Tetrodotoxin: Chemistry, Toxicity, Source, Distribution and Detection
Abstract
:1. Introduction
| Country | Causative Organism | Analogues of TTX found | No. of cases | Year of poisoning incident | Reference |
|---|---|---|---|---|---|
| Australia | Toadfish | TTX | 7 | 2004 | [100] |
| Australia | Puffer fish | TTX | 11 | 1 January 2001 to 13 April 2002 | [101] |
| Bangladesh | Puffer fish, Dora potka i.e., Takifugu oblongus in Natore District and Badami potka i.e., Arothron stellatus in Narsingdi District | TTX | 141 (Three outbreaks) 48 (Narsingdi District) + 10 (Dhaka) + 83 (Natore District) | 9 April 2008 (Narsingdi District) 3 June 2008 (Dhaka) 8 June 2008 (Natore District) | [19] |
| Bangladesh | Puffer fish | TTX | 53 | May 2001–May 2006 | [102] |
| Bangladesh (Khulna district) | Puffer fish | TTX | 37 (8 died) | April 2002 | [9] |
| Bangladesh (Degholia in the Khulna district) | Puffer fish, Takifugu oblongus | TTX | 36 (7 died) | May 2002 | [11] |
| Bangladesh | Puffer fish, Takifugu oblongus | TTX | 8 (5 died) | 1998 | [3] |
| China (Lianyungang) | Gastropod, Nassarius spp. | TTX, trideoxyTTX, 4-epiTTX, anhydroTTX and oxoTTX | - | May–August 2007 | [28] |
| China (South Zheijiang, Mainland) | Gastropod, Zeuxis samiplicutus | TTX | 30 | June 2001 | [5] |
| India (Orissa state, Burla) | Puffer fish | TTX | 8 | October 2007 | [17] |
| Japan | – | TTX | Numerous | 1965–2010 | [46] |
| Japan | Puffer fish “kinfugu”, T. poecilonotus | TTX | 1 | October 2008 | [103] |
| Japan | Thread-sail filefish “Kawahagi” | TTX | 1 | October 2008 | [103] |
| Japan | Marine snail, Nassarius glans | TTX | 1 | July 2007 | [103] |
| Japan | Marine snail, C. saulie | TTX | 2 | December 1987 | [46] |
| Japan | Marine snail, C. saulie | TTX | 1 | December 1979 | [40] |
| Japan | Marine snail (Ivory shell), Babylonia japonica | TTX | 5 | June 1957 | [16] |
| Korea | Unknown fish | TTX | 3 | October 2010 | [104] |
| Mediterranean region (Egypt and Israel) | Puffer fish, L. sceleratus | TTX | 13 (9) Hafia bay, (2) Caesarea coast, (2) Ashkelon coast | November 2005, February 2007, March 2007, November 2007, March 2008 and May 2008 | [105] |
| New Zealand | Grey side-gilled sea slug, Pleurobranchaea maculata | TTX | 15 dogs | July to November 2009 | [46] |
| Spain (Malaga; caught from Portuguese waters) | Trumpet shell, Choronia lampus | TTX | – | October 2007 | [45] |
| Taiwan (Kaohsiung) | Gastropod, Niotha clathrata | TTX | 3 | November 2006 | [13] |
| Taiwan | Gastropod | TTX and PSP | 1 | October 2005 | [12] |
| Taiwan (Southern China Sea) | Marine snail, Nassarius (Alectricon) glans | TTX | 5 | April 2004 | [46] |
| Taiwan (Tungsa Island) | Gastropod, Nassarius glans | TTX | 6 | April 2004 | [10] |
| Taiwan (Western) | Gastropod, Polinices didyma and Natica lineata | TTX | – | 2003 | [8] |
| Taiwan (Tungkang, Southern Taiwan) | Gastropods, Oliva miniacea, Oliva mustelina and Oliva nirasei | TTX | 1 | February 2002 | [7] |
| Taiwan | Unknown fish | TTX | 6 (1 died) | April 2001 | [106] |
| Taiwan | Puffer fish, Lagocephalus lunaris | TTX | 6 (1 died) | April 2001 | [6] |
| Taiwan (Northern) | Gastropods (snails), Zeuxis sufflatus and Niotha clathrata | TTX | 4 | April 2001 | [4] |
| Taiwan (Chunghua Prefecture, Western Taiwan) | Puffer fish, Takifugu niphobles | TTX | 5 | Jan 2000 | [107] |
| Thailand (Chon Buri, Eastern Thailand) | Eggs of horseshoe crab, Carcinoscorpius rotundicauda | TTX | 71 | 1995 | [63] |
| US (New Hampshire, New York, Pennsylvania and Virginia) | Newt, N. viridescens | TTX, 6-epiTTX and 11-oxoTTX | Collected samples for analysis | 2001–2009 | [37] |
| US (Chicago) | Puffer fish | TTX | 2 | May 2007 | [108] |
| US (California) | Puffer fish transported from Japan | TTX | 3 | April 1996 | [109] |
| US (Hawaii) | Puffer fish, Diodon hystrix | TTX | 1 | 1986 | [110] |

2. Structures and Standards for TTX and Its Analogues
| No. | Name of TTX analogue | Source for extraction | Purity (%) | CAS No. | Contact details |
|---|---|---|---|---|---|
| 1 | Tetrodotoxin (citrate free) | Fugu fish organs | 96 (HPLC‚ IR‚ NMR) | 4368-28-9 | [150] |
| 2 | Tetrodotoxin (citrate free) | Fugu spp. | 100 | 4368-28-9 | [151] |
| 3 | Tetrodotoxin (citrate free) | NM | NM | 4368-28-9 | [152] |
| 4 | Tetrodotoxin (citrate free) | NM | NM | 4368-28-9 | [153] |
| 5 | Tetrodotoxin (MW 328.28) | Fugu spp. | NM | 4368-28-9 | [154] |
| 6 | Tetrodotoxin (MW 319.27) C11H17N3O8 | NM | ≥98 (HPLC) | 4368-28-9 | [155] |
| 7 | Tetrodotoxin (citrate free), C11H17N3O8, MW 319.3 | NM | NM | 4368-28-9 | [156] |
| 8 | Tetrodotoxin (citrate free), C11H17N3O8, MW 319.28 | Tetraodon pardalis | >98 | 4368-28-9 | [157] |
| 9 | Tetrodotoxin (citrate free), C11H17N3O8, MW 319.3 | Fugu spp. | ≥95 by TLC | 4368-28-9 | [158] |
| 10 | Tetrodotoxin (citrate free), C11H17N3O8, MW 319.2 | NM | >98 | 4368-28-9 | [159] |
| 11 | Tetrodotoxin citrate, C11H17N3O8, MW 319.27 | Fugu | >98 | 18660-81-6 | [160] |
| 12 | Tetrodotoxin citrate, C11H17N3O8, MW 319.27 | NM | >98 | 4368-28-9 | [161] |
| 13 | Tetrodotoxin (citrate free), C11H17N3O8, MW 319.27 | NM | NM | 4368-28-9 | [162] |
| 14 | Tetrodotoxin (citrate free), C11H17N3O8, MW 319.3 | NM | NM | 4368-28-9 | [163] |
| No. | Analogue | Exact Molar Mass | Molecular Formula | MRM | Source Organism |
|---|---|---|---|---|---|
| 1 | 4-S-Cys TTX | 422.110753 | C14H22N4O9S | NR | Puffer fish: Fugu Pardalis [24,164] |
| 2 | TTX-8-O-hemisuccinate | 418.109787 | C15H20N3O11− | NR | Synthetic analogue [112] |
| 3 | Chiriquitoxin | 392.117945 | C13H20N4O10 | NR | Toad: Atelopus chiriquiensis [30,70,143] |
| 4 | 11-oxoTTX | 335.096482 | C11H17N3O9 | 336/318 336/300 336/282 336/178 336/162 | |
| 5 | TTX-11-carboxylic acid | 332.073007 | C11H14N3O9− | NR | Synthetic analogue [112] |
| 6 | TTX | 319.101567 | C11H17N3O8 | 320/302 320/162 |
|
| 7 | 4-epiTTX | 319.101567 | C11H17N3O8 | 320/302 320/162 |
|
| 8 | 6-epiTTX | 319.101567 | C11H17N3O8 | 320/302 320/162 |
|
| 9 | Tetrodonic acid | 319.101567 | C11H17N3O8 | NR | |
