Characterization of Post-Translational Modifications and Cytotoxic Properties of the Adenylate-Cyclase Hemolysin Produced by Various Bordetella pertussis and Bordetella parapertussis Isolates
Abstract
:1. Introduction
2. Results
2.1. Genomic Analysis of the B. pertussis and B. parapertussis cyaA Genes
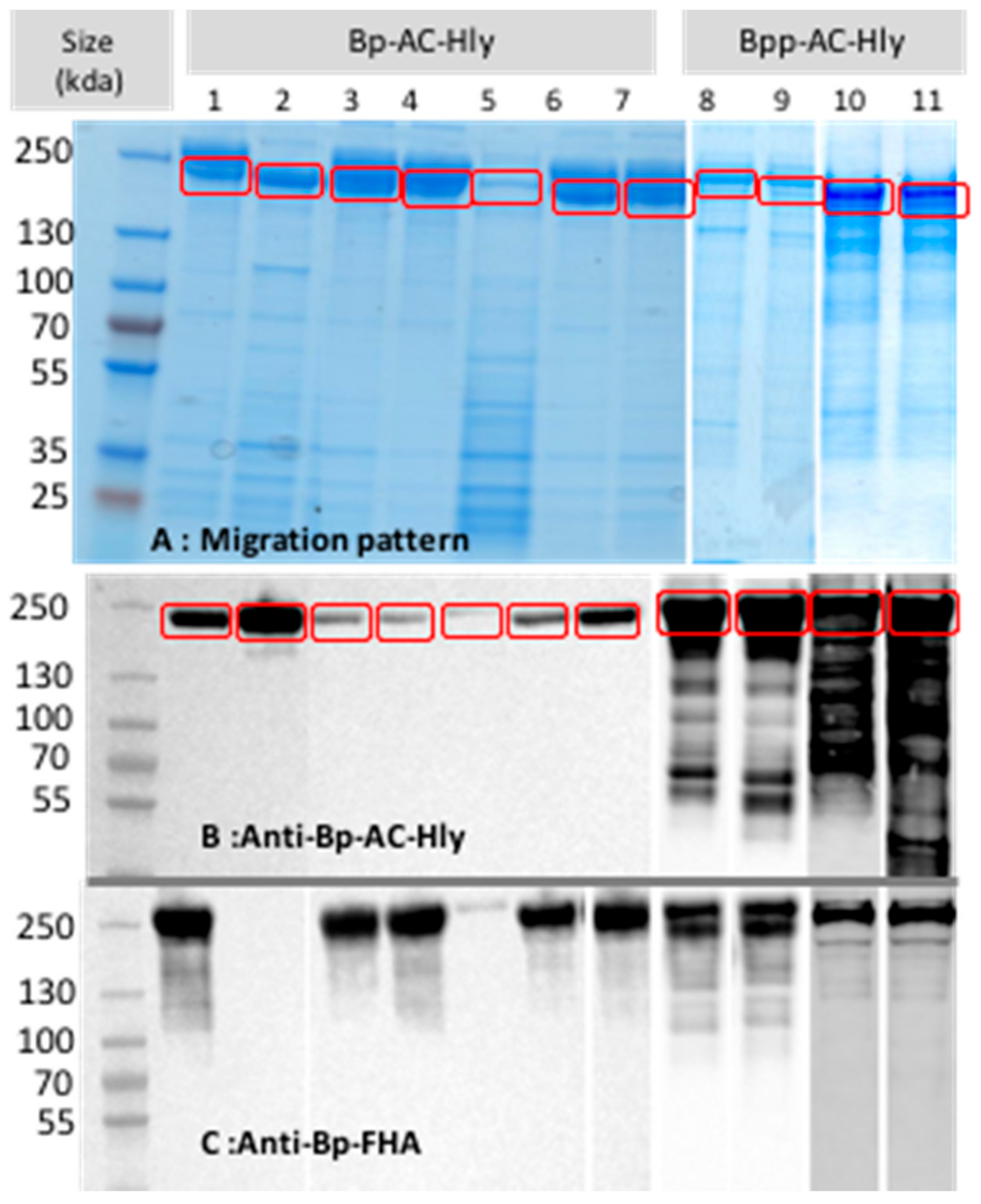
2.2. Partial Purification of AC-Hly
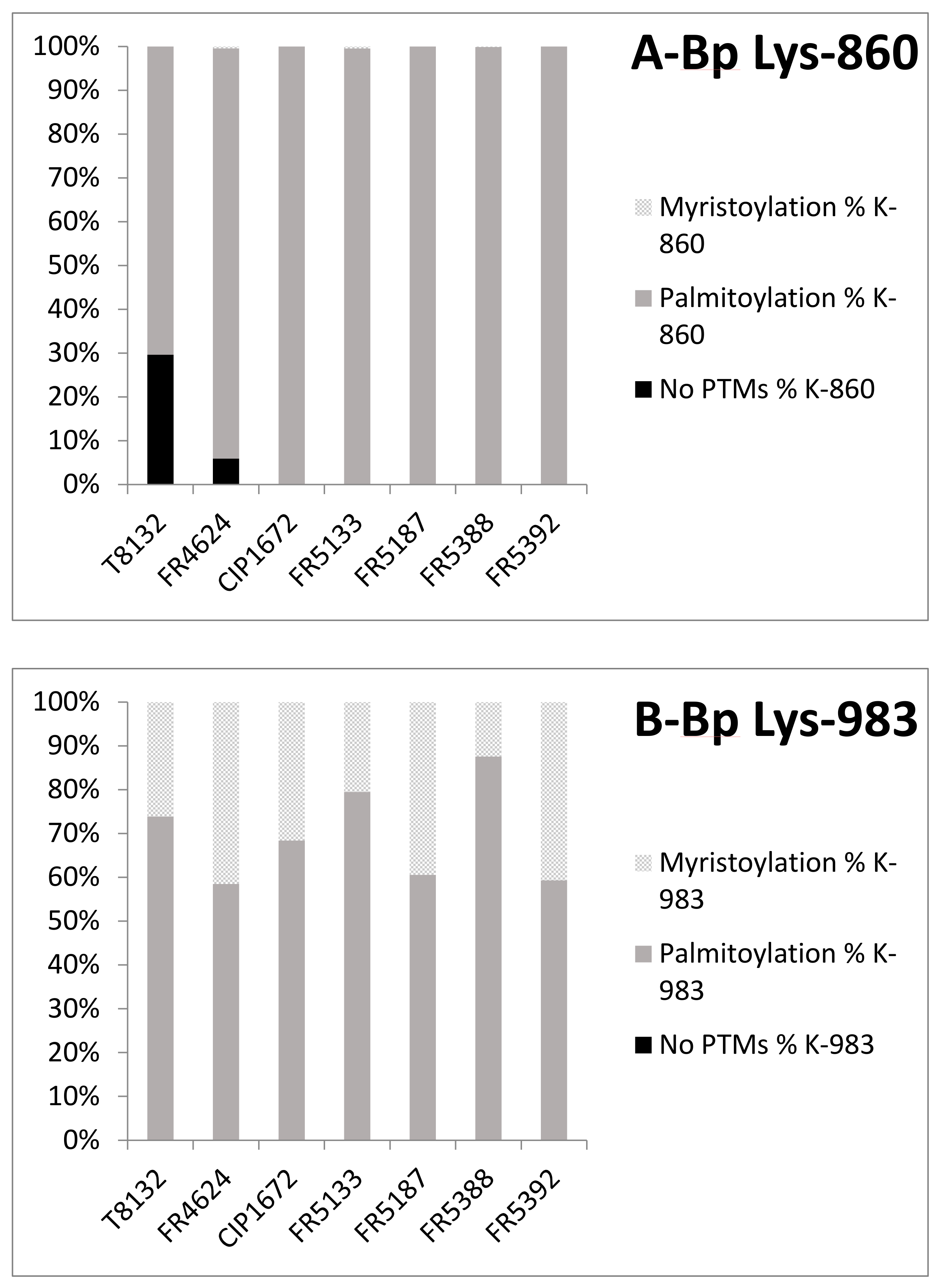
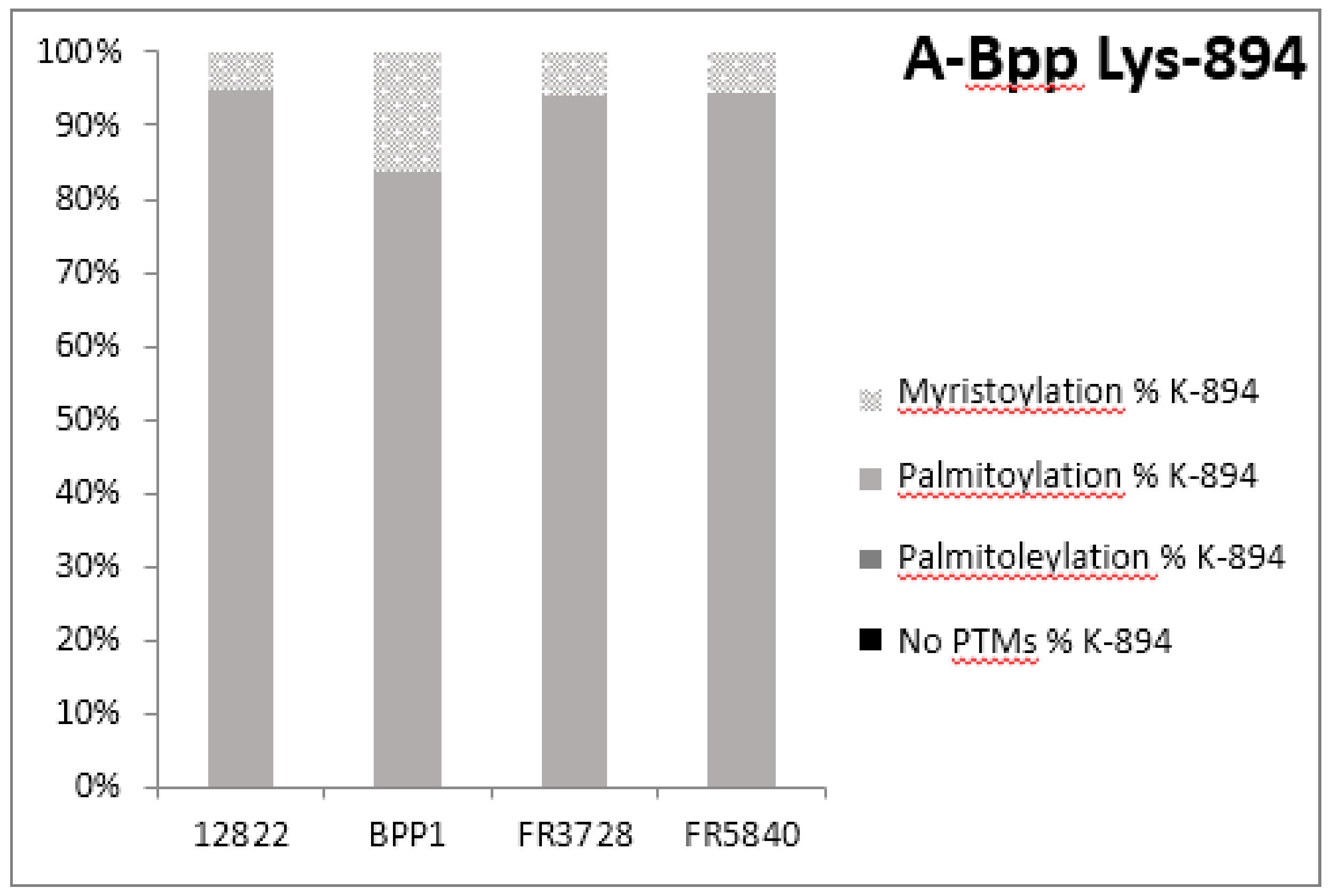
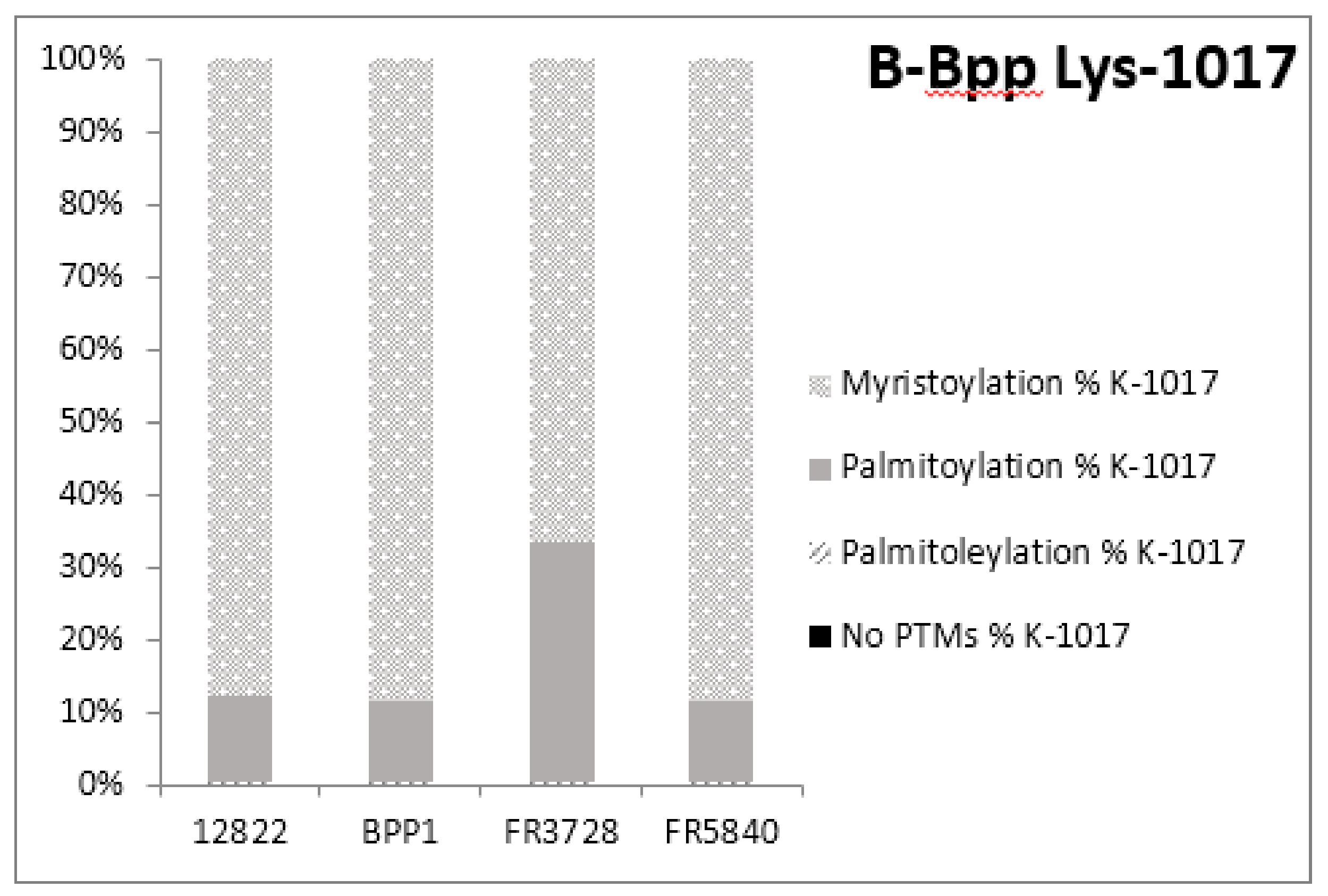
2.3. Post-Translational Modifications
2.4. Cytotoxicity toward Macrophages
3. Discussion
4. Materials and Methods
4.1. Cultures of Bacteria
4.2. AC-Hly Purification
4.3. Western Blots
4.4. Cytotoxicity Assays
4.5. PTM Determination
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guiso, N.; Hegerle, N. Other bordetellas, lessons for and from pertussis vaccines. Expert Rev. Vaccines 2014, 13, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Scarlato, V.; Prugnola, A.; Arico, B.; Rappuoli, R. Positive transcriptional feedback at the bvg locus controls expression of virulence factors in Bordetella pertussis. Proc. Nat. Acad. Sci. USA 1990, 87, 6753–6757. [Google Scholar] [CrossRef] [PubMed]
- Barbier, M.; Boehm, D.T.; Sen-Kilic, E.; Bonnin, C.; Pinheiro, T.; Hoffman, C.; Gray, M.; Hewlett, E.; Damron, F.H. Modulation of pertussis and adenylate cyclase toxins by sigma factor rpoe in Bordetella pertussis. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef] [PubMed]
- Betsou, F.; Sismeiro, O.; Danchin, A.; Guiso, N. Cloning and sequence of the bordetella bronchiseptica adenylate cyclase-hemolysin-encoding gene: Comparison with the Bordetella pertussis gene. Gene 1995, 162, 165–166. [Google Scholar] [CrossRef]
