Deoxynivalenol in the Gastrointestinal Tract of Immature Gilts under per os Toxin Application
Abstract
:1. Introduction
2. Results and Discussion
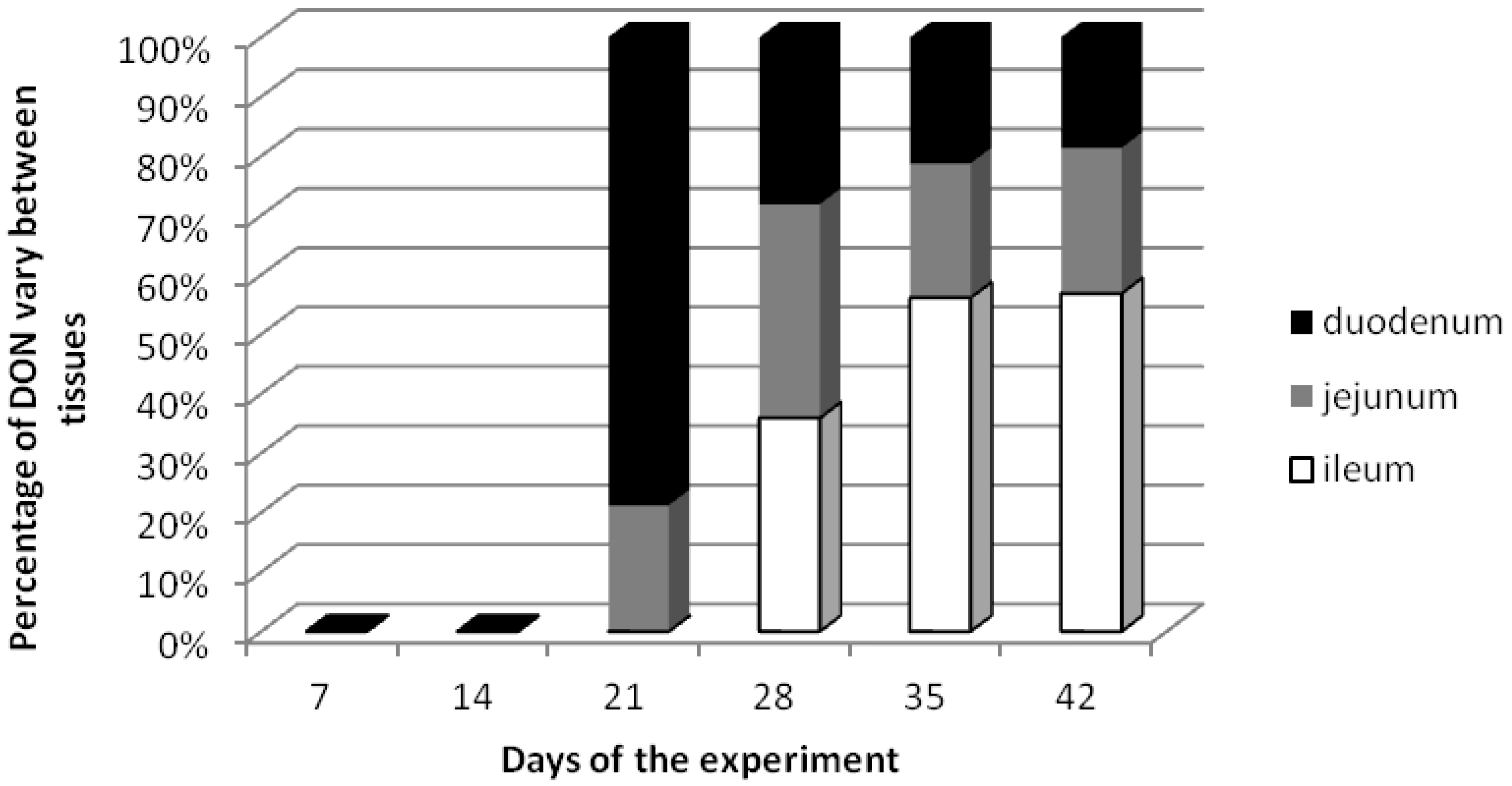
2.1. DON Residues in Small Intestine Tissues
| Days of the experiment | Total doses [µg/kg b.w.] | DON amounts in small intestine [ng/g] ± standard deviation | |||||
|---|---|---|---|---|---|---|---|
| Duodenum | Carry over factor | Jejunum | Carry over factor | Ileum | Carry over factor | ||
| 7 (term I) | 84 | 0 a ± 0 | 0 | 0 a ± 0 | 0 | 0 a ± 0 | 0 |
| 14 (term II) | 168 | 0 a ± 0 | 0 | 0 a ± 0 | 0 | 0 a ± 0 | 0 |
| 21 (term III) | 252 | 6.71 b ± 0.22 | 0.027 | 1.80 a,b ± 0.64 | 0.007 | 0 a ± 0 | 0 |
| 28 (term IV) | 336 | 7.20 b ± 0.16 | 0.021 | 9.20 c ± 2.54 | 0.027 | 9.20 b ± 4.65 | 0.027 |
| 35 (term V) | 420 | 4.24 b ± 1.57 | 0.010 | 4.54 b ± 0.95 | 0.011 | 11.19 b,c ± 2.40 | 0.027 |
| 42 (term VI) | 504 | 6.13 b ± 2.13 | 0.012 | 8.06 c ± 1.08 | 0.016 | 18.60 c ± 4.26 | 0.037 |
2.2. DON Residue in Large Intestine and Liver Tissues
| Days of the experiment | Total doses [µg/kg b.w.] | DON amounts in large intestine [ng/g] ± standard deviation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cecum | Carry over factor | Ascending colon | Carry over factor | Transverse colon | Carry over factor | Descending colon | Carry over factor | ||
| 7 (term I) | 84 | 0 a ± 0 | 0 | 0 a ± 0 | 0 | 0 a ± 0 | 0 | 0 a ± 0 | 0 |
| 14 (term II) | 168 | 3.70 a,b ± 0.47 | 0.022 | 2.50 a ± 0.17 | 0.015 | 0 a ± 0 | 0 | 0 a ± 0 | 0 |
| 21 (term III) | 252 | 6.42 a,b ± 0.22 | 0.025 | 3.10 a ± 1.56 | 0.012 | 0 a ± 0 | 0 | 0 a ± 0 | 0 |
| 28 (term IV) | 336 | 9.81 b ± 4.08 | 0.029 | 7.44 a ± 0.92 | 0.022 | 1.80 b ± 0.18 | 0.005 | 6.75 b ± 2.53 | 0.020 |
| 35 (term V) | 420 | 9.29 b ± 2.54 | 0.022 | 20.52 b ± 5.77 | 0.049 | 0 a ± 0 | 0 | 10.92 b ± 2.62 | 0.026 |
