Chronic Sublethal Effects of Cantharidin on the Diamondback Moth Plutella xylostella (Lepidoptera: Plutellidae)
Abstract
:1. Introduction
2. Results
2.1. Susceptibility of SS, Sub and SubR Strains to Cantharidin
| Strain | N a | Slope ± SE | LC50 (95% confidence interval) (mg·L−1) | χ2 | Toxicity ratio b | LC25 (95% confidence interval) (mg·L−1) |
|---|---|---|---|---|---|---|
| SS c | 320 | 1.48 ± 0.17 | 10.95 (8.35–15.01) | 4.01 | 1.00 | 3.84 (2.73–5.07) |
| Sub-6 d | 320 | 1.50 ± 0.17 | 11.34 (8.66–15.57) | 4.09 | 1.04 | 4.02 (2.87–5.29) |
| Sub-12 e | 320 | 1.90 ± 0.20 | 18.24 (14.51–23.64) | 5.11 | 1.67 | 8.04 (6.08–10.16) |
| SubR-1 f | 320 | 1.59 ± 0.18 | 12.11 (9.35–16.41) | 1.98 | 1.07 | 4.57 (3.33–5.95) |
| SubR-2 g | 320 | 1.53 ± 0.17 | 10.22 (7.87–13.77) | 3.25 | 1.03 | 3.71 (2.65–4.87) |
| SubR-6 h | 320 | 1.63 ± 0.17 | 8.89 (6.95–11.68) | 4.69 | 0.81 | 3.43 (2.49–4.45) |
| SubR-12 i | 320 | 1.56 ± 0.17 | 11.01 (9.35–16.41) | 3.59 | 1.05 | 4.06 (2.93–5.31) |
2.2. Effects of Cantharidin on Life History of Different Strains
| Strain | Life stage | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Egg | Larva | Pupa | Duration of immature stage | Adult longevity | APOP 1 | TPOP 2 | |||
| Female | Male | Female | Male | Female | Female | ||||
| SS | 3.29 ± 0.02 a | 7.59 ± 0.04 c | 4.21 ± 0.02 c | 15.51 ± 0.07 bc | 14.80 ± 0.11 c | 13.34 ± 0.21 a | 14.01 ± 0.28 a | 0.59 ± 0.06 b | 16.09 ± 0.12 c |
| Sub-1 | 3.33 ± 0.05 a | 8.49 ± 0.08 a | 4.58 ± 0.04 a | 16.84 ± 0.13 a | 16.03 ± 0.12 a | 9.77 ± 0.36 d | 11.93 ± 0.30 b | 0.93 ± 0.05 a | 17.77 ± 0.14 a |
| Sub-6 | 3.45 ± 0.07 a | 7.97 ± 0.09 b | 4.33 ± 0.06 bc | 15.93 ± 0.10 b | 15.57 ± 0.09 b | 10.90 ± 0.24 cd | 13.01 ± 0.53 a | 0.86 ± 0.06 ab | 16.79 ± 0.11 b |
| Sub-12 | 3.39 ± 0.02 a | 7.67 ± 0.04 c | 4.42 ± 0.02 ab | 15.52 ± 0.10 bc | 15.21 ± 0.13 bc | 11.90 ± 0.31 bc | 13.50 ± 0.19 a | 0.74 ± 0.09 ab | 16.26 ± 0.05 c |
| SubR-12 | 3.38 ± 0.02 a | 7.71 ± 0.05 bc | 4.25 ± 0.03 c | 15.31 ± 0.11 c | 15.30 ± 0.08 b | 12.33 ± 0.27 ab | 13.95 ± 0.23 a | 0.70 ± 0.09 ab | 16.00 ± 0.17 c |
| p | 0.084 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.001 | 0.018 | <0.0001 |
| F | 2.329 | 33.766 | 14.452 | 34.460 | 18.045 | 11.910 | 6.765 | 3.648 | 35.313 |
| df | 4,25 | 4,25 | 4,25 | 4,25 | 4,25 | 4,25 | 4,25 | 4,25 | 4,25 |
2.3. Effects of Cantharidin on Adult Performance and Reproductive Potential of Different Strains