- Parkhill, J.; Sebaihia, M.; Preston, A.; Murphy, L.D.; Thomson, N.; Harris, D.E.; Holden, M.T.; Churcher, C.M.; Bentley, S.D.; Mungall, K.L.; et al. Comparative analysis of the genome sequences of Bordetella pertussis, bordetella parapertussis and bordetella bronchiseptica. Nat. Genet. 2003, 35, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Masin, J.; Osicka, R.; Bumba, L.; Sebo, P. Bordetella adenylate cyclase toxin: A unique combination of a pore-forming moiety with a cell-invading adenylate cyclase enzyme. Pathog. Dis. 2015, 73, ftv075. [Google Scholar] [CrossRef] [PubMed]
- Carbonetti, N.H. Pertussis toxin and adenylate cyclase toxin: Key virulence factors of Bordetella pertussis and cell biology tools. Future Microbiol. 2010, 5, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Sebo, P.; Osicka, R.; Masin, J. Adenylate cyclase toxin-hemolysin relevance for pertussis vaccines. Expert Rev. Vaccines 2014, 13, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Guiso, N. Bordetella adenylate cyclase-hemolysin toxins. Toxins 2017, 9, 277. [Google Scholar] [CrossRef] [PubMed]
- Fiser, R.; Masin, J.; Bumba, L.; Pospisilova, E.; Fayolle, C.; Basler, M.; Sadilkova, L.; Adkins, I.; Kamanova, J.; Cerny, J.; et al. Calcium influx rescues adenylate cyclase-hemolysin from rapid cell membrane removal and enables phagocyte permeabilization by toxin pores. PLoS Pathog. 2012, 8, e1002580. [Google Scholar] [CrossRef] [PubMed]
- Sotomayor-Perez, A.C.; Ladant, D.; Chenal, A. Calcium-induced folding of intrinsically disordered repeat-in-toxin (rtx) motifs via changes of protein charges and oligomerization states. J. Biol. Chem. 2011, 286, 16997–17004. [Google Scholar] [CrossRef] [PubMed]
- Skopova, K.; Tomalova, B.; Kanchev, I.; Rossmann, P.; Svedova, M.; Adkins, I.; Bibova, I.; Tomala, J.; Masin, J.; Guiso, N.; et al. Cyclic amp-elevating capacity of adenylate cyclase toxin-hemolysin is sufficient for lung infection but not for full virulence of Bordetella pertussis. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef] [PubMed]