| 42 (term VI) | 504 | 23.00 c ± 4.89 | 0.046 | 16.09 b ± 3.57 | 0.032 | 0 a ± 0 | 0 | 20.00 c ± 3.27 | 0.040 |
| Days of the experiment | Total doses [µg/kg b.w.] | DON amounts in liver [ng/g] ± standard deviation | |
|---|---|---|---|
| Liver | Carry over factor | ||
| 7 (term I) | 84 | 6.79 a ± 1.28 | 0.081 |
| 14 (term II) | 168 | 7.51 a ± 0.74 | 0.045 |
| 21 (term III) | 252 | 7.90 a ± 2.87 | 0.031 |
| 28 (term IV) | 336 | 7.78 a ± 3.35 | 0.023 |
| 35 (term V) | 420 | 8.80 a ± 3.69 | 0.021 |
| 42 (term VI) | 504 | 6.70 a ± 4.28 | 0.013 |

3. Experimental Section
3.1. Experimental Animals
3.2. Experimental Design
3.3. Chemicals
3.4. Tissue Samples
3.5. Extraction and Purification Procedure
3.6. HPLC Analysis of Deoxynivalenol
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Gräfenhan, T.; Patrick, S.K.; Roscoe, M.; Trelka, R.; Gaba, D.; Chan, J.M.; McKendry, T.; Clear, R.M.; Tittlemier, S.A. Fusarium damage in cereal grains from Western Canada. 1. Phylogenetic analysis of moniliformin-producing Fusarium species and their natural occurrence in mycotoxin-contaminated wheat, oats, and rye. J. Agric. Food Chem. 2013, 61, 5425–5437. [Google Scholar] [CrossRef]
- Juan, C.; Ritieni, A.; Mañes, J. Occurrence of Fusarium mycotoxins in Italian cereal and cereal products from organic farming. Food Chem. 2013, 141, 1747–1755. [Google Scholar] [CrossRef]
- Slikova, S.; Gavurnikova, S.; Sudyova, V.; Gregova, E. Occurrence of deoxynivalenol in wheat in Slovakia during 2010 and 2011. Toxins 2013, 5, 1353–1361. [Google Scholar] [CrossRef]
- Wiśniewska, H.; Stępień, Ł.; Waśkiewicz, A.; Beszterda, M.; Góral, T.; Belter, J. Toxigenic Fusarium species infecting wheat heads in Poland. Cent. Eur. J. Biol. 2013, 9, 163–172. [Google Scholar]