| Strain | Fecundity 1 | Egg Size (10−2 mm3) 2 | Reproductive effort per female 3 | Oviposition duration in days | Fertility (% egg hatch) |
|---|---|---|---|---|---|
| SS | 203.97 ± 3.40 ab | 1.32 ± 0.02 a | 2.69 ± 0.06 a | 8.37 ± 0.15 a | 86.86 ± 0.75 a |
| Sub-1 | 161.26 ± 2.43 c | 1.21 ± 0.02 b | 1.96 ± 0.05 c | 6.95 ± 0.17 b | 85.80 ± 0.86 ab |
| Sub-6 | 187.41 ± 5.67 b | 1.24 ± 0.02 b | 2.33 ± 0.10 b | 7.18 ± 0.17 b | 83.46 ± 1.10 ab |
| Sub-12 | 217.15 ± 7.87 a | 1.22 ± 0.02 b | 2.64 ± 0.08 a | 7.79 ± 0.25 ab | 75.86 ± 1.44 c |
| SubR-12 | 195.38 ± 4.99 ab | 1.25 ± 0.02 b | 2.43 ± 0.07 ab | 7.89 ± 0.16 a | 82.34 ± 0.52 b |
| p | <0.0001 | 0.001 | <0.0001 | <0.0001 | <0.0001 |
| F | 18.752 | 6.214 | 15.939 | 9.624 | 19.175 |
| df | 4,25 | 4,25 | 4,25 | 4,25 | 4,25 |
2.4. Effects of Cantharidin on Age-Stage Survival Rates (sxj) of Different Strains
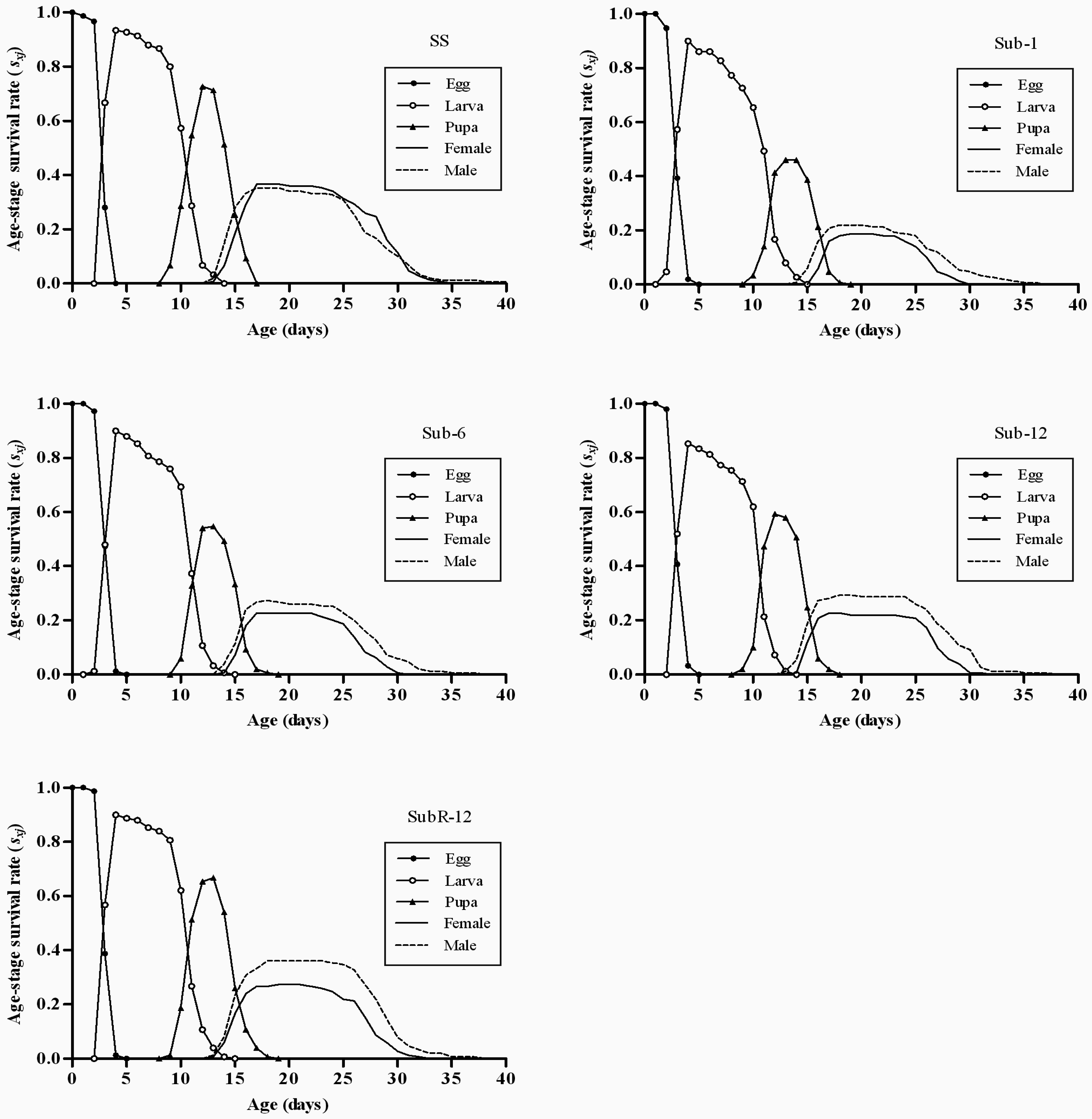
2.5. Effects of Cantharidin on Age-Specific Survival Rate (lx), Age-Specific Fecundity (mx) and Age-Specific Maternity (lxmx) of Different Strains

2.6. Effects of Cantharidin on Demographic Parameters and Fitness
| Strain | Parameter | |||||
|---|---|---|---|---|---|---|
| R0 | r | λ | GRR | T (d) | Rate of relative fitness 1 | |
| SS | 74.80 ± 5.94 a | 0.23 ± 0.01 a | 1.26 ± 0.01 a | 105.25 ± 7.36 a | 18.59 ± 0.15 c | 1.00 |
| Sub-1 | 30.15 ± 3.67 c | 0.18 ± 0.01 d | 1.18 ± 0.01 d | 75.17 ± 7.49 b | 20.34 ± 0.13 a | 0.40 |
| Sub-6 | 43.40 ± 4.91 b | 0.19 ± 0.01 c | 1.21 ± 0.01 c | 87.80 ± 8.52 ab | 19.55 ± 0.13 b | 0.58 |
| Sub-12 | 49.08 ± 5.47 b | 0.20 ± 0.01 c | 1.22 ± 0.01 c | 94.95 ± 9.17 ab | 19.30 ± 0.09 b | 0.66 |
| SubR-12 | 53.41 ± 5.09 b | 0.21 ± 0.01 b | 1.24 ± 0.01 b | 83.68 ± 7.10 b | 18.59 ± 0.14 c | 0.71 |
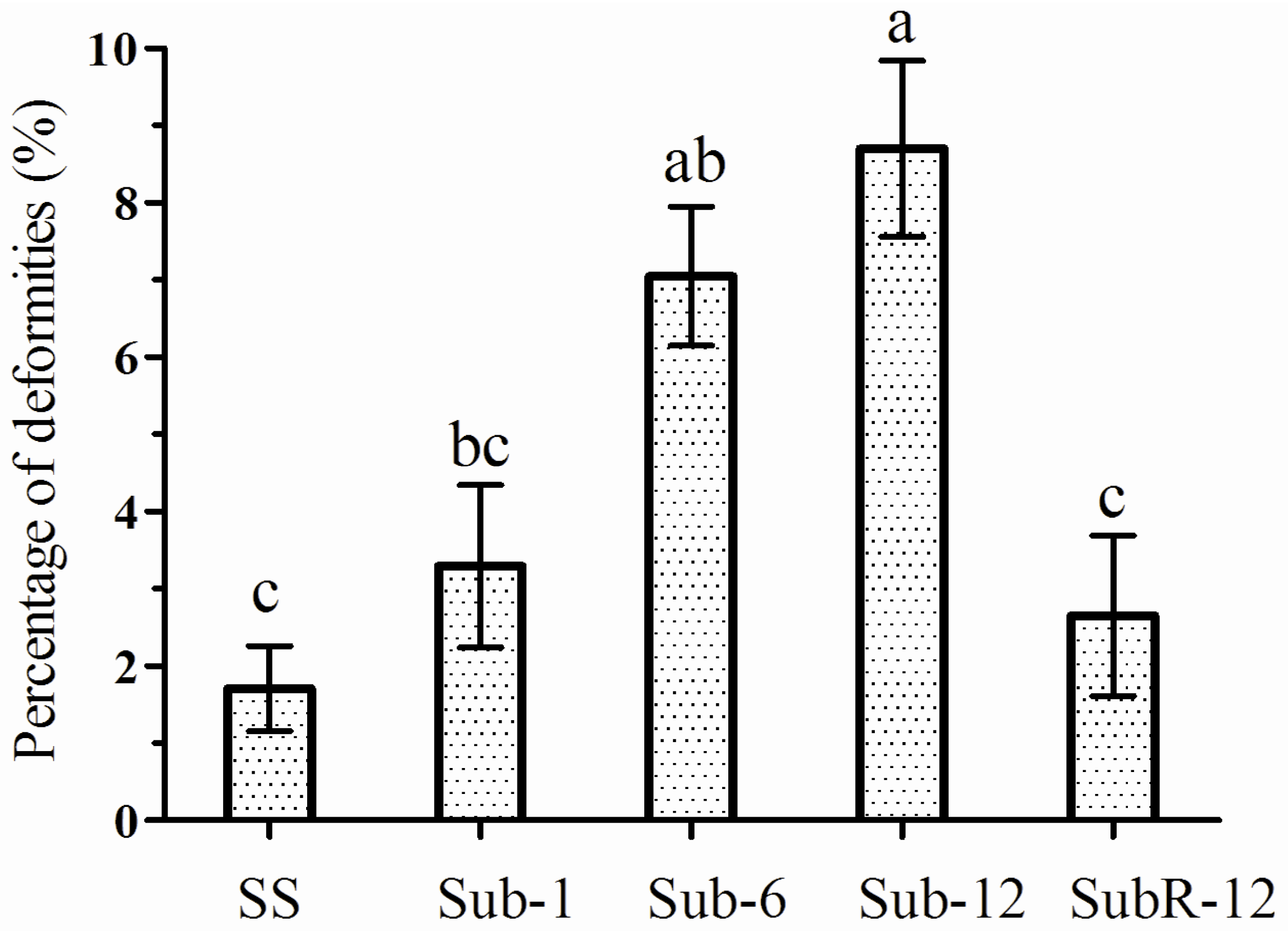
2.7. Abnormalities Caused by Cantharidin Treatment
3. Discussion

4. Materials and Methods
4.1. Insects and Chemicals
4.2. Bioassay
4.3. Selection Experiments
4.4. Biological Characteristics of Plutella xylostella in Different Strains
4.5. Egg Size
4.6. Age-Stage, Two-Sex Life Table Analysis
4.7. Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Furlong, M.J.; Wright, D.J.; Dosdall, L.M. Diamondback moth ecology and management: Problems, progress, and prospects. Annu. Rev. Entomol. 2013, 58, 517–541. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, J.; Li, H.S.; Dai, H.G.; Gu, X.J. Sublethal effects of fenvalerate on the development, fecundity, and juvenile hormone esterase activity of diamondback moth, Plutella xylostella (L.). Agric. Sci. China 2010, 9, 1612–1622. [Google Scholar]