- Hackett, M.; Guo, L.; Shabanowitz, J.; Hunt, D.F.; Hewlett, E.L. Internal lysine palmitoylation in adenylate cyclase toxin from Bordetella pertussis. Science 1994, 266, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Hackett, M.; Walker, C.B.; Guo, L.; Gray, M.C.; Van Cuyk, S.; Ullmann, A.; Shabanowitz, J.; Hunt, D.F.; Hewlett, E.L.; Sebo, P. Hemolytic, but not cell-invasive activity, of adenylate cyclase toxin is selectively affected by differential fatty-acylation in escherichia coli. J. Biol. Chem. 1995, 270, 20250–20253. [Google Scholar] [CrossRef] [PubMed]
- Basar, T.; Havlicek, V.; Bezouskova, S.; Hackett, M.; Sebo, P. Acylation of lysine 983 is sufficient for toxin activity of Bordetella pertussis adenylate cyclase. Substitutions of alanine 140 modulate acylation site selectivity of the toxin acyltransferase cyaC. J. Biol. Chem. 2001, 276, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Basar, T.; Havlicek, V.; Bezouskova, S.; Halada, P.; Hackett, M.; Sebo, P. The conserved lysine 860 in the additional fatty-acylation site of Bordetella pertussis adenylate cyclase is crucial for toxin function independently of its acylation status. J. Biol. Chem. 1999, 274, 10777–10783. [Google Scholar] [CrossRef] [PubMed]
- Betsou, F.; Sebo, P.; Guiso, N. The C-terminal domain is essential for protective activity of the Bordetella pertussis adenylate cyclase-hemolysin. Infect. Immun. 1995, 63, 3309–3315. [Google Scholar] [PubMed]
- Guiso, N.; Szatanik, M.; Rocancourt, M. Protective activity of bordetella adenylate cyclase-hemolysin against bacterial colonization. Microb. Pathog. 1991, 11, 423–431. [Google Scholar] [CrossRef]
- Guiso, N.; Rocancourt, M.; Szatanik, M.; Alonso, J.M. Bordetella adenylate cyclase is a virulence associated factor and an immunoprotective antigen. Microb. Pathog. 1989, 7, 373–380. [Google Scholar] [CrossRef]