- Goliński, P.; Waśkiewicz, A.; Wiśniewska, H.; Kiecana, I.; Mielniczuk, E.; Gromadzka, K.; Kostecki, M.; Bocianowski, J.; Rymaniak, E. Reaction of winter wheat (Triticum aestivum L.) cultivars to infection with Fusarium spp.: Mycotoxin contamination in grain and chaff. Food Add. Contam. 2010, 27, 1015–1024. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Gromadzka, K.; Wiśniewska, H.; Goliński, P. Accumulation of zearalenone in genotypes of spring wheat after inoculation with Fusarium culmorum. Cereal Res. Commun. 2008, 36, 401–404. [Google Scholar]
- Waśkiewicz, A.; Morkunas, I.; Bednarski, W.; Mai, V.-C.; Formela, M.; Beszterda, M.; Wiśniewska, H.; Goliński, P. Deoxynivalenol and oxidative stress indicators in winter wheat inoculated with Fusarium graminearum. Toxins 2014, 6, 575–591. [Google Scholar] [CrossRef]
- Grenier, B.; Bracarense, A.-P.F.L.; Schwartz, H.E.; Lucioli, J.; Cossalter, A.-M.; Moll, W.-D.; Schatzmayr, G.; Oswald, I.P. Biotranformation approaches to alleviate the effects induced by Fusarium mycotoxins in swine. J. Agric. Food Chem. 2013, 61, 6711–6719. [Google Scholar] [CrossRef] [Green Version]
- Gajęcka, M.; Rybarczyk, L.; Jakimiuk, E.; Zielonka, Ł.; Obremski, K.; Zwierzykowski, W.; Gajęcki, M. The effect of experimental long-term exposure to low-dose zearalenone on uterine histology in sexually immature gilts. Exp. Toxicol. Pathol. 2012, 64, 537–542. [Google Scholar] [CrossRef]
- Girish, C.K.; MacDonald, E.J.; Scheinin, M.; Smith, T.K. Effects of feedborne Fusarium mycotoxins on brain regional neurochemistry of turkeys. Poult. Sci. 2008, 87, 1295–1302. [Google Scholar] [CrossRef]
- Wegulo, S.N. Factors influencing deoxynivalenol accumulation in small grain cereals. Toxins 2012, 4, 1157–1180. [Google Scholar]
- SCOOP. Collection of Occurrence Data of Fusarium Toxins in Food and Assessment of Dietary Intake by the Population of EU Member States. Reports on Tasks for Scientific Cooperation. 2003. Available online: http://ec.europa.eu/food/fs/scoop/task3210.pdf (accessed on 31 October 2013).