- Guo, L.; Liang, P.; Zhou, X.; Gao, X. Novel mutations and mutation combinations of ryanodine receptor in a chlorantraniliprole resistant population of Plutella xylostella (L.). Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Talekar, N.S.; Shelton, A.M. Biology, ecology, and management of the diamondback moth. Annu. Rev. Entomol. 1993, 38, 275–301. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhou, Y.; Zhang, Z.Y. Effect of cantharidin on the midgut of oriental armyworm (Mythimnaseparata) and diamondback moth (Plutellaxylostella). Acta Entomol. Sin. 2003, 46, 272–276. [Google Scholar]
- Ma, Y.; Liu, R.R.; Ma, Z.Q.; Zhang, Y.L. Effects of cantharidin on four metabolizing enzymes and PPO in Mythimna separata (Walker) (Lepidoptera: Noctuidae). Acta Entomol. Sin. 2010, 53, 870–875. [Google Scholar]
- Khan, R.A.; Rashid, M.; Wang, D.; Zhang, Y.L. Toxicology and biochemical basis of cantharidin effects on Helicoverpa armigera (Hub.) (Lepidoptera: Noctuidae). Pak. J. Zool. 2013, 45, 769–777. [Google Scholar]
- Wu, Z.W.; Yang, X.Q.; Zhang, Y.L. The Toxicology and Biochemical Characterization of Cantharidin on Cydia pomonella. J. Econ. Entomol. 2015. [Google Scholar] [CrossRef]
- Stark, J.D.; Banks, J.E. Population-level effects of pesticides and other toxicants on arthropods. Annu. Rev. Entomol. 2003, 48, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.D.; Rangus, T.M. Lethal and sublethal effects of the neem insecticide formulation, ‘Margosan-O’, on the pea aphid. Pestic. Sci. 1994, 41, 155–160. [Google Scholar] [CrossRef]
- Mahmoudvand, M.; Abbasipour, H.; Garjan, A.S.; Bandani, A.R. Sublethal effects of indoxacarb on the diamondback moth, P. xylostella (L.) (Lepidoptera,Yponomeutidae). Appl. Entomol. Zool. 2011, 46, 75–80. [Google Scholar] [CrossRef]