| 10 | 11-norTTX-6,6-diol | 305.085917 | C10H15N3O8 | NR | Synthetic analogue [112] |
| 11 | 5-deoxyTTX | 303.106652 | C11H17N3O7 | 304/286 304/176 |
|
| 12 | 11-deoxyTTX | 303.106652 | C11H17N3O7 | 304/286 304/176 |
|
| 13 | 1-hydroxy-5,11-dideoxyTTX | 303.106652 | C11H17N3O7 | NR | Newt: Taricha granulosa [59] |
| 14 | 4,9-anhydro TTX | 301.091002 | C11H15N3O7 | 302/256 302/162 | |
| 15 | 6-epi-4,9-anhydroTTX | 301.091002 | C11H15N3O7 | 302/256 302/162 | Newt: Cynops pyrrhogaster [52], Notophthalmus viridescens 53,57 and Triturus spp 35,175 |
| 16 | AnhydroTTX | 300.083177 | C11H14N3O7− | 302/256 302/162 | |
| 17 | 11-norTTX-6(S)-ol | 289.091002 | C10H15N3O7 | 290/272 290/162 | |
| 18 | 11-norTTX-6(R)-ol | 289.091002 | C10H15N3O7 | 290/272 290/162 | |
| 19 | 1-hydroxy-8-epi-5,6,11-trideoxy TTX | 287.111737 | C11H17N3O6 | 288/162 | |
| 20 | 6,11dideoxyTTX | 287.111737 | C11H17N3O6 | 288/224 |
|
| 21 | 8,11dideoxyTTX | 287.111737 | C11H17N3O6 | NR | Synthetic analogue [114,119] |
| 22 | 5,6,11-trideoxy TTX | 271.116822 | C11H17N3O5 | 272/254 272/162 |
|
| 23 | 8-epi-5,6,11-trideoxy TTX | 271.116822 | C11H17N3O5 | 272/162 | Newt: Cynops ensicauda popei [59] |
| 24 | 4-epi-5,6,11-trideoxyTTX | 271.116822 | C11H17N3O5 | NR | Puffer fish [127] |
| 25 | 1-hydroxy-4,4a-anhydro-8-epi-5,6,11-trideoxyTTX | 269.101172 | C11H15N3O5 | 270/162 | |
| 26 | 4,9-anhydro-5,6,11-trideoxy TTX | 253.106257 | C11H15N3O4 | NR | Puffer fish [59] |
| 27 | 4,9-anhydro-8-epi-5,6,11-trideoxy TTX | 253.106257 | C11H15N3O4 | 254/162 | Newt: Cynops ensicauda popei [59] |
| 28 | 4,4a-anhydro-5,6,11-trideoxy TTX | 253.106257 | C11H15N3O4 | NR | Puffer fish [59] |
| 29 | 4-epi-11-deoxyTTX | NR | NR | NR | Newt (Cynops ensicauda) [173] |
| 30 | 4,9-anhydro-11-deoxyTTX | NR | NR | NR | Newt (Cynops ensicauda) [173] |
3. Chemical Synthesis of TTX and Its Analogues
4. Aetiology of TTX
4.1. Biosynthesis of TTX
4.2. Aetiology of TTX among Marine and Fresh Water Organisms

4.3. Aetiology of TTX among Terrestrial Animals
5. Biochemistry
6. Resistance to TTX in TTX Bearing Organisms
7. Clinical Study
7.1. Time after Ingestion of TTX
7.2. Amount of TTX Ingested
7.3. Physical Status of Victim
7.4. Health Status of Victim
7.5. Clinical Findings
7.6. Treatment
7.7. Application of TTX in the Medical Field
8. The Distribution of TTX and Its Analogues
8.1. Geographic Distribution of TTX
8.1.1. Geographic Distribution of TTX in Freshwater and Marine Organisms
8.1.2. Geographic Distribution of TTX in Terrestrial Animals
8.2. Organism Specific Distribution of TTX and Its Analogues
8.2.1. Distribution of TTX and Its Analogues in Puffer Fish
8.2.2. Distribution of TTX and Its Analogues in Gastropod
8.2.3. Distribution of TTX in Sea Slug, Star Fish, Blue-Ringed Octopus, Ribbon Worm and Bacteria
8.2.4. Distribution of TTX and Its Analogues in Terrestrial Animals
8.3. Tissue Specific Distribution of TTX and Its Analogues in All Organisms
8.4. TTX Co-occurrence with Other Marine Toxins

9. Chemical Stability and Toxicity of Analogues
10. Miscellaneous Studies
11. Historical Perspective on Analytical Methods Used for TTX and Its Analogues
11.1. Bioassays
11.2. Chemical Assays
11.3. Historical Overview of LC-MS/MS Methods for TTX and Its Analogues
11.3.1. Extraction and Clean Up Methodologies
| Species | Extraction | Column | Mobile phase | Method | Analyte * | LOD and LOQ | Linear Range | Reference |
|---|---|---|---|---|---|---|---|---|
| Lagocephalus sceleratus (Gmelin, 1789) (Liver, GI-tract, gonad (ovary/testis), muscle and skin) | 0.1% AA | Zorbax 300SB-C3 Sunfire C18 XBridge™ Amide | Isocratic: 1% ACN + 10mM TMA + 10 mM AF, pH 4 (For Zorbax 300SB-C3) A: 1% ACN + 20 mM AHB + 20 mM Am-OH + 10 mM AF, pH 4 and B: 5% ACN + 20 mM AHB + 20 mM Am-OH + 10 mM AF, pH 4 (For Sunfire C18) A: 10 mM AF + 10 mM FA in H2O B: 5 mM AF + 2 mM FA in ACN:H2O, 95:5 (For XBridge™ Amide) | LC-MS/MS and CID-MS/MS | 6, 7, 11, 12, 14, 17, 18, 22 | LOD:16 ng/mL at S/N > 3 LOQ:63 ng/mL at S/N > 10 | 62.5–2000 ng/mL | [29] |
| Lagocephalus sceleratus (Muscle) | ASE and SE (0.03 M AA) | Acquity UPLC BEH HILIC | A: 5% ACN B: 95% ACN + 1% AA pH 3.5 | LC-M/MS | 6 | For Solvent Std LOD: 0.074 ng/mL LOQ: 0.123 ng/mL For Matrix-matched Std LOD: 7.3 µg/kg and LOQ: 24.5 µg/kg at S/N = 3 and 10 | 5–500 ng/mL (Solvent Std) 50–3000 µg/kg (Matrix-matched Std) | [48] |
| Potka or Tepa fish (Cooked fishAnd blood and urine of victim) | 1% AA + 80% MeOH | C30 UG-5 | A: 30 mM AHB, pH 5 in H2O B: 10 mM AF, pH 5 in 1% ACN | LC-FLD | 6, 7, 14 | NR | NR | [19] |
| Lagocephalus lunaris, L. spadiceus, Tetradon nigroviridis and Arothron reticularis (Reproductive tissue, digestive tissue, liver, muscle and skin) | 0.1% AA, ethyl acetate Defat *, CharAd † | ZIC-HILIC | A: 10 mM AF + 10 mM FA in H2O B: 5 mM AF + 2 mM FA in 80% ACN | LC-MS/MS | 6, 11/12, 16 | NR | NR | [27] |
| Puffer fish (ovary) | 0.05 M AA, ODS-SPE, Ultra filtration (0.22 µ) | Atlantis HILIC Silia | 10 mM AF, pH 3.5 + ACN (22:78, v/v) | LC-MS (SIR) LC-MS/MS (CID) | 6, 7, 9, 11, 14, 17/ 18, 20, 22 | SIR mode LOD: 0.1 ng/mL at S/N = 3 LOQ: 0.25 ng/mL at S/N = 10 | 0.25–100 ng/mL | [127] |
| Takifugu rubripes and Takifugu niphobles (Muscle, skin, liver, gonad) | 1% AA | Puresil C18 | 30 mM HFB + 1 mM Am-acetate, pH 5.0 | LC-MS | 6, 7, 14 | NR | NR | [167] |
| Fugu niphobles (Ovary/testis, liver, intestine, dorsal skin and dorsal muscle) Tetraodon nigroviridis and Tetraodon biocellatus (Whole body) | 0.05 M AA, C18-SPE, CHCl3 Defat *, CharAd † | TSKgel Amide-80 | 16 mM AF, pH 5.5 in ACN (3:7, v/v) | LC-MS/MS | 6, 7, 11, 12, 14, 20, 22 | NR | NR | [26] |
| Lagocephalus sp. (Cooked fish) | 1% AA, CHCl3 Defat * | TSK-GEL Amide-80 | 5 mM AF + 26.5 mM FA in ACN:H2O, 70:30 | LC-MS | 6 | NR | NR | [108] |
| Tetraodon turgidus and Tetraodon sp. (Skin, muscle, liver, intestine, gonad; ovary/testis) | NM | RP-18 | 1 mM TBA-PO4, pH 5.8 | HPLC-FLD | PSP toxins (STX, neoSTX, GTX1–4, dcSTX, dcGTX2 and 3) | NR | NR | [222,243] |