- Waśkiewicz, A.; Irzykowska, L.; Bocianowski, J.; Koralewski, Z.; Kostecki, M.; Weber, Z.; Goliński, P. Occurrence of Fusarium fungi and mycotoxins in marketable asparagus spears. Polish J. Environ. Stud. 2010, 19, 219–225. [Google Scholar]
- Koch, H.-J.; Pringas, C.; Maerlaender, B. Evaluation of environmental and management effects on Fusarium head blight infection and deoxynivalenol concentration in the grain of winter wheat. Eur. J. Agron. 2006, 24, 357–366. [Google Scholar] [CrossRef]
- Sobrowa, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar]
- Nagy, C.M.; Fejer, S.N.; Berek, L.; Molnar, J.; Viskolcz, B. Hydrogen bondings in deoxynivalenol (DON) conformations—A density functional study. J. Mol. Struct. 2005, 726, 55–59. [Google Scholar] [CrossRef]
- Döll, S.; Schrichx, J.A.; Dänicke, S.; Fink-Gremmels, J. Interactions of deoxynivalenol and lipopolysaccharides on cytokine excretion and mRNA expression in porcine hepatocytes and Kupffer cell enriched hepatocyte. Toxicol. Lett. 2009, 190, 96–105. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Anim. Feed Sci. Technol. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef]
- Bonnet, M.S.; Roux, J.; Mounien, L.; Dallaporta, M.; Troadec, J.-D. Advances in deoxynivalenol toxicity mechanisms: The brain as a target. Toxins 2012, 4, 1120–1138. [Google Scholar] [CrossRef]
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health B Crit. Rev. 2005, 8, 39–69. [Google Scholar] [CrossRef]
- Awad, W.A.; Aschenbach, J.R.; Setyabudi, F.M.C.S.; Razzazi-Fazeli, E.; Böhm, J.; Zentek, J. In vitro effects of deoxynivalenol on small intestinal D-glucose uptake and absorption of deoxynivalenol across the isolated jejunal epithelium of laying hens. Poult. Sci. 2007, 86, 15–20. [Google Scholar] [CrossRef]
- Arnold, D.L.; McGuire, P.F.; Nera, E.A; Karpinski, K.F.; Bickis, M.G.; Zawidzka, Z.Z.; Fernie, S.; Vesonder, R.F. The toxicity of orally administered deoxynivalenol (vomitoxin) in rats and mice. Food Chem. Toxicol. 1986, 24, 935–941. [Google Scholar] [CrossRef]
- Maresca, M.; Mahfoud, R.; Garmy, N.; Fantini, F. The mycotoxin deoxynivalenol affects nutrient absorption in human intestinal epithelial Cells. J. Nutr. 2002, 132, 2723–2731. [Google Scholar]
- Pinton, P.; Tsybulskyy, D.; Lucioli, J.; Laffitte, J.; Callu, P.; Lyazhri, F.; Grosjean, F.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: Differential effects on morphology, barrier function, tight function proteins, and mitogen-activated protein kinases. Toxicol. Sci. 2012, 130, 180–190. [Google Scholar] [CrossRef]
- Bensassi, F.; El Golli-Bennour, E.; Abid-Essefi, S.; Bouaziz, C.; Hajlaoui, M.R.; Bacha, H. Pathway of deoxynivalenol-induced apoptosis in human colon carcinoma cells. Toxicology 2009, 264, 104–109. [Google Scholar] [CrossRef]
- Diesing, A.K.; Nossol, C.; Dänicke, S.; Walk, N.; Post, A.; Kahlert, S.; Rothkötter, H.J.; Kluess, J. Vulnerability of polarised intestinal porcine epithelial cells to mycotoxin deoxynivalenol depends on the route of application. PLoS ONE 2011, 6, e17472. [Google Scholar] [CrossRef]