- Boina, D.R.; Onagbola, E.O.; Salyani, M.; Stelinski, L.L. Antifeedant and sublethal effects of imidacloprid on Asian citrus psyllid, Diaphorina citri. Pest Manag. Sci. 2009, 65, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.X.; Qiu, H.; Ren, X.X.; Zhang, W.Z.; Wang, K.Y. Sublethal effects of spinosad on survival, growth and reproduction of Helicoverpa armigera (Lepidoptera: Noctuidae). Pest Manag. Sci. 2009, 65, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Biondi, A.; Desneux, N.; Gao, X.W. Assessment of physiological sublethal effects of imidacloprid on the mirid bug Apolygus lucorum (Meyer-Dür). Ecotoxicology 2012, 21, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Forbes, V.E.; Calow, P. Is the per capita rate of increase a good measure of population-level effects in ecotoxicology? Environ. Toxicol. Chem. 1999, 18, 1544–1556. [Google Scholar] [CrossRef]
- Kerns, D.L.; Stewart, S.D. Sublethal effects of insecticides on the intrinsic rate of increase of cotton aphid. Entomol. Exp. Appl. 2000, 94, 41–49. [Google Scholar] [CrossRef]
- Rashid, M.; Khan, R.A.; Zhang, Y.L. Physiological and population responses of armyworm Mythimna separata (Lepidoptera: Noctuidae) to a sublethal dose of Cantharidin-AC. J. Econ. Entomol. 2013, 106, 2177–2182. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.; Rashid, M.; Wang, D. Zhang, Y.L. Lethal and sublethal effects of cantharidin on the life history traits and population parameters of Helicoverpa armigera (Hübner) (Lepidoptera:Noctuidae). Pest Manag. Sci. 2014, 70, 39–45. [Google Scholar] [PubMed]
- Huang, Z.Y.; Wang, Y.; Zhang, Y.L. Lethal and Sublethal Effects of Cantharidin on Development and Reproduction of Plutella xylostella (Lepidoptera: Plutellidae). J. Econ. Entomol. 2015. [Google Scholar] [CrossRef]
- Young, H.P.; Bailey, W.D.; Roe, R.M. Spinosad selection of a laboratory strain of the tobacco budworm, Heliothis virescens (Lepidoptera: Noctuidae), and characterization of resistance. Crop Prot. 2003, 22, 265–273. [Google Scholar] [CrossRef]
- Vogt, C.; Nowak, C.; Diogo, J.B.; Oetken, M.; Schwenk, K.; Oehlmann, J. Multi-generation studies with Chironomus riparius—Effects of low tributyltin concentrations on life history parameters and genetic diversity. Chemosphere 2007, 67, 2192–2200. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.H.; Wu, Q.J.; Li, X.F.; Zhang, Y.J.; Xu, B.Y. Demographic changes in multigeneration Plutella xylostella (Lepidoptera: Plutellidae) after exposure to sublethal concentrations of spinosad. J. Econ. Entomol. 2009, 102, 357–365. [Google Scholar] [PubMed]
- Tang, B.; Sun, J.; Zhou, X.; Gao, X.; Liang, P. The stability and biochemical basis of fufenozide resistance in a laboratory-selected strain of Plutella xylostella. Pestic. Biochem. Phys. 2011, 101, 80–85. [Google Scholar] [CrossRef]
