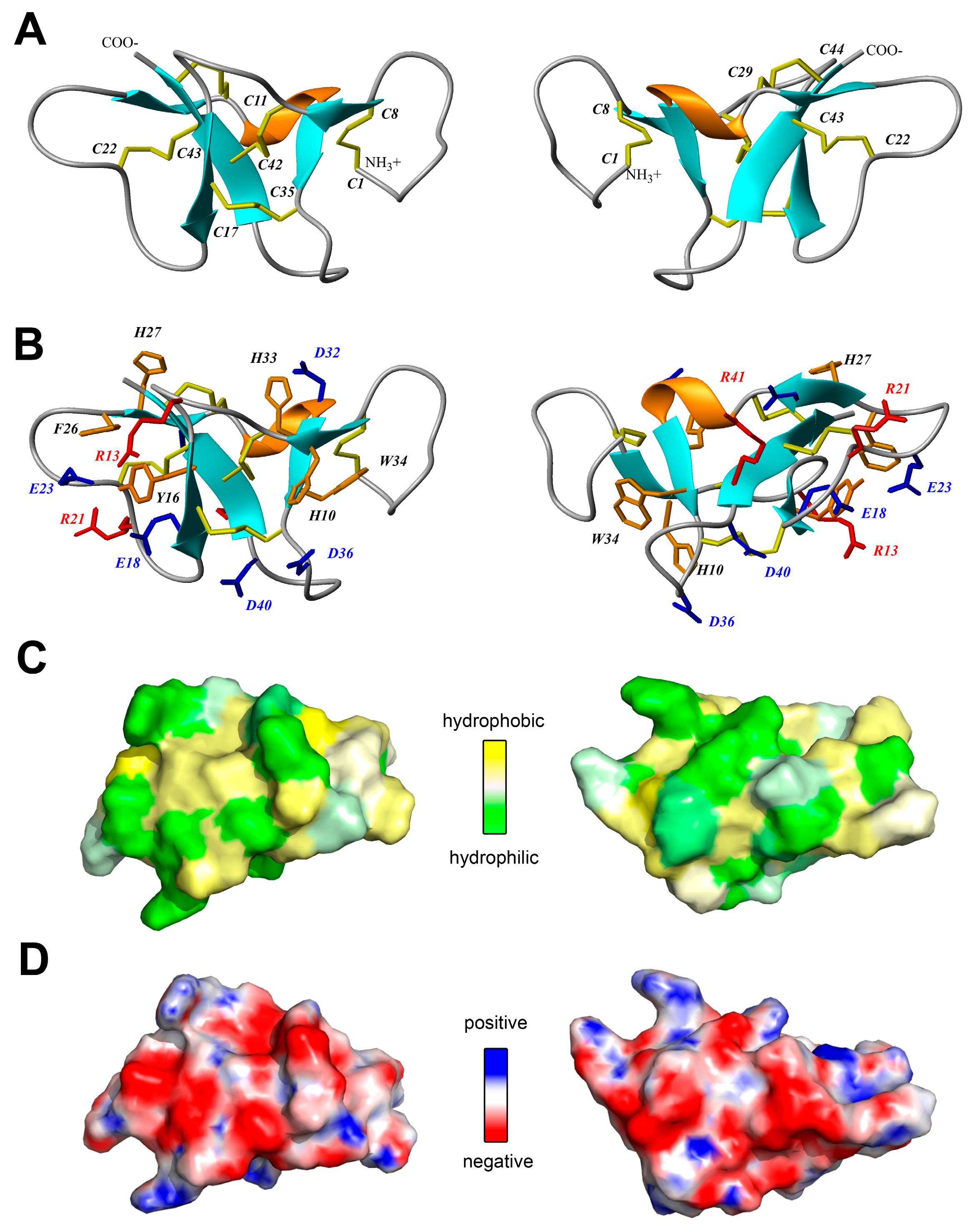
2.4. Spatial Structure of Ueq 12-1 in Water Solution
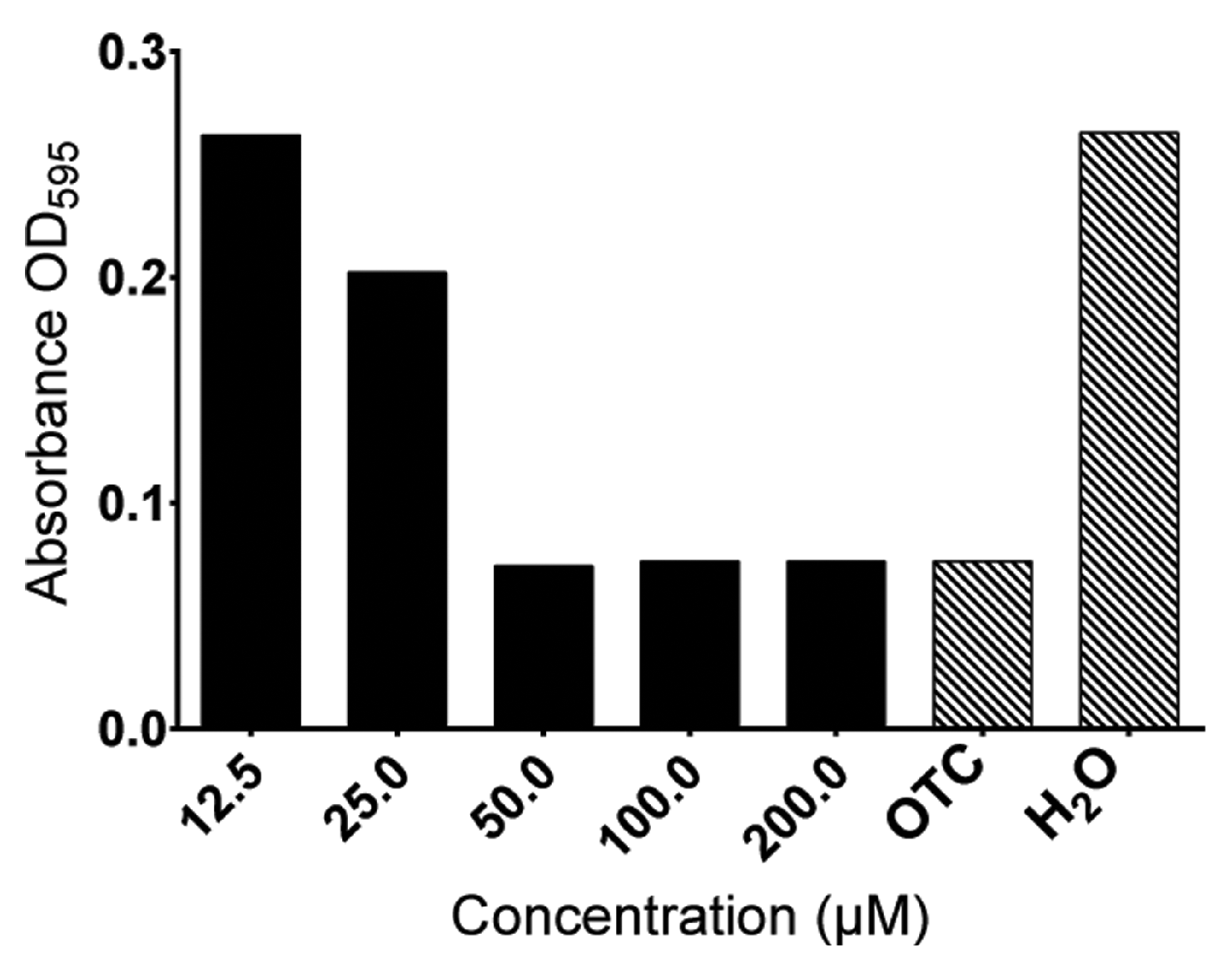
2.5. In Vitro Antimicrobial Activities of Ueq 12-1
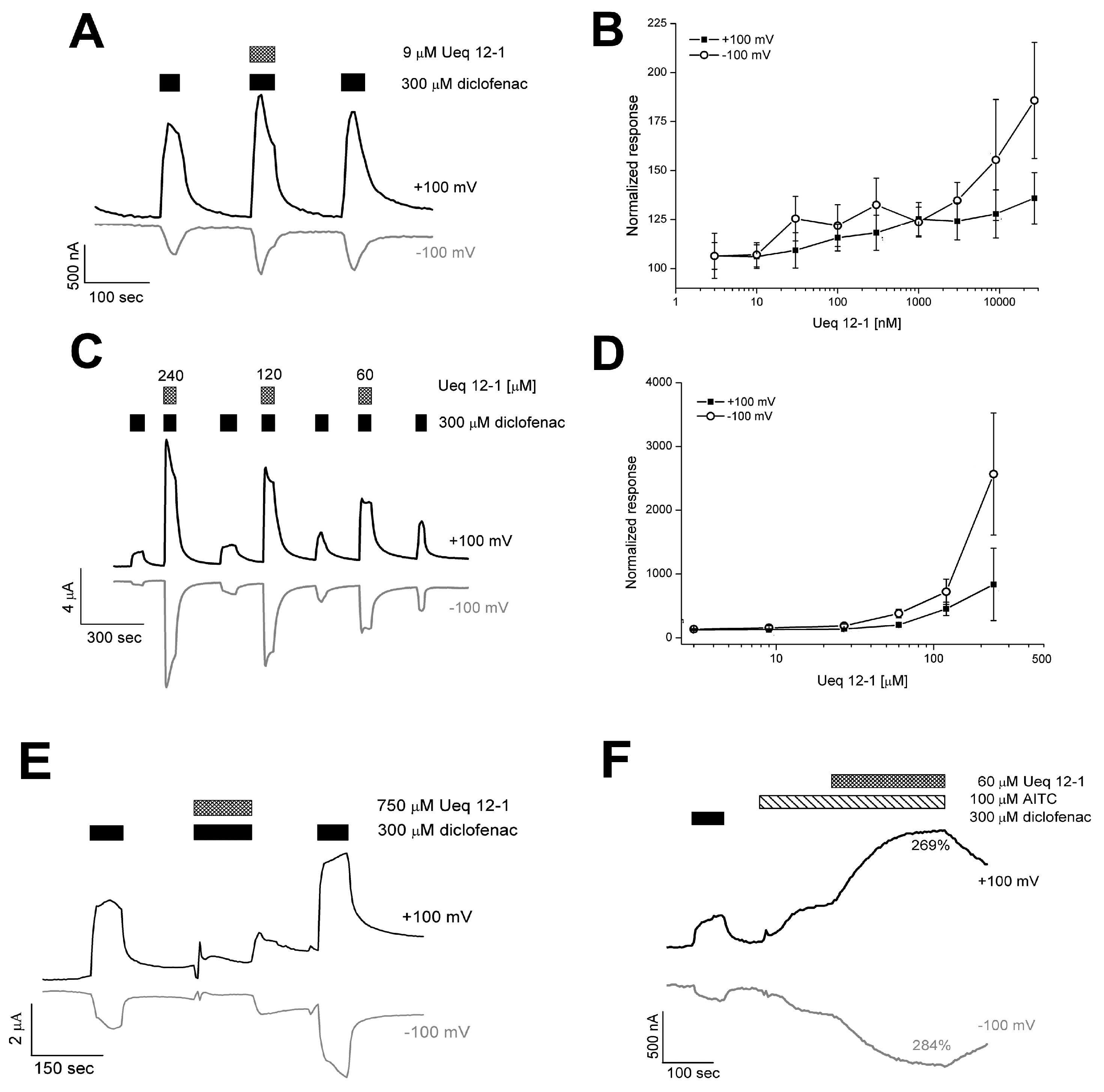
2.6. Effect of Ueq 12-1 on TRPA1 Activity
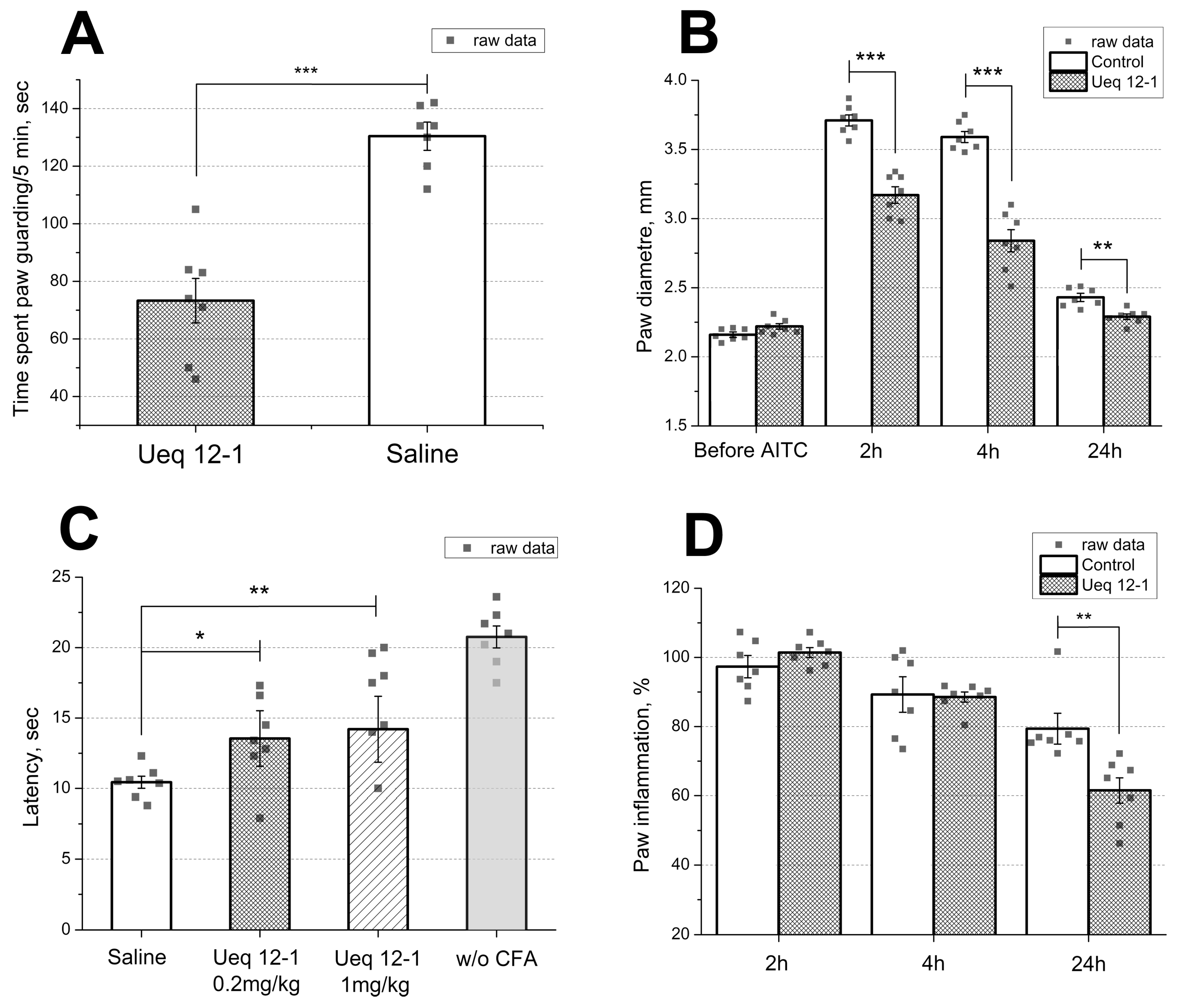
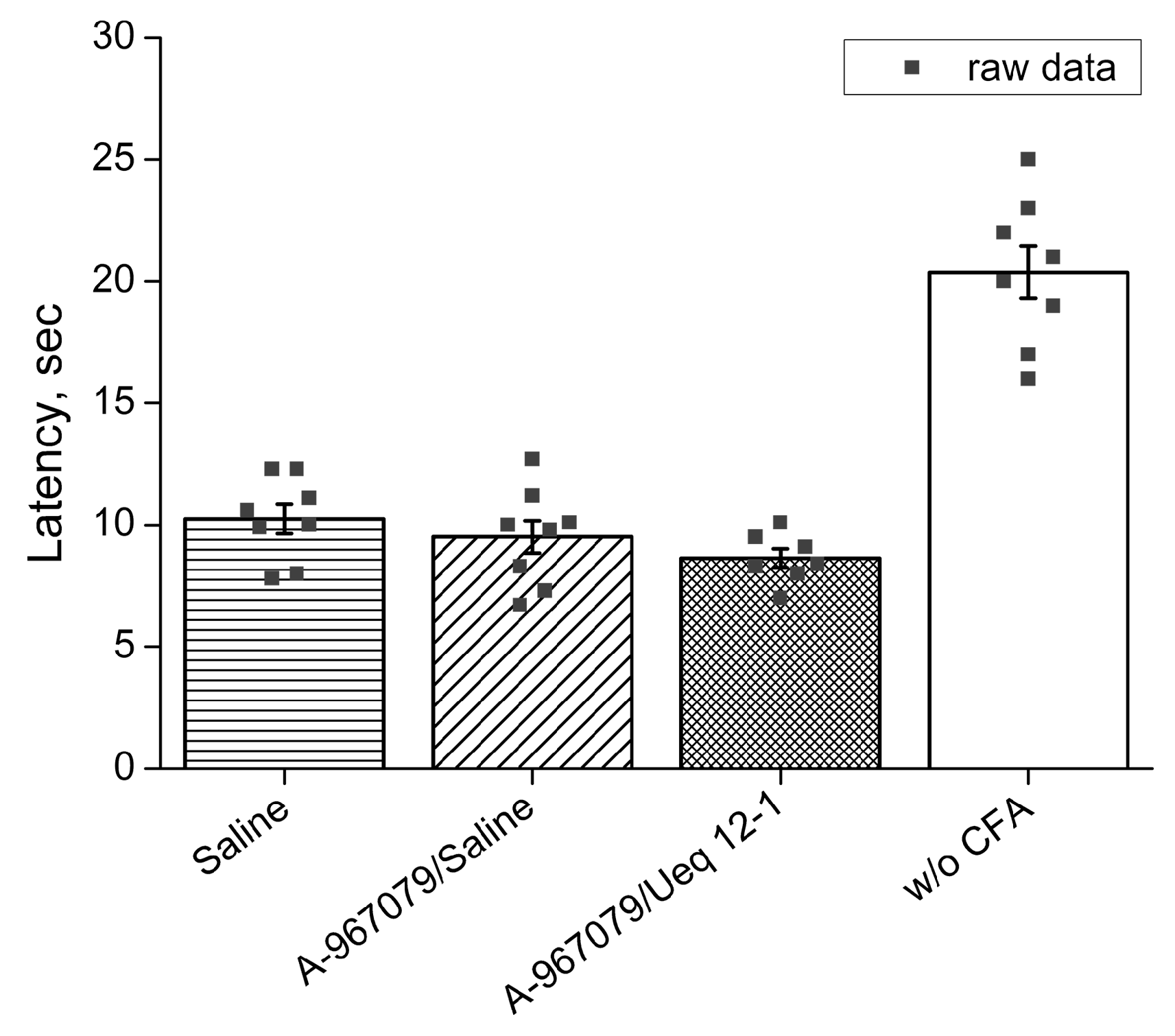
2.7. In Vivo Bioactivites of Ueq 12-1
3. Discussion
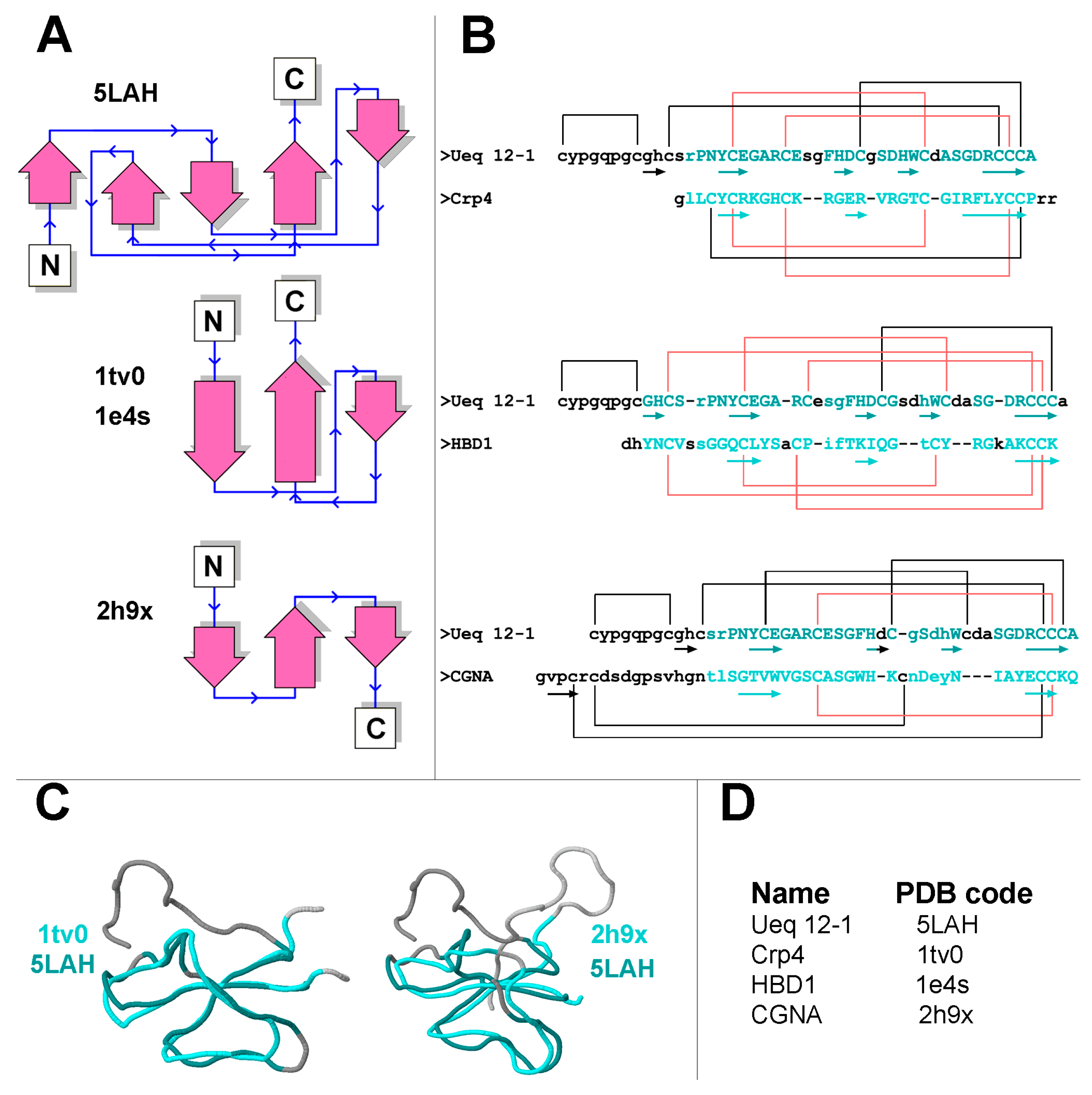
3.1. Primary Structure Analysis of Ueq 12-1
3.2. Spatial Structure Analysis of Ueq 12-1
3.3. Bioactivity Analysis of Ueq 12-1
4. Conclusions
5. Materials and Methods
5.1. Animal Material and Sample Preparation
5.2. High Performance Liquid Chromatography (HPLC) and Mass Spectrometry
5.3. Sequencing and Structure Elucidation
5.4. Precursor Determination and Gene Synthesis
5.5. Bioinformatics
5.6. Recombinant Peptide Production
5.7. NMR Spectroscopy and Calculation of Spatial Structure
5.8. Antibacterial Activity Assay
5.9. TRPA1 Activity Assays
5.9.1. Fluorescent Assay of Calcium Influx
5.9.2. Electrophysiology
5.10. In Vivo Experiments
5.10.1. Animal Models
5.10.2. Test on Pain/Thermal Hyperalgesia-Evoking Activity
5.10.3. “Open Field” Test on Locomotor Activity
5.10.4. Allyl Isothiocyanate (AITC)-Induced Nocifensive Beaviour
5.10.5. Complete Freund’s Adjuvant (CFA)-Induced Inflammation and Thermal Hyperalgesia
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jouiaei, M.; Yanagihara, A.A.; Madio, B.; Nevalainen, T.J.; Alewood, P.F.; Fry, B.G. Ancient Venom Systems: A Review on Cnidaria Toxins. Toxins 2015, 7, 2251–2271. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.G. Phylogeny of Medusozoa and the evolution of cnidarian life cycles. J. Evol. Biol. 2002, 15, 418–432. [Google Scholar] [CrossRef]
- Beckmann, A.; Özbek, S. The nematocyst: A molecular map of the cnidarian stinging organelle. Int. J. Dev. Biol. 2012, 56, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Conklin, E.J.; Bigger, C.H.; Mariscal, R.N. The Formation and Taxonomic Status of the Microbasic Q-Mastigophore Nematocyst of Sea Anemones. Biol. Bull. 1977, 152, 159–168. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Dingley, A.J.; Augustin, R.; Anton-Erxleben, F.; Stanisak, M.; Gelhaus, C.; Gutsmann, T.; Hammer, M.U.; Podschun, R.; Bonvin, A.M.; et al. Hydramacin-1, structure and antibacterial activity of a protein from the basal metazoan Hydra. J. Biol. Chem. 2009, 284, 1896–1905. [Google Scholar] [CrossRef] [PubMed]