- Khelef, N.; Danve, B.; Quentin-Millet, M.J.; Guiso, N. Bordetella pertussis and bordetella parapertussis: Two immunologically distinct species. Infect. Immun. 1993, 61, 486–490. [Google Scholar] [PubMed]
- Sebo, P.; Glaser, P.; Sakamoto, H.; Ullmann, A. High-level synthesis of active adenylate cyclase toxin of Bordetella pertussis in a reconstructed escherichia coli system. Gene 1991, 104, 19–24. [Google Scholar] [CrossRef]
- Bart, M.J.; Harris, S.R.; Advani, A.; Arakawa, Y.; Bottero, D.; Bouchez, V.; Cassiday, P.K.; Chiang, C.S.; Dalby, T.; Fry, N.K.; et al. Global population structure and evolution of Bordetella pertussis and their relationship with vaccination. mBio 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Linz, B.; Ivanov, Y.V.; Preston, A.; Brinkac, L.; Parkhill, J.; Kim, M.; Harris, S.R.; Goodfield, L.L.; Fry, N.K.; Gorringe, A.R.; et al. Acquisition and loss of virulence-associated factors during genome evolution and speciation in three clades of bordetella species. BMC Genom. 2016, 17, 767. [Google Scholar] [CrossRef] [PubMed]
- Havlicek, V.; Higgins, L.; Chen, W.; Halada, P.; Sebo, P.; Sakamoto, H.; Hackett, M. Mass spectrometric analysis of recombinant adenylate cyclase toxin from Bordetella pertussis strain 18323/phsp9. J. Mass Spectrom. 2001, 36, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Brinig, M.M.; Register, K.B.; Ackermann, M.R.; Relman, D.A. Genomic features of bordetella parapertussis clades with distinct host species specificity. Genome Biol. 2006, 7, R81. [Google Scholar] [CrossRef] [PubMed]
- Bouchez, V.; Brun, D.; Dore, G.; Njamkepo, E.; Guiso, N. Bordetella parapertussis isolates not expressing pertactin circulating in france. Clin. Microbiol. Infect. 2011, 17, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Bouchez, V.; Guiso, N. Bordetella pertussis, B. parapertussis, vaccines and cycles of whooping cough. Pathog. Dis. 2015, 73. [Google Scholar] [CrossRef] [PubMed]