- Nemoto, H. Factors inducing resurgence in the diamondback moth after application of methomyl. In Diamondback Moth Management, Proceedings of First International Workshop, Tainan, Taiwan, 11–15 March 1985; Talekar, N.S., Griggs, T.D., Eds.; Asian Vegetable Research and Development Ceter: Shanhua, Taiwan, 1986; pp. 387–394. [Google Scholar]
- Nemoto, H. Mechanism of resurgence of the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Yponomeutidae). Jpn. Agr. Res. Q. 1993, 27, 27–32. [Google Scholar]
- Sota, N.; Motoyama, N.; Fujisaki, K.; Nakasuji, F. Possible amplification of insecticide hormoligosis from resistance in the diamondback moth, Plutella xylostella (Lepidoptera: Yponomeutidae). Appl. Entomol. Zool. 1998, 33, 435–440. [Google Scholar]
- Mahmoudvand, M.; Abbasipour, H.; Garjan, A.S.; Bandani, A.R. Sublethal effects of hexaflumuron on development and reproduction of the diamondback moth, Plutella xylostella (Lepidoptera: Yponomeutidae). Insect Sci. 2011, 18, 689–696. [Google Scholar] [CrossRef]
- Pineda, S.; Martinez, A.M.; Figueroa, J.I.; Schneider, M.I.; Estal, P.D.; Vinuela, E.; Gomez, B.; Smagghe, G.; Budia, F. Influence of azadirachtin and methoxyfenozide on life parameters of Spodoptera littoralis (Lepidoptera:Noctuidae). J. Econ. Entomol. 2009, 102, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ansari, M.S. Effect of neemarin on life table indices of Plutella xylostella (L.). Crop Prot. 2012, 38, 7–14. [Google Scholar] [CrossRef]
- Sibly, R.M.; Hone, J. Population growth rate and its determinants: An overview. Philos. Trans. R. Soc. B 2002, 357, 1153–1170. [Google Scholar] [CrossRef] [PubMed]
- Sáenz-de-Cabezón, F.J.; Zalom, F.G.; Thompson, P.B. Residual toxicity of acaricides to Galendromus occidentalis and Phytoseiulus persimilis reproductive potential. Biol. Control 2007, 40, 153–159. [Google Scholar] [CrossRef]
- Postma, J.F.; Davids, C. Tolerance induction and life cycle changes in cadmium-exposed Chironomus riparius (Diptera) during consecutive generations. Ecotox. Environ. Safe. 1995, 30, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Walthall, W.K.; Stark, J.D. A comparison of acute mortality and population growth rate as endpoints of toxicological effect. Ecotoxicol. Environ. Saf. 1997, 37, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D.; Nakasuji, F. Diminished egg size in fenvalerate resistant strains of the diamondback moth Plutella xylostella (Lepidoptera: Yponomeutidae). Appl. Entomol. Zool. 2004, 39, 335–341. [Google Scholar] [CrossRef]