- Augustin, R.; Anton-Erxleben, F.; Jungnickel, S.; Hemmrich, G.; Spudy, B.; Podschun, R.; Bosch, T.C.G. Activity of the Novel Peptide Arminin against Multiresistant Human Pathogens Shows the Considerable Potential of Phylogenetically Ancient Organisms as Drug Sources. Antimicrob. Agents Chemother. 2009, 53, 5245–5250. [Google Scholar] [CrossRef] [PubMed]
- Bosch, T.C.; Augustin, R.; Anton-Erxleben, F.; Fraune, S.; Hemmrich, G.; Zill, H.; Rosenstiel, P.; Jacobs, G.; Schreiber, S.; Leippe, M.; et al. Uncovering the evolutionary history of innate immunity: The simple metazoan Hydra uses epithelial cells for host defence. Dev. Comp. Immunol. 2009, 33, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikova, T.V.; Balandin, S.V.; Aleshina, G.M.; Tagaev, A.A.; Leonova, Y.F.; Krasnodembsky, E.D.; Men’shenin, A.V.; Kokryakov, V.N. Aurelin, a novel antimicrobial peptide from jellyfish Aurelia aurita with structural features of defensins and channel-blocking toxins. Biochem. Biophys. Res. Commun. 2006, 348, 514–523. [Google Scholar] [CrossRef] [PubMed]
- De Lima, L.A.; Migliolo, L.; Barreiro e Castro, C.; Pires Dde, O.; Lopez-Abarrategui, C.; Goncalves, E.F.; Vasconcelos, I.M.; de Oliveira, J.T.; Otero-Gonzalez Ade, J.; Franco, O.L.; et al. Identification of a novel antimicrobial peptide from Brazilian coast coral Phyllogorgia dilatata. Protein Pept. Lett. 2013, 20, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Trapani, M.R.; Parisi, M.G.; Toubiana, M.; Coquet, L.; Jouenne, T.; Roch, P.; Cammarata, M. First evidence of antimicrobial activity of neurotoxin 2 from Anemonia sulcata (Cnidaria). ISJ Invertebr. Surviv. J. 2014, 11, 182–191. [Google Scholar]
- Béress, L.; Béress, R.; Wunderer, G. Purification of three polypeptides with neuroand cardiotoxic activity from the sea anemone Anemonia sulcata. Toxicon 1975, 13, 359–364. [Google Scholar] [CrossRef]
- Béress, L.; Béress, R.; Wunderer, G. Isolation and characterisation of three polypeptides with neurotoxic activity from Anemonza sulcata. FEBS Lett. 1975, 50, 311–314. [Google Scholar] [CrossRef]