| Fugu poecilonotus (Liver) Fugu niphobles (Whole body) | Sephadex G-10 Gel filtration (For F. poecilonotus) 0.1% AA, 50% CharAd † (For Fugu niphobles) | For F. poecilonotus: ODS-5; LC-FLD For Fugu niphobles: HILIC; LC-MS | 5 mM AHB + 50 mM Am-acetate, pH 5 in 3% ACN; LC-FLD (For F. poecilonotus) 5 mM AHB + 50 mM Am-acetate, pH 5 in 3% ACN; LC-FLD (For Fugu niphobles) | LC-FLD LC-FLD and LC-MS | 1, 4, 6, 7, 14 (on ODS-5) and 11, 12, 20, 22 (on HILIC) | NR | NR | [89,166] |
| Fugu niphobles (Liver, intestine, gonad, bone, muscle, skin, other organs; viscera) | 0.1% AA | ODS-5 | 20 mM AHB, pH 5 + 10 mM Am-acetate, pH 5 in 3% MeCN | LC-FLD | 6, 7, 14 | NR | NR | [89] |
| Takifugu oblongus (Liver, gonad; ovary/testis, muscle, skin, other organs; viscera) | 0.1% AA | SeQuant ZIC- HILIC | A: 10 mM AF + 10 mM FA in water B: 5 mM AF + 2 mM FA in 80:20 ACN:H2O | LC-MS/MS | 6, 7, 12, 16, 22 | LOD:0.09 ng (TTX), 0.14 ng(AnhydroTTX), 0.20 ng (11-deoxy TTX) | 0.25–10 ng (TTX) 0.25-5.8 ng (AnhydroTTX) 0.20-5 ng (11-deoxy TTX) | [138] |
| Fugu pardalis (Ovary) | 0.05 M AA, EtOAc Defat *, CharAd †, Bio-Gel P2 and Hitachi gel 3011C filtration | TSK gel Amide-80 | 16 mM AF, pH 5.5 in ACN (3:7, v/v) | LC-MS | 6, 7, 11, 12, 14, 17, 20, 22 | NR | NR | [24,125] |
| Fugu pardalis (Ovary, testis, liver, spleen, gall, skin, intestine, kidney and muscle) | 0.1% AA, Cosmosil 75 C18-OPN resin | C30 UG-5 (LC-FLD) TSK-GEL Amide-80 (LC/MS) | 30 mM AHB + 10 mM AF in 1% ACN, pH 5 (C30 UG-5) 16 mM AF, pH 5.5 in ACN (3:7, v/v) (TSK-GEL Amide-80) | LC-FLD LC-MS | 1, 6, 11, 12, 14, 22, | LOD 0.07 pmole (LC-MS) | NR | [24,149,175] |
| Fugu poecilonotus and F. pardalis (Ovary) | 0.05 M AA, CharAd † | C30 UG-5 (LC-MS/MS and LC-FLD) | 20 mM AHB + 10 mM AF in 1% ACN, pH 4 (LC-MS/MS) 30 mM AHB + 10 mM AF in 1% ACN, pH 5 (LC-LFD) | LC-MS/MS LC-LFD | 6, 7, 10, 11, 14, 17, 18, 22 | LOD 0.7 pmol at S/N 2 | 50–1000 pmol | [149] |
| Takifugu xanthopterus (Liver) | 0.05 M Tris-Ac, pH 8.2; Sephacryl S-400 column filtration, 0.03 M AA, DCM Defat *, Bio-Gel P-2 filtration | YMC AM OSD | [218] | LC-FLD | 6, 7, 9, 11 | NR | NR | [218] |
| Species | Extraction | Column | Mobile phase | MS | Analyte * | LOD and LOQ | Linear Range | Reference |
|---|---|---|---|---|---|---|---|---|
| Trumpet shell | ||||||||
| Charonia lampas (Viscera and muscle) | ASE ‡ and SE ‡‡ (0.03 M AA) | Acquity UPLC BEH HILIC | A: 5% ACN B: 95% ACN + 1% AA pH 3.5 | LC-MS/MS | 6 | For Solvent Std LOD: 0.074 ng/mL LOQ: 0.123 ng/mL For Matrix-matched Std LOD: 7.3 µg/kg LOQ: 24.5 µg/kg at S/N = 3 and 10 | 5–500 ng/mL (Solvent Std) 50–3000 µg/kg (Matrix-matched Std) | [48] |
| Charonia lampas lampas (Digestive gland) | NM | NM | NM | LC-MS/MS | 6, 22 | NR | NR | [45] |
| Gastropod | ||||||||
| Nassarius spp. | [28] | [28] | [28] | HPLC-MSn (Ion trap) and HPLC-FLD | 4, 6, 7, 16, 22 | NR | NR | [28] |
| Gibbula umbilicalis, Monodonta lineata and Charonia lampas | 1% AA, DCM Defat *, C18 SPE | XBridge™ Amide (LC- MS/MS) Waters Acquity UPLC BEH Amide (UPLC-MS/MS) | For both, A: 10 mM FA + 10 mM AF in H2O B: 2 mM FA + 5 mM AF in ACN: H2O, 95:5 | LC-MS/MS UPLC-MS/MS | 6, 7, 11/12, 14, 17/18, 22 | For LC- MS/MS LOD: 16 ng/mL at S/N > 3 LOQ: 63 ng/mL S/N > 10 For UPLC-MS/MS LOD: 1.7 ng/mL at S/N > 3 LOQ: 5 ng/mL S/N > 10 | 50–2000 ng/mL (LC-MS/MS) 31.25–3000 ng/mL (UPLC-MS/MS) | [47] |
| Grey side-gilled sea slug, Pleurobranchaea maculata (Whole body) | 50% MeOH, Strata Phenomonex SPE | TSK-GEL amide 80 | A: 10% ACN + (90% 50 mM FA + 2 mM AF in H2O) B: 90% ACN + (10% 50 mM FA + 2 mM AF in H2O) | LC-MS/MS | 4, 6, 12, 16, 17/18 | LOD: 5 ng/mL (S/N = 50) | 5–250 ng/mL | [73] |
| Blue-ringed octopus | ||||||||
| Hapalochlaena fasciata and H. lunulata | 0.05 N AA | Synergi 4 µ Hydro-RP 80A C18 | 0.97% Heptafluorobutyric acid + 0.29% AA in 3% ACN (pH adjusted to 5.0 with NH4OH) | Q-TOF MS | 6 | NR | 500 ng/mL to 0.5 mg/mL | [76] |
| Blue-ringed octopus (Hapalochlaena fasiata and H. lunulata) (Posterior salivary gland, arm, dorsal mantle, ventral mantle, anterior salivary gland, digestive gland, testes conts./egg/paralarva, oviducal gland, brachial heart, nephridia, gill) | 0.05 N AA | Synergi 4 µ Hydro-RP 80A C18 | 3% ACN + 0.97% HFB + 0.29% AA, pH 5 | LC-FLD | 6 | NR | 500 ng/mL–0.5 mg/mL | [178] |
| Species | Extraction | Column | Mobile phase | MS | Analytes * | LOD and LOQ | Linear Range | Reference |
|---|---|---|---|---|---|---|---|---|
| Cynops ensicauda popei (Whole body) | O.2 M AA, Hexane Defat *, CharAd †, Bio-Rex 70 and Hitachi gel 3011C SPE | TSK gel G1000PW (HILIC) | 16 mM AF, pH 5.5 + ACN (3:7, v/v) | LC-MS/MS | 6, 7, 8, 11, 12, 14, 17, 19, 20, 23, 25, 27 | NR | NR | [24,26,59,175] |
| Notophthalmus viridescens (Whole body) | 0.1% AA + 70% EtOH, CharAd † | Develosil C30 UG-5 | 1% ACN + 20 mM AHB + 10 mM AF, pH 4.0 | LC-FLD | 4, 6, 8 | NR | NR | [37] |
| Notophthalmus viridescens (Whole body, liver and skin) | 0.1% AA in 70% MeOH | Develosil C30 UG-5 | 1% ACN + 30 mM AHB + 10 mM AF, pH 5.0 | LC-FLD | 6, 7, 8, 14, 15, | LOD 0.4 pmol | 50–1000 pmol | [57,149] |
| Triturus spp. (Whole body) | 0.1% AA in 70% MeOH, CharAd † | Develosil (C30-UG-5) | 30 mM AHB + 10 mM AF, pH 5 | LC-FLD | 6, 8 | LOD 100 ng/g (TTX) 40 ng/g (6-epi TTX) | NR | [35] |
| Taricha granulosa (Skin) | 0.1 M AA | Synergi 4 µ Hydro-RP 80A | 50 mM Am-acetate + 60 mM AHB, pH 5 in 1% ACN | LC-FLD | 6 | NR | NR | [54,176] |
| Notophthalmus viridescens (Whole body) | 1% AA in 70% MeOH | Develosil C30-UG-5 | 1:11 vol.% ACN, 30 mM AHB + 10 mMAF, pH 5.0 | LC-FLD | 4, 6, 7, 8, 14, 15 | NR | NR | [53] |
| Taricha granulosa (Skin) | 0.1 M AA | Synergi 4 µ Hydro-RP 80A | 50 mM Am-acetate + 60 mM AHB, pH 5 in 1% ACN | LC-FLD | 6, 7, 14 | NR | NR | [51] |
| Cynopus ensicauda (Skin) | 0.05 M AA, CharAd † | C30- UG-5 (LC-MS/MS and LC-FLD) | 20 mM AHB + 10 mM AF in 1% ACN, pH 4 (LC-MS/MS) 30 mM AHB + 10 mM AF in 1% ACN, pH 5 (LC-LFD) | LC-MS/MS LC-LFD | 6, 7, 8, 11, 12, 14, 15, 17, 18 | LOD: 0.7 pmol at S/N 2 | 50–1000 pmol | [149] |