- Bouhet, S.; Oswald, I.P. The effects of mycotoxins, fungal food contaminants, on the intestinal epithelial cell-derived innate immune response. Vet. Immunol. Immunopathol. 2005, 108, 199–209. [Google Scholar] [CrossRef]
- Sergent, T.; Parys, M.; Garsou, S.; Pussemier, L.; Schneider, Y.J.; Larondelle, Y. Deoxynivalenol transport across human intestinal Caco-2 cells and its effects on cellular metabolism at realistic intestinal concentrations. Toxicol. Lett. 2006, 164, 167–176. [Google Scholar] [CrossRef]
- Seeling, K.; Dänicke, S.; Valenta, H.; van egmond, H.P.; Schothorst, R.C.; Jekel, A.A.; Lebzien, P.; Schollenberger, M.; Razzazi-Fazeli, E.; Flachowsky, G. Effects of Fusarium toxin-contaminated wheat and feed intake level on the biotransformation and carry-over of deoxynivalenol in dairy cows. Food Addit. Contam. 2006, 23, 1008–1020. [Google Scholar] [CrossRef]
- Gutzwiller, A. Effects of deoxynivalenol (DON) in the lactation diet on the feed intake and fertility of sows. Mycotoxin Res. 2010, 26, 211–215. [Google Scholar] [CrossRef]
- Collins, T.F.X.; Sprando, R.L.; Black, T.N.; Olejnik, N.; Eppley, R.M.; Hines, F.A.; Rorie, J.; Ruggles, D.I. Effects of deoxynivalenol (DON, vomitoxin) on in utero development in rats. Food Chem. Toxicol. 2006, 44, 747–757. [Google Scholar] [CrossRef]
- Prelusky, D.B.; Gerdes, R.G.; Underhill, K.L.; Rotter, B.A.; Jui, P.Y.; Trenholm, H.L. Effects of low-level dietary deoxynivalenol on the hematological and clinical parameters of the pig. Nat. Toxins 1994, 2, 97–104. [Google Scholar] [CrossRef]
- Swamy, H.V.L.N.; Smith, T.K.; MacDonald, E.J.; Boermans, H.J.; Squires, E.J. Effects of feeding a blend of grains naturally contaminated with Fusarium mycotoxins on growth and immunological measurements of starter pigs, and the efficacy of a polymeric glucomannan mycotoxin adsorbent. J. Anim. Sci. 2002, 80, 3257–3267. [Google Scholar]
- Bretz, M.; Beyer, M.; Cramer, B.; Knecht, A.; Humpf, H.-U. Thermal degradation of the Fusarium mycotoxin deoxynivalenol. J. Agric. Food Chem. 2006, 54, 6445–6451. [Google Scholar] [CrossRef]
- Dänicke, S.; Valenta, H.; Döll, S. On the toxicokinetics and the metabolism of deoxynivalenol (DON) in the pig. Arch. Anim. Nutr. 2004, 58, 169–180. [Google Scholar] [CrossRef]
- Zielonka, Ł.; Wiśniewska, M.; Gajecka, M.; Obremski, K.; Gajecki, M. Influence of low doses of deoxynivalenol on histopathology of selected organs of pigs. Pol. J. Vet. Sci. 2009, 12, 89–95. [Google Scholar]
- Goyarts, T.; Dänicke, S.; Valenta, H.; Ueberschär, K.H. Carry-over of Fusarium toxins (deoxynivalenol and zearalenone) from naturally contaminated wheat to pigs. Food Addit. Contam. 2007, 24, 369–380. [Google Scholar] [CrossRef]
- Dänicke, S.; Brezina, U. Kinetics and metabolism of the Fusarium toxin deoxynivalenol in farm animals: Consequences for diagnosis of exposure and intoxication and carry over. Food Chem. Toxicol. 2013, 60, 58–75. [Google Scholar] [CrossRef]
- Eriksen, G.S.; Pettersson, H.; Lindberg, J.E. Absorption, metabolism and excretion of 3-acetyl DON in pigs. Arch. Tierernähr. 2003, 57, 335–345. [Google Scholar]
- Gajęcka, M.; Stopa, E.; Tarasiuk, M.; Zielonka, Ł.; Gajęcki, M. The expression of type-1 and type-2 nitric oxide synthase in selected tissues of the gastrointestinal tract during mixed mycotoxicosis. Toxins 2013, 5, 2281–2292. [Google Scholar] [CrossRef]