- Moran, Y.; Gordon, D.; Gurevitz, M. Sea anemone toxins affecting voltage-gated sodium channels—Molecular and evolutionary features. Toxicon 2009, 54, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
- Norton, R.S. Structures of sea anemone toxins. Toxicon 2009, 54, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Osmakov, D.I.; Kozlov, S.A.; Andreev, Y.A.; Koshelev, S.G.; Sanamyan, N.P.; Sanamyan, K.E.; Dyachenko, I.A.; Bondarenko, D.A.; Murashev, A.N.; Mineev, K.S.; et al. V Sea anemone peptide with uncommon beta-hairpin structure inhibits acid-sensing ion channel 3 (ASIC3) and reveals analgesic activity. J. Biol. Chem. 2013, 288, 23116–23127. [Google Scholar] [CrossRef] [PubMed]
- Andreev, Y.A.; Kozlov, S.A.; Koshelev, S.G.; Ivanova, E.A.; Monastyrnaya, M.M.; Kozlovskaya, E.P.; Grishin, E. V Analgesic compound from sea anemone Heteractis crispa is the first polypeptide inhibitor of vanilloid receptor 1 (TRPV1). J. Biol. Chem. 2008, 283, 23914–23921. [Google Scholar] [CrossRef] [PubMed]
- Hoenderop, J.G.; Voets, T.; Hoefs, S.; Weidema, F.; Prenen, J.; Nilius, B.; Bindels, R.J. Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J. 2003, 22, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Moiseenkova-Bell, V.Y.; Stanciu, L.A.; Serysheva, I.I.; Tobe, B.J.; Wensel, T.G. Structure of TRPV1 channel revealed by electron cryomicroscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 7451–7455. [Google Scholar] [CrossRef] [PubMed]
- Jaquemar, D.; Schenker, T.; Trueb, B. An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J. Biol. Chem. 1999, 274, 7325–7333. [Google Scholar] [CrossRef] [PubMed]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef]
- Levine, J.D.; Alessandri-Haber, N. TRP channels: Targets for the relief of pain. Biochim. Biophys. Acta Mol. Basis Dis. 2007, 1772, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.S.; Fernandes, M.A.; Keeble, J.E. The functions of TRPA1 and TRPV1: Moving away from sensory nerves. Br. J. Pharmacol. 2012, 166, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Johansen, S.D.; Emblem, A.; Karlsen, B.O.; Okkenhaug, S.; Hansen, H.; Moum, T.; Coucheron, D.H.; Seternes, O.M. Approaching marine bioprospecting in hexacorals by RNA deep sequencing. New Biotechnol. 2010, 27, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Razpotnik, A.; Krizaj, I.; Sribar, J.; Kordis, D.; Macek, P.; Frangez, R.; Kem, W.R.; Turk, T. A new phospholipase A2 isolated from the sea anemone Urticina crassicornis—Its primary structure and phylogenetic classification. FEBS J. 2010, 277, 2641–2653. [Google Scholar] [CrossRef] [PubMed]