| Notophthalmus viridescens (Whole body) | 1% AA in 70% MeOH | Develosil C30-UG-5 (HPLC-FLD and LC-MS) | 30 mM AHB in 1% ACN, pH 5 (HPLC-FLD) 20 mM AHB + 10 mM AF in 1% ACN, pH 4 (LC-MS) | HPLC-FLD LC-MS | 6, 7, 8, 11, 12, 14, 17, 18 | NR | NR | [50] |
| Cynops pyrrhogaster (Whole body) | 0.1% AA | Puresil C18 | 30 mM HFB + 1 mM Am-acetate, pH 5 | LC-MS | 6, 7, 8, 15 | NR | NR | [52] |
| Species | Extraction | Column | Mobile phase | MS | Analytes * | LOD and LOQ | Linear Range | Reference |
|---|---|---|---|---|---|---|---|---|
| Crabs | ||||||||
| Demania cultripes, D. toxica, D. reynaudi, Lophozozymus incisus, L.pictor and Atergatopsis germaini (Appendage, cephalo-thorax and viscera) | 1%AA in MeOH, C18 cartridge | ODS-3 | 30 mM HFB + 1 mM Am-acetate, pH 5 | LC-MS | 6, 7, 16 | LOD: 0.005 µg/mL | 0.03–3 µg/mL | [52,139,] |
| Xanthias lividus (Appendage, cephalothorax and viscera) | 1%AA in MeOH, DCM Defat *, Bio-Gel P-2 filtration | [67] | [67] | HPLC | 6, 16 | NR | NR | [67] |
| Frogs | ||||||||
| Brachycephalus ephippium, B. nodoterga and B. pernix (Whole Body, skin, liver and ovary) | MeOH:AA (96:4), Amberlite GC-50 SPE, CharAd† | CLC-ODS (LC-FLD) Puresil C18 (LC-MS/MS) | 0.06N HFB + 0.001N Am-acetate, pH 5 (CLC-ODS) 30 mM HFB + 1 mM Am-acetate, pH 5 (Puresil C18) | LC-FLD LC-MS/MS | 4, 6, 7, 8, 9, 11, 12, 14, 17 | NR | NR | [33,52,72,176] |
| Brachycephalus ephippium (Skin) | 1%AA in MeOH, Petroleum ether Defat*, CharAd† | CLC-ODS (LC-FLD) Puresil C18 (LC-MS/MS) | 0.06N HFB + 0.001N Am-acetate, pH 5 (CLC-ODS) 30 mM HFB + 1 mM Am-acetate, pH 5 (Puresil C18) | LC-FLD LC-MS/MS | 4, 6, 7, 8, 9, 14 | NR | NR | [33,34,52,176] |
| Polypedates sp. (Skin, muscle and viscera) | 80% EtOH, pH 2, DCM Defat*, CharAd†, 1% AA in 20% EtOH, Bio-Gel P2 and Bio-Rex 70 filtration | Inertsil ODS-3 | 60 mM (NH4)3PO4, pH 5 + 10 mM HSA in 2% ACN | LC-FLD | 6, 8, 14, 15 | NR | NR | [71] |
| Species | Extraction | Column | Mobile phase | MS | Analytes * | LOD and LOQ | Linear Range | Reference |
|---|---|---|---|---|---|---|---|---|
| Aeromonas strain from ovary of puffer fish, Takifugu obscurus | 0.1% AA, CharAd †, Bio-Gel P2 and C18 SPE | ACQUITY UPLC BEH HILIC | A: 0.2% FA in H2O B: 0.2% FA in ACN | Q-TOF MS | 6 | NR | 0–250 ng/mL | [88] |
| Shewanella woodyi and Rosebacter sp. from copepod, Pseudocaligus fugu; ectoparasite of puffer fish, Takifugu pardalis | 0.1% AA, C18 SPE, CharAd † | [85,169] | Asakawa et al. 2003 and Ito et al. 2006 | Asakawa et al. 2003 and Ito et al. 2006 | 6, 7, 16 | NR | NR | [85,169] |
| Vibrio strain, LM-1 from the intestine of puffer fish, Fugu vermicularis radiates | DCM Defat *, 0.03 M AA, Bio-Gel P-2 filtration | YMC-pack AM-314 octyldecyl silane | 0.05M HSA + 0.05M KH2PO4, pH 7 in MeOH | LC-FLD | 6, 7, 16 | NR | NR | [91] |
| Nocardiopsis dassonvillei from the ovary of puffer fish, Fugu rubripes | 0.1% AA, CharAd †, Bio-Gel P2 and Bio-Rex 70 filtration | Bio-Rex 70 | MeOH | LC-MS | 6 | NR | NR | [82,92] |
| Sample | Extraction | Column | Mobile phase | MS | Analytes * | LOD and LOQ | Linear Range | Reference |
|---|---|---|---|---|---|---|---|---|
| Postmortem whole blood | MeOH, SPE | PC(Phosphorychloline)–HILIC | 1% AA + ACN in MeOH | LC-MS/MS | 6 and voglibose | LOD: 0.32 ng/mL LOQ: 1.08 ng/mL | 2–1200 ng/mL | [104] |
| Urine and plasma | 2% AA, C18 and ZIC-HILIC SPE | Atlantics dC18 | 10 mM AF + FA (95:5, v/v) + 5 mM HFB in 2% ACN | LC-MS/MS | 6 | LOD: LOQ: | 10–500 ng/mL | [135] |
| Blood and urine | C18 and Oasis MCX SPE | Allsphere ODS-2 (LC-UV) Nova-Pak C18 (LC-LFD) Zorax 300SB-C3 (LC-MS/MS) HILIC (LC-MS/MS) Atlantics dC18 (LC-MS/MS) | 4.8 mM 1-HSA + 41.8 mM SDP + 10% MeOH, pH (Allsphere ODS-2) 5 mM PIC B7 (HSA) + 3% MeCN in H2O, pH 4.5 (Nova-Pak C18) 10 mM TMA, 10 mM AF in 1% ACN, pH 4 (Zorax 300SB-C3) 0.1% FA in MeOH (HILIC) 10 mM AF + FA, (95:5, v/v) + 5 mM HFB + 2% ACN | LC-UV LC-LFD LC-MS/MS LC-MS/MS LC-MS/MS | 6 | LOD: 10 ng/mL (LC-UV) LOQ: 5 and 20 ng/mL for serum and urine (LC-LFD) LOD: 15.6 nM (LC-MS/MS) LOD: 0.1 ng/mL LOQ: 1 ng/mL (LC-MS/MS) LOD: 0.13 ngmL−1 LOQ: 2.5 ngmL−1 for urine and plasma | 10–50,000 ng/mL (LC-UV) 20–300 for urine and 5–20 ng/mL for serum (LC-LFD) 93.75–9375 nM (LC-MS/MS) 1–100 ng/mL 0–500 ngmL−1 for urine and 0–20 ngmL−1 for plasma | [13,106,135,196] |
| Cooked and raw puffer fish (liver) and human urine | 1% AA in MeOH | TosoHaas TSK-GEL Amide-80 | 5 mM AF + 26.5 mM FA in ACN: H2O, 70:30 | LC-MS/MS | 6 | 20 µg/100g tissue | 1–10,000 ng/mL | [136] |
| Urine and blood | 0.5 M AA, C18 SPE | Zorax 300SB-C3 | 1% ACN + 10 mM TMA + 10 mM AF, pH 4 | LC-MS | 6 | LOD: 15.6nM | 93.75–9375 nM | [106] |
| Std mixture | Not used | TSKgel Amide-80 | 16 mM AF, pH 5.5 + ACN (3:7, v/v) | LC-MS/MS | 6, 7, 14, 22 | NR | 64 pg–2 ng 64 pg–2 ng 128 pg–1 ng 180 pg–1.4 ng | [128] |
| Serum | 0.5 M AA in MeOH, Oasis MCX SPE | Cosmosil HILIC 4.6 × 150 mm | 0.1% FA in water + MeOH | LC-MS/MS (M. Horie et al., 2002) | 6, 7, 16 | LOD: 0.1 ng/mL LOQ: 1 ng/mL | 1–100 ng/mL | [13,100] |
| Urine and serum | Urine Extraction: C18 Sep-Pak SPE (0.2 M HCl in 20% MeOH) followed by Strata X-C 33 µm Cation Mixed-Mode Polymer SPE (0.1 M HCl+MeCN+MeOH+Water) Serum Extraction: Oasis MCX SPE (0.2 M HCl in 20% MeOH +MeCN+MeOH+Water) | Nova-Pak C18 4 µm, 8 × 100 mm | PIC B7 (Heptane sulfonic acid), 5 mM + 3% MeCN, pH 4.5 (adjusted with conc. NH3) | LC-FLD | 6 | LOD: 20 ng/mL (Urine) 5 ng/mL (Serum) LOQ: 20 ng/mL (Urine) 5 ng/mL (Serum) | 20–300 ng/mL (Urine) 5–20 ng/mL (Serum) | [100] |
| Matrix | Extraction Method | % Recovery | Reference |
|---|---|---|---|
| Trumpet shell | ASE (Accelerated solvent extraction) and SE (Solvent Extraction) (0.03 M AA) (UPLC–MS/MS) | 80–92 | [48] |
| Gastropod tissue | 1% AA in MeOH, C18-SPE, ultrafiltration (<3000 MW), (HPLC-FLD) | 90 | [13] |
| Xanthid crab, Demania cultripes | 1%AA in MeOH, C18-SPE, ultrafiltration (<3000 MW), (LC-MS) | 86.3 ± 2.9 | [139] |
| Puffer fish ovary | 0.05M AA, ODS-SPE, ultrafiltration (0.22 µ), (LC-MS) | 94.2–9108.3 | [127] |