- Diavatopoulos, D.A.; Cummings, C.A.; Schouls, L.M.; Brinig, M.M.; Relman, D.A.; Mooi, F.R. Bordetella pertussis, the causative agent of whooping cough, evolved from a distinct, human-associated lineage of B. bronchiseptica. PLoS Pathog. 2005, 1, e45. [Google Scholar] [CrossRef] [PubMed]
- Bouchez, V.; Brun, D.; Cantinelli, T.; Dore, G.; Njamkepo, E.; Guiso, N. First report and detailed characterization of B. pertussis isolates not expressing pertussis toxin or pertactin. Vaccine 2009, 27, 6034–6041. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Zhang, Y.; Buboltz, A.M.; Zhang, X.; Schuster, S.C.; Ahuja, U.; Liu, M.; Miller, J.F.; Sebaihia, M.; Bentley, S.D.; et al. Comparative genomics of the classical bordetella subspecies: The evolution and exchange of virulence-associated diversity amongst closely related pathogens. BMC Genom. 2012, 13, 545. [Google Scholar] [CrossRef] [PubMed]
- Dalet, K.; Weber, C.; Guillemot, L.; Njamkepo, E.; Guiso, N. Characterization of adenylate cyclase-hemolysin gene duplication in a Bordetella pertussis isolate. Infect. Immun. 2004, 72, 4874–4877. [Google Scholar] [CrossRef] [PubMed]
- Chenal-Francisque, V.; Caro, V.; Boursaux-Eude, C.; Guiso, N. Genomic analysis of the adenylate cyclase-hemolysin C-terminal region of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Res. Microbiol. 2009, 160, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Zaretzky, F.R.; Gray, M.C.; Hewlett, E.L. Mechanism of association of adenylate cyclase toxin with the surface of Bordetella pertussis: A role for toxin-filamentous haemagglutinin interaction. Mol. Microbiol. 