- Razpotnik, A.; Krizaj, I.; Kem, W.R.; Macek, P.; Turk, T. A new cytolytic protein from the sea anemone Urticina crassicornis that binds to cholesterol- and sphingomyelin-rich membranes. Toxicon 2009, 53, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Cline, E.I.; Wolowyk, M.W.; Wiebe, L.I.; George, R.; Samuel, J. Isolation and characterization of a novel cardiac stimulatory and haemolytic protein from the sea anemone Urticina piscivora (Sebens and Laakso). Pharm. Pharmacol. Commun. 1995, 1, 155–162. [Google Scholar]
- LaVallie, E.R.; DiBlasio, E.A.; Kovacic, S.; Grant, K.L.; Schendel, P.F.; McCoy, J.M. A thioredoxin gene fusion expression system that circumvents inclusion body formation in the E. coli cytoplasm. Biotechnology 1993, 11, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, T.; Guntert, P.; Wuthrich, K. Protein NMR structure determination with automated NOE assignment using the new software CANDID and the torsion angle dynamics algorithm DYANA. J. Mol. Biol. 2002, 319, 209–227. [Google Scholar] [CrossRef]
- Efremov, R.G.; Vergoten, G. Hydrophobic Nature of Membrane-Spanning Alpha-Helical Peptides as Revealed by Monte-Carlo Simulations and Molecular Hydrophobicity Potential Analysis. J. Phys. Chem. 1995, 99, 10658–10666. [Google Scholar] [CrossRef]
- Paulsen, V.S.; Blencke, H.M.; Benincasa, M.; Haug, T.; Eksteen, J.J.; Styrvold, O.B.; Scocchi, M.; Stensvåg, K. Structure-Activity Relationships of the Antimicrobial Peptide Arasin 1—And Mode of Action Studies of the N-Terminal, Proline-Rich Region. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Mikov, A.N.; Kozlov, S.A. Structural features of cysteine-rich polypeptides from sea anemone venoms. Russ. J. Bioorgan. Chem. 2015, 41, 455–466. [Google Scholar] [CrossRef]
- Pineda, S.S.; Undheim, E.A.; Rupasinghe, D.B.; Ikonomopoulou, M.P.; King, G.F. Spider venomics: Implications for drug discovery. Future Med. Chem. 2014, 6, 1699–1714. [Google Scholar] [CrossRef] [PubMed]
- Frazao, B.; Vasconcelos, V.; Antunes, A. Sea anemone (Cnidaria, Anthozoa, Actiniaria) toxins: An overview. Mar. Drugs 2012, 10, 1812–1851. [Google Scholar] [CrossRef] [PubMed]