- Wilson, T.G.; Cryan, J.R. Lufenuron, a chitin-synthesis inhibitor, interrupts development of Drosophila melanogaster. J. Exp. Zool. 1997, 278, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Rossitor, M.C. Maternal effects generate variation in life history: Consequences of egg weight plasticity in the gypsy moth. Funct. Ecol. 1991, 5, 386–393. [Google Scholar] [CrossRef]
- Brady, M.E. The significance of egg size variation in butterflies in relation to host plant quality. Oikos 1994, 71, 119–129. [Google Scholar]
- Fox, C.W. Egg-size manipulations in the seed beetle Stator limbatus: Consequences for progeny growth. Can. J. Zool. 1997, 75, 1465–1473. [Google Scholar]
- Bechmann, R.K. Use of life tables and LC50 tests to evaluate chronic and acute toxicity effects of copper on the marine copepod Tisbe furcata (Baird). Environ. Toxicol. Chem. 1994, 13, 1509–1517. [Google Scholar] [CrossRef]
- Stark, J.D.; Banks, J.E.; Acheampong, S. Estimating susceptibility of biological control agents to pesticides: Influence of life history strategies and population structure. Biol. Control 2004, 29, 392–398. [Google Scholar] [CrossRef]
- Hoy, C.W.; Head, G.P.; Hall, F.R. Spatial heterogeneity and insect adaptation to toxins. Annu. Rev. Entomol. 1998, 43, 571–594. [Google Scholar] [CrossRef] [PubMed]
- Pineda, S.; Schneider, M.I.; Smagghe, G.; Martínez, A.M.; Del Estal, P.; Viñuela, E.; Valle, J.; Budia, F. Lethal and sublethal effects of methoxyfenozide and spinosad on Spodoptera littoralis (Lepidoptera: Noctuidae). J. Econ. Entomol. 2007, 100, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Wanner, K.W.; Helson, B.V.; Harris, B.J. Laboratory and field evaluation of spinosad against the gypsy moth, Lymantria dispar. Pest Manag. Sci. 2000, 56, 855–860. [Google Scholar] [CrossRef]
- Sun, W.B.; Liu, Z.Y.; Zhang, Y.L. Cantharidin and its anhydride-modified derivatives: Relation of structure to insecticidal activity. Int. J. Mol. Sci. 2013, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sayyed, A.H.; Rizvi, M.R.; Alvi, A.H. Management of diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae): A lesson from South East Asia for sustainable integrated pest management. Pak. J. Biol. Sci. 2002, 5, 234–245. [Google Scholar]