2002, 45, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Perez Vidakovics, M.L.; Lamberti, Y.; van der Pol, W.L.; Yantorno, O.; Rodriguez, M.E. Adenylate cyclase influences filamentous haemagglutinin-mediated attachment of Bordetella pertussis to epithelial alveolar cells. FEMS Immunol. Med. Microbiol. 2006, 48, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, C.; Eby, J.; Gray, M.; Heath Damron, F.; Melvin, J.; Cotter, P.; Hewlett, E. Bordetella adenylate cyclase toxin interacts with filamentous haemagglutinin to inhibit biofilm formation in vitro. Mol. Microbiol. 2017, 103, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Bouchez, V.; Hegerle, N.; Strati, F.; Njamkepo, E.; Guiso, N. New data on vaccine antigen deficient Bordetella pertussis isolates. Vaccines 2015, 3, 751–770. [Google Scholar] [CrossRef] [PubMed]
- Hegerle, N.; Guiso, N. Antibody-mediated inhibition of Bordetella pertussis adenylate cyclase-haemolysin-induced macrophage cytotoxicity is influenced by variations in the bacterial population. Microbiology 2014, 160, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Ramjeet, M.; Cox, A.D.; Hancock, M.A.; Mourez, M.; Labrie, J.; Gottschalk, M.; Jacques, M. Mutation in the lps outer core biosynthesis gene, galu, affects lps interaction with the rtx toxins apxi and apxii and cytolytic activity of actinobacillus pleuropneumoniae serotype 1. Mol. Microbiol. 2008, 70, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.E.; Welch, R.A. Pleiotropic effects of a mutation in rfac on escherichia coli hemolysin. Infect. Immun. 1997, 65, 2218–2224. [Google Scholar] [PubMed]
- Novotny, P.; Chubb, A.P.; Cownley, K.; Montaraz, J.A. Adenylate cyclase activity of a 68,000-molecular-weight protein isolated from the outer membrane of Bordetella bronchiseptica. Infect. Immun. 1985, 50, 199–206. [Google Scholar] [PubMed]