- Jouiaei, M.; Sunagar, K.; Federman Gross, A.; Scheib, H.; Alewood, P.F.; Moran, Y.; Fry, B.G. Evolution of an ancient venom: Recognition of a novel family of cnidarian toxins and the common evolutionary origin of sodium and potassium neurotoxins in sea anemone. Mol. Biol. Evol. 2015, 32, 1598–1610. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, A.; Howorth, D.; Chandonia, J.M.; Brenner, S.E.; Hubbard, T.J.; Chothia, C.; Murzin, A.G. Data growth and its impact on the SCOP database: New developments. Nucleic Acids Res. 2008, 36, D419–D425. [Google Scholar] [CrossRef] [PubMed]
- Krissinel, E.; Henrick, K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 2256–2268. [Google Scholar] [CrossRef] [PubMed]
- Pazgier, M.; Hoover, D.M.; Yang, D.; Lu, W.; Lubkowski, J. Human beta-defensins. Cell. Mol. Life Sci. 2006, 63, 1294–1313. [Google Scholar] [CrossRef] [PubMed]
- Pallaghy, P.K.; Scanlon, M.J.; Monks, S.A.; Norton, R.S. 3-Dimensional Structure in Solution of the Polypeptide Cardiac Stimulant Anthopleurin-A. Biochemistry 1995, 34, 3782–3794. [Google Scholar] [CrossRef] [PubMed]
- Salceda, E.; Perez-Castells, J.; Lopez-Mendez, B.; Garateix, A.; Salazar, H.; Lopez, O.; Aneiros, A.; Standker, L.; Beress, L.; Forssmann, W.G.; et al. CgNa, a type I toxin from the giant Caribbean sea anemone Condylactis gigantea shows structural similarities to both type I and II toxins, as well as distinctive structural and functional properties. Biochem. J. 2007, 406, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Norton, R.S. Structure and Structure-Function-Relationships of Sea-Anemone Proteins That Interact with the Sodium-Channel. Toxicon 1991, 29, 1051–1084. [Google Scholar] [CrossRef]
- Nilius, B.; Flockerzi, V. Mammalian transient receptor potential (TRP) cation channels. Preface. In Handbook of Experimental Pharmacology; Springer: Berlin, Germany, 2014. [Google Scholar]
- Mahoney, J.L.; Graugnard, E.M.; Mire, P.; Watson, G.M. Evidence for involvement of TRPA1 in the detection of vibrations by hair bundle mechanoreceptors in sea anemones. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2011, 197, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, R.I.; Lu, W. alpha-Defensins in human innate immunity. Immunol. Rev. 2012, 245, 84–112. [Google Scholar] [CrossRef] [PubMed]