- Saeed, R.; Sayyed, A.H.; Shad, S.A.; Zaka, S.M. Effect of different host plants on the fitness of diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Crop Prot. 2010, 29, 178–182. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Takahashi, T.; Yoshioka, T.; Nakasuji, F. Changes in egg size of the diamondback moth Plutella xylostella (Lepidoptera: Yponomeutidae) treated with fenvalerate at sublethal doses and viability of the eggs. Appl. Entomol. Zool (Jpn.) 2002, 37, 103–109. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rate among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Goodman, D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 1982, 119, 803–823. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A computer program for the age-stage, two-sex life table analysis. Available online: http://140.120.197.173/Ecology/Download/Twosex-MSChart.rar (accessed on 25 November 2014).
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Huang, Y.B.; Chi, H. Life tables of Bactrocera cucurbitae (Diptera: Tephritidae): With an invalidation of the jackknife technique. J. Appl. Entomol. 2013, 137, 327–339. [Google Scholar] [CrossRef]
- Alinejad, M.; Kheradmand, K.; Fathipour, Y. Sublethal effects of fenazaquin on life table parameters of the predatory mite Amblyseius swirskii (Acari: Phytoseiidae). Exp. Appl. Acarol. 2014, 64, 361–373. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Zhang, Y. Chronic Sublethal Effects of Cantharidin on the Diamondback Moth Plutella xylostella (Lepidoptera: Plutellidae). Toxins 2015, 7, 1962-1978. https://doi.org/10.3390/toxins7061962
Huang Z, Zhang Y. Chronic Sublethal Effects of Cantharidin on the Diamondback Moth Plutella xylostella (Lepidoptera: Plutellidae). Toxins. 2015; 7(6):1962-1978. https://doi.org/10.3390/toxins7061962
Chicago/Turabian StyleHuang, Zhengyu, and Yalin Zhang. 2015. "Chronic Sublethal Effects of Cantharidin on the Diamondback Moth Plutella xylostella (Lepidoptera: Plutellidae)" Toxins 7, no. 6: 1962-1978. https://doi.org/10.3390/toxins7061962
APA StyleHuang, Z., & Zhang, Y. (2015). Chronic Sublethal Effects of Cantharidin on the Diamondback Moth Plutella xylostella (Lepidoptera: Plutellidae). Toxins, 7(6), 1962-1978. https://doi.org/10.3390/toxins7061962