| Puffer fish tissues, Muscle, Skin and Liver | 2% AA, methacrylate-styrene divinyl benzene cartridge (LC-MS) C18 column (50 mm × 2.1 mm i.d.) using 10 mmol/L IPCC-MS7-methanol (65:35) as the mobile phase at a flow rate of 0.2 mL/min | Muscle 79–83 Skin 85–88 Liver 85–90 (LOD 0.01 µg/g tissue) | [244] |
| Puffer fish eggs and newt | 0.1% AA, Cosmosil 75C18-OPN resin-SPE, CHCl3 wash, (LC-MS) | >90 | [175] |
| Newt (Whole body) | 0.1% AA in 70% MeOH, charcoal adsorption, (HPLC-FLD) | 50 | [35] |
| Blood serum | 0.5 M AA and Oasis MCX-SPE, ultrafiltration (<3000 MW) (LC-MS/MS) | >95 | [13] |
| Whole Blood | 1% AA in MeOH, PCX-SPE, (LC-MS/MS) | TTX 61.4Voglibose 62.8 | [104] |
| Human urine and plasma | C-18 and HILIC SPE (LC-MS/MS) | 75–81 | [135] |
| Human urine and blood | 0.5 M AA, C18 SPE, ultrafiltration (<3000 MW), (LC-MS) | Urine 90.9 ± 1.4 Blood 90.6 ± 0.2 | [106] |
| Human urine and blood | 2% AA, methacrylate-styrene divinyl benzene cartridge (LC-MS) C18 column (50 mm × 2.1 mm i.d.) using 10 mmol/L IPCC-MS7-methanol (65:35) as the mobile phase at a flow rate of 0.2 mL/min | Human serum 93–96 (0.1 ng/mL) Human urine 93-101 (0.1 ng/mL) | [244] |
| Combined muscle, liver and ovary from tiger puffers and muscle and ovary from balloon fishes | 1% AA in MeOH, defatted with chloroform (HPLC-FLD) | 91.0 ± 5.2 | [43] |
| Puffer fish muscle, liver and phosphate buffered saline | 1% AA in MeOH, defatted with chloroform (HPLC-FLD) | 86.4 ± 18.9 | [136] |
11.3.2. Development in Chromatography
11.3.3. Development in Mass Spectrometry
11.3.4. Quantitative TTX Analysis
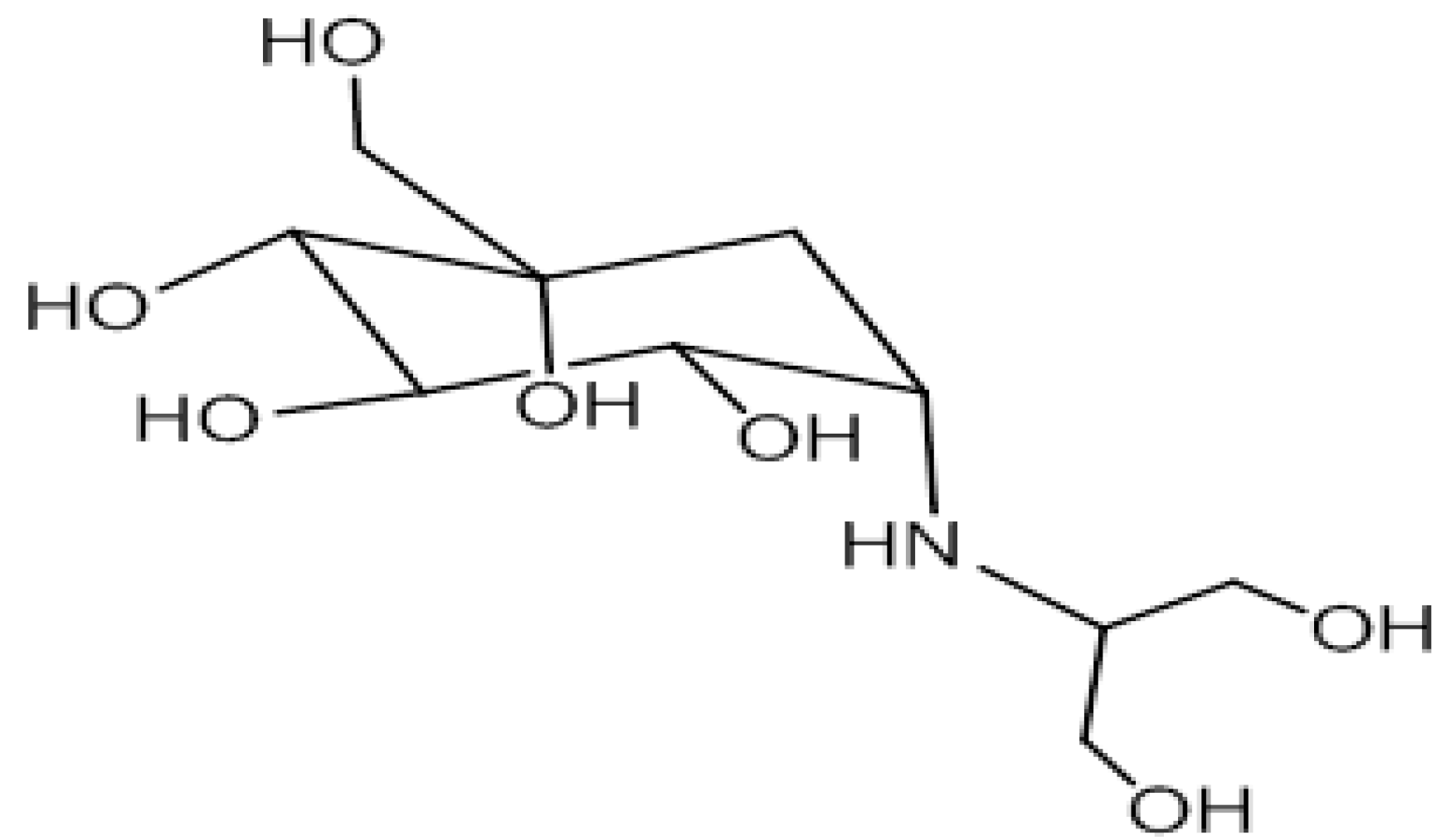
11.3.5. Matrix Effect in Puffer Fish, Trumpet Shell and Human Urine/Blood Sample
12. Measures to Ensure Human Safety (Legislation)
| Description | Value | Reference |
|---|---|---|
| Human median lethal dose | 8.7 µg/kg | [127] |
| MLD for mammals (IP or IV) | 2.7–10 µg/kg for rats 4.5 µg/kg for guinea pigs 8–10 µg/kg for mice, rabbits, dogs and cats | [97] |
| Lethal potency | 5,000–6,000 MU/mg | [46] |
| MLD for human | 10,000 MU (≈2 mg) | [46] |
| Regulatory limit in food in US | Zero | [247] |
| LD50 of TTX in mice | 9 µg/kg | [19] |
| LD99 of 5,6,11-trideoxy TTX | 750 µg/kg | [124] |
| IC50 for nine human functional voltage-gated Na+ channels | ≥1 μM | [97] |
| MLD of TTX to humans | 2 mg/50 kg BW | [131] |
| Minimum acute dose of TTX to humans | 0.2 mg/50 kg BW | [131] |
| Acceptability limit in puffer fish as food in Japan | 10 MU TTX eq/g or 2.2 µg TTX eq/g of puffer flesh | [131] |
| LD50 of TTX in mice, dogs and rabbits | 8–14 µg/kg by injection | [76] |
| Lethal dose | 2 mg | [25] |
| LD50 of TTX | 10 µg/kg (IP in mice) | [129] |
| LD50 of 11-deoxy TTX | 70 µg/kg (IP in mice) | [31] |
| IC50 for 6,11-dideoxy TTX | 420 µg/kg (IP in mice) | [125] |
| Regulatory limit in food in Japan | 2000 µg/kg TTX eq | [246] |
| Lethal doses in KM mice | LD1: 9.4 µg/kg LD50: 11.3 µg/kg LD99: 13.5 µg/kg | [130] |
13. Conclusions
Acronyms
| 4-AP | 4-aminopyridine |
| AA | Acetic acid |
| ACN | Acetonitrile |
| AF | Ammonium formate |
| AHB | Ammonium heptafluoro butyric acid |
| Am-acetate | Ammonium acetate |
| Am-OH | Ammonium hydroxide |
| ASE | Accelerated solvent extraction |
| BW | Body weight |
| CharAd † | Charcoal adsorption |
| CID | Collision induced dissociation |
| DCM | Dichloromethane |
| Defat * | Defatting |
| ELISA | Enzyme linked immunosorbent assay |
| Eq | Equivalent |
| EtOH | Ethanol |
| FA | Formic acid |
| GC-MS | Gas chromatography- Mass spectrometry |
| GTX | Gonyautoxin |
| HCD | High collision induced dissociation |
| HFB | Heptafluoro butyric acid |
| HLB-HILIC | Hydrophilic-lipophilic balance- HILIC |
| HILIC | Hydrophilic interaction liquid chromatography |
| HPLC | High performance liquid chromatography |
| HAS | Heptane sulfonic acid |
| IC50 | Half maximal inhibitory concentration is a measure of the effectiveness of a compound in inhibiting biological or biochemical function |
| IP | Intraperitoneal |
| IR | Infrared resonance |
| IV | Intravenous |
| LC-FLD | Liquid chromatography-fluorescent detection |
| LC-MS | Liquid chromatography-Mass spectrometry |
| LC-PDA | Liquid chromatography-Photo diode array detection |
| LC-UV | Liquid chromatography-Ultra violet detection |
| LD50 | Lethal dose50 of a toxin is the dose required to kill 50% of the members of a tested population after a specified test duration. |
| LD99 | Lethal dose99 of a toxin is the dose required to kill 99% of the members of a tested population after a specified test duration. |
| LOD | Limit of detection |
| LOQ | Limit of quantitation |
| MAX-HILIC | Mixed-mode anion exchange- HILIC |
| MCX-HILIC | Mixed-mode cation exchange- HILIC |
| MeCN | Methyl cyanide |
| MeOH | Methanol |
| MLD | Median lethal dose |
| MRM | Multiple reaction monitoring |
| MU | Mouse unit; 1 MU is defined as the amount of toxin required to kill a 20 g ICR (Institute of Cancer Research) strain mouse in 30 min after intraperitoneal injection [81] |
| MW | Molecular weight |
| m/z | mass/charge ratio |
| NMR | Nuclear magnetic resonance |
| PSP | Paralytic shell fish poisoning |
| Q-TOF MS | Quadrupole-time-of-flight mass spectrometry |
| SE | solvent extraction |
| SIR | Selected ion recording |
| S/N | Signal/Noise ratio |
| SPE | Solid phase extraction |
| STX | Saxitoxin |
| TBA-PO4 | Tetrabutyl ammonium phosphate |
| TLC | Thin layer chromatography |
| TMA | Trimethyl amine |
| Tris-Ac | Tris- acetate buffer |
| TTX | Tetrodotoxin |
| UPLC | Ultra performance liquid chromatography |
| ZIC-HILIC | Zwitter ionic hydrophilic interaction liquid chromatography |
Acknowledgments
Conflicts of Interest
References
- Kan, S.K.; Chan, M.K.; David, P. Nine fatal cases of puffer fish poisoning in Sabah, Malaysia. Med. J. Malays. 1987, 42, 199–200. [Google Scholar]
- U.S. Department of Health and Human Services. Tetrodotoxin Poisoning Associated with Eating Puffer Fish Transported from Japan—California, 1996; Morbidity and Mortality Weekly Report; Centre For Disease Control and Prevention: Atlanta, GA, USA, 1996; Volume 45, pp. 389–412.