- Satchell, D.P.; Sheynis, T.; Kolusheva, S.; Cummings, J.; Vanderlick, T.K.; Jelinek, R.; Selsted, M.E.; Ouellette, A.J. Quantitative interactions between cryptdin-4 amino terminal variants and membranes. Peptides 2003, 24, 1795–1805. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Pellegrino, M.; Tsunozaki, M. TRPA1: A gatekeeper for inflammation. Annu. Rev. Physiol. 2013, 75, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Logashina, Y.A.; Mosharova, I.V.; Korolkova, Y.V.; Shelukhina, I.V.; Dyachenko, I.A.; Palikov, V.A.; Palikova, Y.A.; Murashev, A.N.; Kozlov, S.A.; Stensvag, K.; et al. Peptide from Sea Anemone Metridium senile Affects Transient Receptor Potential Ankyrin-repeat 1 (TRPA1) Function and Produces Analgesic Effect. J. Biol. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Schweitz, H.; Bruhn, T.; Guillemare, E.; Moinier, D.; Lancelin, J.M.; Beress, L.; Lazdunski, M. Kalicludines and kaliseptine. Two different classes of sea anemone toxins for voltage sensitive K+ channels. J. Biol. Chem. 1995, 270, 25121–25126. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernandez, R.; Peigneur, S.; Pons, T.; Alvarez, C.; Gonzalez, L.; Chavez, M.A.; Tytgat, J. The Kunitz-Type Protein ShPI-1 Inhibits Serine Proteases and Voltage-Gated Potassium Channels. Toxins 2016, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Andreev, Y.A.; Kozlov, S.A.; Korolkova, Y.V.; Dyachenko, I.A.; Bondarenko, D.A.; Skobtsov, D.I.; Murashev, A.N.; Kotova, P.D.; Rogachevskaja, O.A.; Kabanova, N.V.; et al. Polypeptide modulators of TRPV1 produce analgesia without hyperthermia. Mar. Drugs 2013, 11, 5100–5115. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Feng, J.; Xiang, F.; Xie, Z.; Zhang, G.; Sabatier, J.M.; Cao, Z.; Li, W.; Chen, Z.; Wu, Y. Endogenous animal toxin-like human beta-defensin 2 inhibits own K+ channels through interaction with channel extracellular pore region. Cell. Mol. Life Sci. 2015, 72, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Lu, Z.Y.; Li, N.; Yu, L.H.; Zhao, Y.F.; Ma, B. The role of large-conductance, calcium-activated potassium channels in a rat model of trigeminal neuropathic pain. Cephalalgia 2015, 35, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Materazzi, S.; Benemei, S.; Fusi, C.; Gualdani, R.; De Siena, G.; Vastani, N.; Andersson, D.A.; Trevisan, G.; Moncelli, M.R.; Wei, X.; et al. Parthenolide inhibits nociception and neurogenic vasodilatation in the trigeminovascular system by targeting the TRPA1 channel. Pain 2013, 154, 2750–2758. [Google Scholar] [CrossRef] [PubMed]