- Welch, R.A. Rtx toxin structure and function: A story of numerous anomalies and few analogies in toxin biology. Curr. Top. Microbiol. Immunol. 2001, 257, 85–111. [Google Scholar] [PubMed]
- Caroff, M.; Aussel, L.; Zarrouk, H.; Martin, A.; Richards, J.C.; Therisod, H.; Perry, M.B.; Karibian, D. Structural variability and originality of the bordetella endotoxins. J. Endotoxin Res. 2001, 7, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Goebel, E.M.; Rodriguez, M.E.; Preston, A.; Harvill, E.T. The O antigen is a critical antigen for the development of a protective immune response to Bordetella parapertussis. Infect. Immun. 2009, 77, 5050–5058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Hamidi, A.; Novikov, A.; Karibian, D.; Perry, M.B.; Caroff, M. Structural characterization of Bordetella parapertussis lipid A. J. Lipid Res. 2009, 50, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Hegerle, N.; Paris, A.S.; Brun, D.; Dore, G.; Njamkepo, E.; Guillot, S.; Guiso, N. Evolution of french Bordetella pertussis and Bordetella parapertussis isolates: Increase of bordetellae not expressing pertactin. Clin. Microbiol. Infect. 2012, 18, E340–E346. [Google Scholar] [CrossRef] [PubMed]
- Stainer, D.W.; Scholte, M.J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J. Gen. Microbiol. 1970, 63, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Ladant, D.; Brezin, C.; Alonso, J.M.; Crenon, I.; Guiso, N. Bordetella pertussis adenylate cyclase. Purification, characterization, and radioimmunoassay. J. Biol. Chem. 1986, 261, 16264–16269. [Google Scholar] [PubMed]
- Weber, C.; Boursaux-Eude, C.; Coralie, G.; Caro, V.; Guiso, N. Polymorphism of Bordetella pertussis isolates circulating for the last 10 years in France, where a single effective whole-cell vaccine has been used for more than 30 years. J. Clin. Microbiol. 2001, 39, 4396–4403. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.V.; Mann, M. Andromeda: A peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef] [PubMed]