- Mahmud, Y.; Tanu, M.B.; Noguchi, T. First occurrence of a food poisoning incident due to ingestion of Takifugu oblongus, along with a toxicological report on three marine puffer species in Bangladesh. J. Food Hyg. Soc. Jpn. 1999, 40, 473–480. [Google Scholar] [CrossRef]
- Hwang, D.F.; Shiu, Y.C.; Hwang, P.A.; Lu, Y.H. Tetrodotoxin in gastropods (snails) implicated in food poisoning in Northern Taiwan. J. Food Prot. 2002, 65, 1341–1344. [Google Scholar]
- Sui, L.M.; Chen, K.; Hwang, P.A.; Hwang, D.F. Identification of tetrodotoxin in marine gastropods implicated in food poisoning. J. Nat. Toxins 2002, 11, 213–220. [Google Scholar]
- How, C.-K.; Chern, C.-H.; Huang, Y.-C.; Wang, L.-M.; Lee, C.-H. Tetrodotoxin poisoning. Am. J. Emerg. Med. 2003, 21, 51–54. [Google Scholar] [CrossRef]
- Hwang, P.-A.; Tsai, Y.-H.; Lu, Y.-H.; Hwang, D.-F. Paralytic toxins in three new gastropod (Olividae) species implicated in food poisoning in southern Taiwan. Toxicon 2003, 41, 529–533. [Google Scholar] [CrossRef]
- Shiu, Y.-C.; Lu, Y.-H.; Tsai, Y.-H.; Chen, S.-K.; Hwang, D.-F. Occurrence of Tetrodotoxin in the Causative Gastropod Polinices didyma and another Gastropod Natica lineata collected from western Taiwan. J. Food Drug Anal. 2003, 11, 159–163. [Google Scholar]
- Ahasan, H.A.M.N.; Mamun, A.A.; Karim, S.R.; Bakar, M.A.; Gazi, E.A.; Bala, C.S. Paralytic complications of puffer fish (tetrodotoxin) poisoning. Singap. Med. J. 2004, 45, 73–74. [Google Scholar]
- Hwang, P.A.; Tsai, Y.H.; Deng, J.F.; Cheng, C.A.; Ho, P.H.; Hwang, D.F. Identification of tetrodotoxin in a marine gastropod (Nassarius glans) responsible for human morbidity and mortality in Taiwan. J. Food Prot. 2005, 68, 1696–1701. [Google Scholar]
- Ahmed, S. Puffer fish tragedy in Bangladesh: An incident of Takifugu oblongus poisoning in Degholia, Khulna. Afr. J. Mar. Sci. 2006, 28, 457–458. [Google Scholar] [CrossRef]
- Jen, H.C.; Lin, S.J.; Lin, S.Y.; Huang, Y.W.; Liao, I.C.; Arakawa, O.; Hwang, D.F. Occurrence of tetrodotoxin and paralytic shellfish poisons in a gastropod implicated in food poisoning in southern Taiwan. Food Addit. Contam. 2007, 8, 902–909. [Google Scholar]
- Jen, H.-C.; Lin, S.-J.; Tsai, Y.-H.; Chen, C.-H.; Lin, Z.-C.; Hwang, D.-F. Tetrodotoxin poisoning evidenced by solid-phase extraction combining with liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2008, 871, 95–100. [Google Scholar] [CrossRef]
- Noguchi, T.; Arakawa, O.; Takatani, T. TTX accumulation in pufferfish—Review. Comp. Biochem. Physiol. D 2006, 1, 145–152. [Google Scholar]
- Saoudi, M.; Rabeh, F.B.; Jammoussi, K.; Abdelmouleh, A.; Belbahri, L.; Feki, A.E. Biochemical and physiological responses in Wistar rat after administration of puffer fish (Lagocephalus lagocephalus) flesh. J. Food Agric. Environ. 2007, 5, 107–111. [Google Scholar]
- Machida, S. Food poisoning due to ingestion of “ivory shell” Babylonia japonica. J. Food Hyg. Soc. Jpn. 1965, 6, 87–89. [Google Scholar]
- Behera, A.; Dash, B.K.; Barik, B.K. Rare puffer fish poisoning—A case report. Medico-Leg. Update 2008, 8, 5–6. [Google Scholar]
- Homaira, N.; Rahman, M.; Luby, S.P.; Rahman, M.; Haider, M.S.; Faruque, L.I.; Khan, D.; Parveen, S.; Gurley, E.S. Multiple outbreaks of puffer fish intoxication in Bangladesh, 2008. Am. J. Trop. Med. Hyg. 2010, 83, 440–444. [Google Scholar] [CrossRef]
- Islam, Q.T.; Razzak, M.A.; Islam, M.A.; Bari, M.I.; Basher, A.; Chowdhury, F.R.; Sayeduzzaman, A.B.M.; Ahasan, H.A.M.N.; Faiz, M.A.; Arakawa, O.; et al. Puffer fish poisoning in Bangladesh: Clinical and toxicological results from large outbreaks in 2008. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 74–80. [Google Scholar] [CrossRef]
- Suehiro, M. Historical review on chemical and medical studies of globefish toxin before World War II. Jpn. Soc. Hist. Pharm. 1994, 29, 428–434. [Google Scholar]
- Do, H.K.; Kogure, K.; Simidu, U. Identification of deep-sea-sediment bacteria which produce tetrodotoxin. Appl. Environ. Microbiol. 1990, 56, 1162–1163. [Google Scholar]
- Asakawa, M.; Toyoshima, T.; Shida, Y.; Noguchi, T.; Miyazawa, K. Paralytic toxins in a ribbon worm Cephalothrix species (Nemertean) adherent to cultured oysters in Hiroshima Bay, Hiroshima Prefecture, Japan. Toxicon 2000, 38, 763–773. [Google Scholar] [CrossRef]
- Mahmud, Y.; Okada, K.; Takatani, T.; Kawatsu, K.; Hamano, Y.; Arakawa, O.; Noguchi, T. Intra-tissue distribution of tetrodotoxin in two marine puffers Takifugu vermicularis and Chelonodon patoca. Toxicon 2003, 41, 13–18. [Google Scholar] [CrossRef]
- Jang, J.; Yotsu-Yamashita, M. Distribution of tetrodotoxin, saxitoxin, and their analogs among tissues of the puffer fish Fugu pardalis. Toxicon 2006, 48, 980–987. [Google Scholar] [CrossRef]
- Huang, H.-N.; Lin, J.; Lin, H.-L. Identification and quantification of tetrodotoxin in the marine gastropod Nassarius by LC-MS. Toxicon 2008, 51, 774–779. [Google Scholar] [CrossRef]
- Jang, J.-H.; Lee, J.-S.; Yotsu-Yamashita, M. LC/MS analysis of tetrodotoxin and its deoxy analogs in the marine puffer fish Fugu niphobles from the southern coast of Korea, and in the brackishwater puffer fishes Tetraodon nigroviridis and Tetraodon biocellatus from Southeast Asia. Mar. Drugs 2010, 8, 1049–1058. [Google Scholar] [CrossRef]
- Chulanetra, M.; Sookrung, N.; Srimanote, P.; Indrawattana, N.; Thanongsaksrikul, J.; Sakolvaree, Y.; Chongsa-Nguan, M.; Kurazono, H.; Chaicumpa, W. Toxic marine puffer fish in Thailand Seas and tetrodotoxin they contained. Toxins 2011, 3, 1249–1262. [Google Scholar] [CrossRef]
- Luo, X.; Yu, R.C.; Wang, X.J.; Zhou, M.J. Toxin composition and toxicity dynamics of marine gastropod Nassarius spp. collected from Lianyungang, China. Food Addit. Contam. A 2012, 29, 117–127. [Google Scholar] [CrossRef]
- Rodríguez, P.; Alfonso, A.; Otero, P.; Katikou, P.; Georgantelis, D.; Botana, L.M. Liquid chromatography-mass spectrometry method to detect Tetrodotoxin and Its analogues in the puffer fish Lagocephalus sceleratus (Gmelin, 1789) from European waters. Food Chem. 2012, 132, 1103–1111. [Google Scholar] [CrossRef]
- Kim, Y.H.; Brown, G.H.; Mosher, H.S.; Fuhrman, F.A. Tetrodotoxin: Occurrence in Atelopid frogs of Costa Rica. Science 1975, 189, 151–152. [Google Scholar]
- Yasumoto, T.; Yotsu, M.; Murate, M.; Naoki, H. New tetrodotoxin analogues from the newt Cynops ensicauda. J. Am. Chem. Soc. 1988, 110, 2344–2345. [Google Scholar] [CrossRef]
- Yotsu, M.; Iorizzi, M.; Yasumoto, T. Distribution of tetrodotoxin, 6-epitetrodotoxin, and 11-deoxytetrodotoxin in newts. Toxicon 1990, 28, 238–241. [Google Scholar] [CrossRef]
- Pires, O.R., Jr.; Sebben, A.; Schwartz, E.F.; Largura, S.W.R.; Bloch, C., Jr.; Morales, R.A.V.; Schwartz, C.A. Occurrence of tetrodotoxin and its analogues in the Brazilian frog Brachycephalus ephippium (Anura: Brachycephalidae). Toxicon 2002, 40, 761–766. [Google Scholar] [CrossRef]
- Pires, O.R., Jr.; Sebben, A.; Schwartz, E.F.; Bloch, C., Jr.; Morales, R.A.V.; Schwartz, C.A. The occurrence of 11-oxotetrodotoxin, a rare tetrodotoxin analogue, in the brachycephalidae frog Brachycephalus ephippium. Toxicon 2003, 42, 563–566. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Mebs, D.; Kwet, A.; Schneider, M. Tetrodotoxin and its analogue 6-epitetrodotoxin in newts (Triturus spp.; Urodela, Salamandridae) from southern Germany. Toxicon 2007, 50, 306–309. [Google Scholar] [CrossRef]
- Mebs, D.; Yotsu-Yamashita, M. Tetrodotoxin in North-American newts. Toxicon 2012, 60, 1–120. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Gilhen, J.; Russell, R.W.; Krysko, K.L.; Melaun, C.; Kurz, A.; Kauferstein, S.; Kordis, D.; Mebs, D. Variability of tetrodotoxin and of its analogues in the red-spotted newt, Notophthalmus viridescens (Amphibia: Urodela: Salamandridae). Toxicon 2012, 59, 257–264. [Google Scholar] [CrossRef]
- Noguchi, T.; Ebesu, J.S.M. Puffer poisoning: Epidemiology and treatment. Toxin Rev. 2001, 20, 1–10. [Google Scholar] [CrossRef]
- Saoudi, M.; Abdelmouleh, A.; El Feki, A. Tetrodotoxin: A potent marine toxin. Toxin Rev. 2010, 29, 60–70. [Google Scholar] [CrossRef]