- Tyler, M.J.; Stone, D.J.; Bowie, J.H. A novel method for the release and collection of dermal, glandular secretions from the skin of frogs. J. Pharmacol. Toxicol. Methods 1992, 28, 199–200. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Improved methods for classification, prediction, and design of antimicrobial peptides. Methods Mol. Biol. 2015, 1268, 43–66. [Google Scholar] [PubMed]
- Jones, P.; Binns, D.; Chang, H.-Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-Scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.C.; Ryan, J.F.; Watson, J.A.; Webb, J.; Mullikin, J.C.; Rokhsar, D.; Finnerty, J.R. StellaBase: The Nematostella vectensis Genomics Database. Nucleic Acids Res. 2006, 34, D495–D499. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.N.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Expasy program. Avaliable online: http://us.expasy.org/ (accessed on 28 April 2017).
- Patiny, L.; Borel, A. ChemCalc: A building block for tomorrow’s chemical infrastructure. J. Chem. Inf. Model. 2013, 53, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Andreev, Y.A.; Kozlov, S.A.; Vassilevski, A.A.; Grishin, E.V. Cyanogen bromide cleavage of proteins in salt and buffer solutions. Anal. Biochem. 2010, 407, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Wuthrich, K. NMR of Proteins and Nucleic Acids; John Wiley Sons: New York, NY, USA, 1986. [Google Scholar]
- Delaglio, F.; Wu, Z.; Bax, A. Measurement of homonuclear proton couplings from regular 2D COSY spectra. J. Magn. Reson. 2001, 149, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Koradi, R.; Billeter, M.; Wuthrich, K. MOLMOL: A program for display and analysis of macromolecular structures. J. Mol. Graph. 1996, 14, 51–55. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Hutchinson, E.G.; Michie, A.D.; Wallace, A.C.; Jones, M.L.; Thornton, J.M. PDBsum: A Web-based database of summaries and analyses of all PDB structures. Trends Biochem. Sci. 1997, 22, 488–490. [Google Scholar] [CrossRef]
- APDS software. Avaliable online: http: www.poissonboltzmann.org (accessed on 28 April 2017).