| Name | Species | Year of Collection | Antigen Deficiency | References |
|---|---|---|---|---|
| Tohama(CIP8132) | B. pertussis | 1954 | none | [29] |
| FR4624 | B. pertussis | 2009 | FHA/PRN | [45] |
| 1672 | B. pertussis | 1950 | FHA | [37,45] |
| FR5133 | B. pertussis | 2012 | none | [37] |
| FR5187 | B. pertussis | 2012 | PRN | [37] |
| FR5388 | B. pertussis | 2012 | PRN | [37] |
| FR5392 | B. pertussis | 2012 | none | [37] |
| Bpp12822 | B. parapertussis | 1993 | none | [26] |
| BPP1 | B. parapertussis | 1990 | none | [26] |
| FR3728 | B. parapertussis | 2007 | PRN | [26] |
| FR5840 | B. parapertussis | 2014 | PRN | this study |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouchez, V.; Douché, T.; Dazas, M.; Delaplane, S.; Matondo, M.; Chamot-Rooke, J.; Guiso, N. Characterization of Post-Translational Modifications and Cytotoxic Properties of the Adenylate-Cyclase Hemolysin Produced by Various Bordetella pertussis and Bordetella parapertussis Isolates. Toxins 2017, 9, 304. https://doi.org/10.3390/toxins9100304
Bouchez V, Douché T, Dazas M, Delaplane S, Matondo M, Chamot-Rooke J, Guiso N. Characterization of Post-Translational Modifications and Cytotoxic Properties of the Adenylate-Cyclase Hemolysin Produced by Various Bordetella pertussis and Bordetella parapertussis Isolates. Toxins. 2017; 9(10):304. https://doi.org/10.3390/toxins9100304
Chicago/Turabian StyleBouchez, Valérie, Thibaut Douché, Mélody Dazas, Sophie Delaplane, Mariette Matondo, Julia Chamot-Rooke, and Nicole Guiso. 2017. "Characterization of Post-Translational Modifications and Cytotoxic Properties of the Adenylate-Cyclase Hemolysin Produced by Various Bordetella pertussis and Bordetella parapertussis Isolates" Toxins 9, no. 10: 304. https://doi.org/10.3390/toxins9100304