- Narita, H.; Noguchi, T.; Maruyama, J.; Ueda, Y.; Hashlmoto, K.; Watanade, Y.; Hida, K. Occurrence of tetrodotoxin in a trumpet shell, “boshubora” Charonia sauliae. Bull. Jpn. Soc. Sci. Fish 1981, 47, 935–941. [Google Scholar] [CrossRef]
- Hwang, D.F.; Lin, L.C.; Jeng, S.S. Variation and secretion of toxins in gastropod mollusc Niotha clathrata. Toxicon 1992, 30, 1189–1194. [Google Scholar] [CrossRef]
- Hwang, D.F.; Cheng, C.A.; Tsai, H.T.; Shih, D.Y.C.; Ko, H.C.; Yang, R.Z.; Jeng, S.S. Identification of tetrodotoxin and paralytic shellfish toxins in marine gastropods implicated in food poisoning. Fish. Sci. 1995, 61, 675–679. [Google Scholar]
- Chen, C.-Y.; Chou, H.-N. Detection of tetrodotoxin by high performance liquid chromatography in lined-moon shell and puffer fish. Acta Zool. Taiwan 1998, 9, 41–48. [Google Scholar]
- Cassiday, L. First report of TTX in a European trumpet shell. Anal. Chem. 2008, 80. [Google Scholar] [CrossRef]
- Rodriguez, P.; Alfonso, A.; Vale, C.; Alfonso, C.; Vale, P.; Tellez, A.; Botana, L.M. First toxicity report of tetrodotoxin and 5,6,11-TrideoxyTTX in the trumpet shell Charonia lampas lampas in Europe. Anal. Chem. 2008, 80, 5622–5629. [Google Scholar] [CrossRef]
- Noguchi, T.; Onuki, K.; Arakawa, O. Tetrodotoxin poisoning due to pufferfish and gastropods, and their intoxication mechanism. ISRN Toxicol. 2011, 2011, 1–10. [Google Scholar] [CrossRef]
- Silva, M.; Azevedo, J.; Rodriguez, P.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. New gastropod vectors and tetrodotoxin potential expansion in temperate waters of the Atlantic Ocean. Mar. Drugs 2012, 10, 712–726. [Google Scholar] [CrossRef]
- Nzoughet, J.K.; Campbell, K.; Barnes, P.; Cooper, K.M.; Chevallier, O.P.; Elliott, C.T. Comparison of sample preparation methods, validation of an UPLC-MS/MS procedure for the quantification of tetrodotoxin present in marine gastropods and analysis of pufferfish. Food Chem. 2013, 136, 1584–1589. [Google Scholar] [CrossRef]
- Kotaki, Y.; Shimizu, Y. 1-Hydroxy-5,11-dideoxytetrodotoxin, the first N-hydroxy and ring-deoxy derivative of tetrodotoxin found in the newt Taricha granulosa. J. Am. Chem. Soc. 1993, 115, 827–830. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Mebs, D. The levels of tetrodotoxin and its analogue 6-epitetrodotoxin in the red-spotted newt, Notophthalmus viridescens. Toxicon 2001, 39, 1261–1263. [Google Scholar] [CrossRef]
- Hanifin, C.T.; BrodieIII, E.D.; Brodie, E.D., Jr. Tetrodotoxin levels of the rough-skin newt, Taricha granulosa, increase in long-term captivity. Toxicon 2002, 40, 1149–1153. [Google Scholar] [CrossRef]
- Tsuruda, K.; Arakawa, O.; Kawatsu, K.; Hamano, Y.; Takatani, T.; Noguchi, T. Secretory glands of tetrodotoxin in the skin of the Japanese newt Cynops pyrrhogaster. Toxicon 2002, 40, 131–136. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Mebs, D. Occurrence of 11-oxotetrodotoxin in the red-spotted newt, Notophthalmus viridescens, and further studies on the levels of tetrodotoxin and its analogues in the newt’s efts. Toxicon 2003, 41, 893–897. [Google Scholar] [CrossRef]
- Cardall, B.L.; Brodie, E.D., Jr.; Brodie, E.D., III; Hanifin, C.T. Secretion and regeneration of tetrodotoxin in the rough-skin newt (Taricha granulosa). Toxicon 2004, 44, 933–938. [Google Scholar] [CrossRef]
- Zimmer, R.K.; Schar, D.W.; Ferrer, R.P.; Krug, P.J.; Kats, L.B.; Michel, W.C. The scent of danger: Tetrodotoxin (TTS) as an olfactory cue of predation risk. Ecol. Monogr. 2006, 76, 585–600. [Google Scholar] [CrossRef]
- Ferrer, R.P.; Zimmer, R.K. The scent of danger: Arginine as an olfactory cue of reduced predation risk. J. Exp. Biol. 2007, 210, 1768–1775. [Google Scholar] [CrossRef]
- Mebs, D.; Arakawa, O.; Yotsu-Yamashita, M. Tissue distribution of tetrodotoxin in the red-spotted newt Notophthalmus viridescens. Toxicon 2010, 55, 1353–1357. [Google Scholar] [CrossRef]
- Gall, B.G.; Stokes, A.N.; French, S.S.; Schlepphorst, E.A.; Brodie, E.D., 3rd.; Brodie, E.D., Jr. Tetrodotoxin levels in larval and metamorphosed newts (Taricha granulosa) and palatability to predatory dragonflies. Toxicon 2011, 57, 978–983. [Google Scholar] [CrossRef]
- Kudo, Y.; Yasumoto, T.; Konoki, K.; Cho, Y.; Yotsu-Yamashita, M. Isolation and structural determination of the first 8-epi-type tetrodotoxin analogs from the newt, Cynops ensicauda popei, and comparison of tetrodotoxin analogs profiles of this newt and the puffer fish, Fugu poecilonotus. Mar. Drugs 2012, 10, 655–667. [Google Scholar] [CrossRef]
- Mebs, D.; Yotsu-Yamashita, M.; Seitz, H.M.; Arakawa, O. Tetrodotoxin does not protect red-spotted newts, Notophthalmus viridescens, from intestinal parasites. Toxicon 2012, 60, 66–69. [Google Scholar] [CrossRef]
- Noguchi, T.; Jeon, J.K.; Arakawa, O.; Sugita, H.; Deguchi, Y.; Hashimoto, K. Occurrence of tetrodotoxin and anhydrotetrodotoxin in Vibrio sp. isolated from the intestines of xanthid crab Atergatis floridus. J. Biochem. 1986, 99, 311–314. [Google Scholar]
- Arakawa, O.; Noguchi, T.; Shida, Y.; Onoue, Y. Occurence of 11-oxotetrodotoxin and 11-nortetrodotoxin-6(R)-ol in a xanthid crab Atergatis floridus collected at Kojima, Ishigaki Island. Fish. Sci. 1994, 60, 769–771. [Google Scholar]
- Kanchanapongkul, J.; Krittayapoositpot, P. An epidemic of tetrodotoxin poisoning following ingestion of the horseshoe crab Carcinoscorpius rotundicauda. Southeast Asian J. Trop. Med. Public Health 1995, 26, 364–367. [Google Scholar]
- Hwang, D.F.; Tsai, Y.H. Toxicological study on the xanthid crab Zosimus aeneus in Taiwan. Toxicon 1997, 35. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Hwang, D.-F.; Chai, T.-J.; Jeng, S.-S. Toxicity and toxic components of two xanthid crabs, Atergatis floridus and Demania reynaudi, in Taiwan. Toxicon 1997, 35, 1327–1335. [Google Scholar] [CrossRef]
- Llewellyn, L.E.; Dodd, M.J.; Robertson, A.; Ericson, G.; de Koning, C.; Negri, A.P. Post-mortem analysis of samples from a human victim of a fatal poisoning caused by the xanthid crab, Zosimus aeneus. Toxicon 2002, 40, 1463–1469. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Ho, P.-H.; Jeng, S.-S.; Hwang, D.-F. Paralytic toxins in Taiwanese crab Xanthias lividus. Fish. Sci. 2002, 68, 659–661. [Google Scholar]
- Jester, R.; Rhodes, L.; Beuzenberg, V. Uptake of paralytic shellfish poisoning and spirolide toxins by paddle crabs (Ovalipes catharus) via a bivalve vector. Harmful Algae 2009, 8, 369–376. [Google Scholar] [CrossRef]
- Lin, H.; Nagashima, Y.; Jiang, P.; Qin, X.; Lu, Y.; Zhang, C. Screening for toxicity and resistance to paralytic shellfish toxin of shore crabs inhabiting at Leizhou peninsula, China. Mar. Environ. Res. 2012, 78, 48–52. [Google Scholar] [CrossRef]
- Yang, L.; Kao, C.Y. Actions of chiriquitoxin on frog skeletal muscle fibers and implications for the tetrodotoxin/saxitoxin receptor. J. Gen. Physiol. 1992, 100, 609–622. [Google Scholar] [CrossRef]
- Tanu, M.B.; Mahmud, Y.; Tsuruda, K.; Arakawa, O.; Noguchi, T. Occurrence of tetrodotoxin in the skin of a rhacophoridid frog Polypedates sp. from Bangladesh. Toxicon 2001, 39, 937–941. [Google Scholar] [CrossRef]
- Pires, O.R., Jr.; Sebben, A.; Schwartz, E.F.; Morales, R.A.V.; Bloch, C., Jr.; Schwartz, C.A. Further report of the occurrence of tetrodotoxin and new analogues in the Anuran family Brachycephalidae. Toxicon 2005, 45, 73–79. [Google Scholar] [CrossRef]
- McNabb, P.; Selwood, A.I.; Munday, R.; Wood, S.A.; Taylor, D.I.; MacKenzie, L.A.; van Ginkel, R.; Rhodes, L.L.; Cornelisen, C.; Heasman, K.; et al. Detection of tetrodotoxin from the grey side-gilled sea slug—Pleurobranchaea maculata, and associated dog neurotoxicosis on beaches adjacent to the Hauraki Gulf, Auckland, New Zealand. Toxicon 2010, 56, 466–473. [Google Scholar] [CrossRef]
- Wood, S.; Casas, M.; Taylor, D.; McNabb, P.; Salvitti, L.; Ogilvie, S.; Cary, S.C. Depuration of tetrodotoxin and changes in bacterial communities in Pleurobranchea maculata adults and egg masses maintained in captivity. J. Chem. Ecol. 2012, 38, 1342–1350. [Google Scholar] [CrossRef]