- Protein Data Bank. Available online: www.pdb.org (accessed on 28 January 2017).
- Solstad, R.G.; Li, C.; Isaksson, J.; Johansen, J.; Svenson, J.; Stensvag, K.; Haug, T. Novel Antimicrobial Peptides EeCentrocins 1, 2 and EeStrongylocin 2 from the Edible Sea Urchin Echinus esculentus Have 6-Br-Trp Post-Translational Modifications. PLoS ONE 2016, 11, e0151820. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Tian, J.; Zhu, Y.; Wang, C.; Xiao, R.; Herz, J.M.; Wood, J.D.; Zhu, M.X. Activation of TRPA1 channels by fenamate nonsteroidal anti-inflammatory drugs. Pflugers Arch. 2010, 459, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Raisinghani, M.; Zhong, L.; Jeffry, J.A.; Bishnoi, M.; Pabbidi, R.M.; Pimentel, F.; Cao, D.S.; Evans, M.S.; Premkumar, L.S. Activation characteristics of transient receptor potential ankyrin 1 and its role in nociception. Am. J. Physiol. Cell Physiol. 2011, 301, C587–C600. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.R.; Crown, E.D.; Moore, E.L.; Liang, H.A.; Choong, K.C.; Dima, S.; Henze, D.A.; Kane, S.A.; Urban, M.O. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol. Pain 2008, 4, 48. [Google Scholar] [CrossRef] [PubMed]



| Distance and Angle Restraints | |
| Total NOEs | 308 |
| intraresidual | 88 |
| interresidual | 220 |
| sequential (|i − j| = 1) | 104 |
| Medium range (1 < |i − j| ≤ 4) | 36 |
| Long range (|i − j| > 4) | 80 |
| Hydrogen bond restraints (upper/lower) | 56/56 |
| S-S bond restraints (upper/lower) | 15/15 |
| J-couplings | 70 |
| JHNHα | 34 |
| JHαHβ | 36 |
| Total restraints/per residue: | 523/12 |
| Statistics of calculated set of structures | |
| CYANA target function (Å2) | 1.77 ± 0.04 |
| Restraint violations | |
| distance (>0.2 Å) | 3 |
| angle (>5°) | 0 |
| RMSD (Å) Elements with defined structure (6–27) | |
| Backbone | 0.38 ± 0.15 |
| All heavy atoms | 1.03 ± 0.14 |
| Ramachandran analysis | |
| % residues in most favored regions | 75.8 |
| % residues in additional allowed regions | 21.2 |
| % residues in generously allowed regions | 3.0 |
| % residues in disallowed regions | 0.0 |
| Peptides | PDB Code | N res | N Align | % Iden | Score | RMSD | |
|---|---|---|---|---|---|---|---|
| Q | Z | ||||||
| Cryptdin-4, alpha-defensin from mouse, a-defenM4, Crp4 | 1tv0 | 32 | 29 | 21 | 0.46 | 4.2 | 1.54 |
| Revised solution structure of platypus DLP-2 | 1zue | 42 | 38 | 13 | 0.40 | 4.0 | 2.85 |
| Human defensin HBD-2 | 1e4q | 37 | 34 | 18 | 0.39 | 3.7 | 2.67 |
| DLP-4 from platypus | 1zuf | 42 | 38 | 13 | 0.38 | 3.1 | 3.00 |
| Human defensin HBD-1 | 1e4s | 36 | 31 | 19 | 0.38 | 5.5 | 2.24 |
| Kalata B12 from Oldenlandia affinis | 2kvx | 28 | 22 | 14 | 0.31 | 2.8 | 1.42 |
| Anthopleurin-A from the sea anemone Anthopleura xanthogrammica | 1ahl | 49 | 30 | 23 | 0.26 | 5.3 | 2.27 |
| CGNA toxin from the sea anemone Condylactis gigantea | 2h9x | 47 | 30 | 20 | 0.25 | 5.0 | 2.50 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Logashina, Y.A.; Solstad, R.G.; Mineev, K.S.; Korolkova, Y.V.; Mosharova, I.V.; Dyachenko, I.A.; Palikov, V.A.; Palikova, Y.A.; Murashev, A.N.; Arseniev, A.S.; et al. New Disulfide-Stabilized Fold Provides Sea Anemone Peptide to Exhibit Both Antimicrobial and TRPA1 Potentiating Properties. Toxins 2017, 9, 154. https://doi.org/10.3390/toxins9050154
Logashina YA, Solstad RG, Mineev KS, Korolkova YV, Mosharova IV, Dyachenko IA, Palikov VA, Palikova YA, Murashev AN, Arseniev AS, et al. New Disulfide-Stabilized Fold Provides Sea Anemone Peptide to Exhibit Both Antimicrobial and TRPA1 Potentiating Properties. Toxins. 2017; 9(5):154. https://doi.org/10.3390/toxins9050154
Chicago/Turabian StyleLogashina, Yulia A., Runar Gjerp Solstad, Konstantin S. Mineev, Yuliya V. Korolkova, Irina V. Mosharova, Igor A. Dyachenko, Victor A. Palikov, Yulia A. Palikova, Arkadii N. Murashev, Alexander S. Arseniev, and et al. 2017. "New Disulfide-Stabilized Fold Provides Sea Anemone Peptide to Exhibit Both Antimicrobial and TRPA1 Potentiating Properties" Toxins 9, no. 5: 154. https://doi.org/10.3390/toxins9050154
APA StyleLogashina, Y. A., Solstad, R. G., Mineev, K. S., Korolkova, Y. V., Mosharova, I. V., Dyachenko, I. A., Palikov, V. A., Palikova, Y. A., Murashev, A. N., Arseniev, A. S., Kozlov, S. A., Stensvåg, K., Haug, T., & Andreev, Y. A. (2017). New Disulfide-Stabilized Fold Provides Sea Anemone Peptide to Exhibit Both Antimicrobial and TRPA1 Potentiating Properties. Toxins, 9(5), 154. https://doi.org/10.3390/toxins9050154