- Silva-Campa, E.; Mata-Haro, V.; Mateu, E.; Hernández, J. Porcine reproductive and respiratory syndrome virus induces CD4+CD8+CD25+Foxp3+ regulatory T cells (Tregs). Virology 2012, 430, 73–80. [Google Scholar] [CrossRef]
- Döll, S.; Dänicke, S.; Valenta, H. Residues of deoxynivalenol (DON) in pig tissue after feeding mash or pellet diets containing low concentrations. Mol. Nutr. Food Res. 2008, 52, 727–734. [Google Scholar] [CrossRef]
- Dänicke, S.; Goyarts, T.; Döll, S.; Grove, N.; Spolders, M.; Flachowsky, G. Effects of the Fusarium toxin deoxynivalenol on tissue protein synthesis in pigs. Toxicol. Lett. 2006, 165, 297–311. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, A.C. Despite minimal hemodynamic alterations endotoxemia modulates NOS and p38-MAPK phosphorylation via metalloendopeptidases. Mol. Cell. Biochem. 2004, 265, 4–56. [Google Scholar]
- Grześk, E.; Grześk, G.; Koziński, M.; Stolarek, W.; Zieliński, M.; Kubica, J. Nitric oxide as a cause and a potential place therapeutic intervention in hypo responsiveness vascular in early sepsis. Folia Cardiol. 2011, 6, 36–43. [Google Scholar]
- Castro, M.; Muńoz, J.M.; Arruebo, M.P.; Murillo, M.D.; Arnal, C.; Bonafonte, J.I.; Plaza, M.A. Involvement of neuronal nitric oxide synthase (nNOS) in the regulation of migrating motor complex (MMC) in sheep. Vet. J. 2012, 192, 352–358. [Google Scholar] [CrossRef]
- Lucioli, J.; Pinton, P.; Callu, P.; Laffitte, J.; Grosjean, F.; Kolf-Clauw, M.; Oswald, I.P.; Bracarense, A.P.F.R.L. The food contaminant deoxynivalenol activates the mitogen activated protein kinases in the intestine: Interest of ex vivo models as an alternative to in vivo experiments. Toxicon 2013, 66, 31–36. [Google Scholar] [CrossRef]
- Alonso-Pozos, I.; Rosales-Torres, A.M.; Avalos-Rodriguez, A.; Vergara-Onofre, M.; Rosado-Garcia, A. Mechanism of granulosa cell death during follicular atresia depends on follicular size. Theriogenology 2003, 60, 1071–1081. [Google Scholar] [CrossRef]
- Waché, Y.J.; Valat, C.; Postollec, G.; Bougeard, S.; Burel, C.; Oswald, I.P.; Fravalo, P. Impact of deoxynivalenol on the intestinal microflora of pigs. Int. J. Mol. Sci. 2009, 10, 1–17. [Google Scholar]
- Alassane-Kpembi, I.; Kolf-Clauw, M.; Gauthier, T.; Abrami, R.; Abiola, F.A.; Oswald, I.P.; Puel, O. New insights into mycotoxin mixtures: The toxicity of low doses of type B trichothecenes on intestinal epithelial cells is synergistic. Toxicol. Appl. Pharmacol. 2013, 272, 191–198. [Google Scholar] [CrossRef]
- Awad, W.A.; Aschenbach, J.R.; Zentek, J. Cytotoxicity and metabolic stress induced by deoxynivalenol in the porcine intestinal IPEC-J2 cell line. J. Anim. Physiol. Anim. Nutr. 2012, 96, 709–716. [Google Scholar] [CrossRef]
- Grenier, B.; Loureiro-Bracarense, A.-P.; Lucioli, J.; Pacheco, G.D.; Cossalter, A.-M.; Moll, W.-D.; Schatzmayr, G.; Oswald, I.P. Individual and combined effects of subclinical doses of deoxynivalenol and fumonisins in piglets. Mol. Nutr. Food Res. 2011, 55, 761–771. [Google Scholar] [CrossRef]
- Weaver, A.C.; See, M.T.; Hansen, J.A.; Kim, Y.B.; de Souza, A.L.P.; Middleton, T.F.; Kim, S.W. The use of feed additives to reduce the effects of aflatoxin and deoxynivalenol on pig growth, organ health and immune status during chronic exposure. Toxins 2013, 5, 1261–1281. [Google Scholar] [CrossRef]
- Awad, W.A.; Razzazi-Fazeli, E.; Böhm, J.; Ghareeb, K.; Zentek, J. Effect of addition of a probiotic microorganism to broiler diets contaminated with deoxynivalenol on performance and histological alterations of intestinal villi of broiler chickens. Poult. Sci. 2006, 85, 974–979. [Google Scholar] [CrossRef]
- Arunachalam, C.; Doohan, F.M. Trichothecene toxicity in eukaryotes: Cellular and molecular mechanisms in plants and animals. Toxicol. Lett. 2013, 217, 149–158. [Google Scholar] [CrossRef]
- Coppock, R.W.; Swanson, S.P.; Gelberg, H.B.; Koritz, G.D.; Hoffman, W.E.; Buck, W.B. Preliminary study of the pharmacokinetics and toxicopathy of deoxynivalenol (vomitoxin) in swine. Am. J. Vet. Res. 1985, 46, 169–174. [Google Scholar]
- Kolf-Clauw, M.; Castellote, J.; Joly, B.; Bourges-Abella, N.; Raymond-Letron, I.; Pinton, P. Development of a pig jejunal explant culture for studying the gastrointestinal toxicity of the mycotoxin deoxynivalenol: Histopathological analysis. Toxicol. in Vitro 2009, 23, 1580–1584. [Google Scholar] [CrossRef]
- D’Mello, J.P.F. Antinutritional factors and mycotoxins. In Farm Animal Metabolism and Nutrition; D’Mello, J.P.F., Ed.; CAB International: Wallingford, UK, 2000; pp. 383–403. [Google Scholar]
- Bracarense, A.P.; Lucioli, J.; Grenier, B.; Drociunas Pacheco, G.; Moll, W.D.; Schatzmayr, G. Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. Br. J. Nutr. 2012, 107, 1776–1786. [Google Scholar] [CrossRef]
- Pinton, P.; Nougayrede, J.P.; Del Rio, J.C.; Moreno, C.; Marin, D.E.; Ferrier, L. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol. 2009, 237, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Vandenbroucke, V.; Croubels, S.; Martel, A.; Verbrugghe, E.; Goossens, J.; van Deun, K. The mycotoxin deoxynivalenol potentiates intestinal inflammation by Salmonella typhimurium in porcine ileal loops. PLoS ONE 2011, 6, e23871. [Google Scholar]
- Davila, A.-M.; Blachier, F.; Gotteland, M.; Andriamihaja, M.; Benetti, P.-H.; Sanz, Y.; Tomé, D. Re-print of “Intestinal luminal nitrogen metabolism: Role of the gut microbiota and consequences for the host”. Pharmacol. Res. 2013, 69, 114–126. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Waśkiewicz, A.; Beszterda, M.; Kostecki, M.; Zielonka, Ł.; Goliński, P.; Gajęcki, M. Deoxynivalenol in the Gastrointestinal Tract of Immature Gilts under per os Toxin Application. Toxins 2014, 6, 973-987. https://doi.org/10.3390/toxins6030973
Waśkiewicz A, Beszterda M, Kostecki M, Zielonka Ł, Goliński P, Gajęcki M. Deoxynivalenol in the Gastrointestinal Tract of Immature Gilts under per os Toxin Application. Toxins. 2014; 6(3):973-987. https://doi.org/10.3390/toxins6030973
Chicago/Turabian StyleWaśkiewicz, Agnieszka, Monika Beszterda, Marian Kostecki, Łukasz Zielonka, Piotr Goliński, and Maciej Gajęcki. 2014. "Deoxynivalenol in the Gastrointestinal Tract of Immature Gilts under per os Toxin Application" Toxins 6, no. 3: 973-987. https://doi.org/10.3390/toxins6030973