- Lin, S.-J.; Hwang, D.-F. Possible source of tetrodotoxin in the starfish Astropecten scoparius. Toxicon 2001, 39, 573–579. [Google Scholar] [CrossRef]
- Williams, B.L.; Caldwell, R.L. Intra-organismal distribution of tetrodotoxin in two species of blue-ringed octopuses (Hapalochlaena fasciata and H. lunulata). Toxicon 2009, 54, 345–353. [Google Scholar] [CrossRef]
- Williams, B.; Hanifin, C.; Brodie, E.; Caldwell, R. Ontogeny of tetrodotoxin levels in blue-ringed octopuses: Maternal investment and apparent independent production in offspring of Hapalochlaena lunulata. J. Chem. Ecol. 2011, 37, 10–17. [Google Scholar]
- Williams, B.L.; Stark, M.R.; Caldwell, R.L. Microdistribution of tetrodotoxin in two species of blue-ringed octopuses (Hapalochlaena lunulata and Hapalochlaena fasciata) detected by fluorescent immunolabeling. Toxicon 2012, 60, 1307–1313. [Google Scholar] [CrossRef]
- Ali, A.E.; Arakawa, O.; Noguchi, T.; Miyazawa, K.; Shida, Y.; Hashimoto, K. Tetrodotoxin and related substances in a ribbon worm Cephalothrix linearis (Nemertean). Toxicon 1990, 28, 1083–1093. [Google Scholar] [CrossRef]
- Cheng, C.A.; Hwang, D.F.; Tsai, Y.H.; Chen, H.C.; Jeng, S.S.; Noguchi, T.; Ohwada, K.; Hasimoto, K. Microflora and tetrodotoxin-producing bacteria in a gastropod, Niotha clathrata. Food Chem. Toxicol. 1995, 33, 929–934. [Google Scholar] [CrossRef]
- Yu, C.-F.; Yu, P.H.-F.; Chan, P.-L.; Yan, Q.; Wong, P.-K. Two novel species of tetrodotoxin-producing bacteria isolated from toxic marine puffer fishes. Toxicon 2004, 44, 641–647. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, Y.; Xie, L.; Xia, G.; Hu, J.; Wang, S.; Zhang, R. Toxicity and distribution of tetrodotoxin-producing bacteria in puffer fish Fugu rubripes collected from the Bohai Sea of China. Toxicon 2005, 46, 471–476. [Google Scholar]
- Yan, Q.; Yu, P.H.-F.; Li, H.-Z. Detection of tetrodotoxin and bacterial production by Serratia Marcescens. World J. Microbiol. Biotechnol. 2005, 21, 1255–1258. [Google Scholar] [CrossRef]
- Croci, L.; Cozzi, L.; Suffredini, E.; Ciccaglioni, G.; Toti, L.; Milandri, A.; Ceredi, A.; Benzi, M.; Poletti, R. Characterization of microalgae and associated bacteria collected from shellfish harvesting areas. Harmful Algae 2006, 5, 266–274. [Google Scholar] [CrossRef]
- Venmathi Maran, B.A.; Iwamoto, E.; Okuda, J.; Matsuda, S.; Taniyama, S.; Shida, Y.; Asakawa, M.; Ohtsuka, S.; Nakai, T.; Boxshall, G.A. Isolation and characterization of bacteria from the copepod Pseudocaligus fugu ectoparasitic on the panther puffer Takifugu pardalis with the emphasis on TTX. Toxicon 2007, 50, 779–790. [Google Scholar] [CrossRef]
- Wang, X.J.; Yu, R.C.; Luo, X.; Zhou, M.J.; Lin, X.T. Toxin-screening and identification of bacteria isolated from highly toxic marine gastropod Nassarius semiplicatus. Toxicon 2008, 52, 55–61. [Google Scholar] [CrossRef]
- Wang, J.; Fan, Y. Isolation and characterization of a Bacillus species capable of producing tetrodotoxin from the puffer fish Fugu obscurus. World J. Microbiol. Biotechnol. 2010, 26, 1755–1760. [Google Scholar] [CrossRef]
- Yang, G.; Xu, J.; Liang, S.; Ren, D.; Yan, X.; Bao, B. A novel TTX-producing Aeromonas isolated from the ovary of Takifugu obscurus. Toxicon 2010, 56, 324–329. [Google Scholar] [CrossRef]
- Kono, M.; Matsui, T.; Furukawa, K.; Yotsu-Yamashita, M.; Yamamori, K. Accumulation of tetrodotoxin and 4,9-anhydrotetrodotoxin in cultured juvenile kusafugu Fugu niphobles by dietary administration of natural toxic komonfugu Fugu poecilonotus liver. Toxicon 2008, 51, 1269–1273. [Google Scholar] [CrossRef]
- Noguchi, T.; Arakawa, O.; Takatani, T. Toxicity of pufferfish Takifugu rubripes cultured in netcages at sea or aquaria on land. Comp. Biochem. Phys. D 2006, 1, 153–157. [Google Scholar]
- Lee, M.-J.; Jeong, D.-Y.; Kim, W.-S.; Kim, H.-D.; Kim, C.-H.; Park, W.-W.; Park, Y.-H.; Kim, K.-S.; Kim, H.-M.; Kim, D.-S. A tetrodotoxin-producing Vibrio strain, LM-1, from the puffer fish Fugu vermicularis radiatus. Appl. Environ. Microbiol. 2000, 66, 1698–1701. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, L.; Xia, G.; Zhang, J.; Nie, Y.; Hu, J.; Wang, S.; Zhang, R. A new tetrodotoxin-producing actinomycete, Nocardiopsis dassonvillei, isolated from the ovaries of puffer fish Fugu rubripes. Toxicon 2005, 45, 851–859. [Google Scholar] [CrossRef]
- Noguchi, T.; Arakawa, O. Tetrodotoxin—Distribution and accumulation in aquatic organisms and cases of human intoxication. Mar. Drugs 2008, 6, 220–242. [Google Scholar] [CrossRef]
- Hwang, D.F.; Arakawa, O.; Saito, T.; Noguchi, T.; Simidu, U.; Tsukamoto, K.; Shida, Y.; Hashimoto, K. Tetrodotoxin-producing bacteria from the blue-ringed octopus Octopus maculosus. Mar. Biol. Heidelb. Ger. 1989, 100, 327–332. [Google Scholar] [CrossRef]
- Do, H.K.; Hamasaki, K.; Ohwada, K.; Simidu, U.; Noguchi, T.; Shida, Y.; Kogure, K. Presence of tetrodotoxin and tetrodotoxin-producing bacteria in freshwater sediments. Appl. Environ. Microbiol. 1993, 59, 3934–3937. [Google Scholar]
- Hasan, S.; Nikkon, F.; Pervin, F.; Rahman, M.M.; Khatun, S.; Hossain, T.; Khan, A.; Sarker, S.K.; Mosaddik, A.; Absar, N. Biochemical and histopathological effects of tetrodotoxin isolated from puffer fish Tetraodon patoca available in Bangladesh. Res. J. Med. Med. Sci. 2008, 3, 177–181. [Google Scholar]
- Zimmer, T. Effects of tetrodotoxin on the mammalian cardiovascular system. Mar. Drugs 2010, 8, 741–762. [Google Scholar] [CrossRef]
- Denac, H.; Mevissen, M.; Scholtysik, G. Structure, function and pharmacology of voltage-gated sodium channels. Pharmacol. 2000, 362, 453–479. [Google Scholar]
- Danovaro, R.; Fonda Umani, S.; Pusceddu, A. Climate change and the potential spreading of marine mucilage and microbial pathogens in the mediterranean sea. PLoS ONE 2009, 4, 1–8. [Google Scholar]
- O’Leary, M.A.; Schneider, J.J.; Isbister, G.K. Use of high performance liquid chromatography to measure tetrodotoxin in serum and urine of poisoned patients. Toxicon 2004, 44, 549–553. [Google Scholar] [CrossRef]
- Isbister, G.K.; Son, J.; Wang, F.; Maclean, C.J.; Lin, C.S.; Ujma, J.; Balit, C.R.; Smith, B.; Milder, D.G.; Kiernan, M.C. Puffer fish poisoning: A potentially life-threatening condition. Med. J. Aust. 2002, 177, 650–653. [Google Scholar]
- Chowdhury, F.R.; Nazmul Ahasan, H.A.; Mamunur Rashid, A.K.; Al Mamun, A.; Khaliduzzaman, S.M. Tetrodotoxin poisoning: A clinical analysis, role of neostigmine and short-term outcome of 53 cases. Singapore Med. J. 2007, 48, 830–833. [Google Scholar]
- Arakawa, O.; Hwang, D.-F.; Taniyama, S.; Takatani, T. Toxins of Pufferfish that Cause Human Intoxications. In Coastal Environmental and Ecosystem Issues of the East China Sea; Ishimatsu, A., Lie, H.-J., Eds.; Terrapub and Nagasaki University: Tokyo, Japan, 2010; pp. 227–244. [Google Scholar]
- Cho, H.E.; Ahn, S.Y.; Son, I.S.; In, S.; Hong, R.S.; Kim, D.W.; Woo, S.H.; Moon, D.C.; Kim, S. Determination and validation of tetrodotoxin in human whole blood using hydrophilic interaction liquid chromatography-tandem mass spectroscopy and its application. Forensic Sci. Int. 2012, 217, 76–80. [Google Scholar] [CrossRef]
- Bentur, Y.; Ashkar, J.; Lurie, Y.; Levy, Y.; Azzam, Z.S.; Litmanovich, M.; Golik, M.; Gurevych, B.; Golani, D.; Eisenman, A. Lessepsian migration and tetrodotoxin poisoning due to Lagocephalus sceleratus in the eastern Mediterranean. Toxicon 2008, 52, 964–968. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Hwang, D.F.; Cheng, C.A.; Hwang, C.C.; Deng, J.F. Determination of tetrodotoxin in human urine and blood using C18 cartridge column, ultrafiltration and LC-MS. J. Chromatogr. B 2006, 832, 75–80. [Google Scholar] [CrossRef]
- Hwang, D.-F.; Noguchi, T.; Steve, L.T. Tetrodotoxin Poisoning. In Advances in Food and Nutrition Research; Elsevier: Lincoln, NE, USA, 2007; Volume 52, pp. 141–236. [Google Scholar]
- Cohen, N.J.; Deeds, J.R.; Wong, E.S.; Hanner, R.H.; Yancy, H.F.; White, K.D.; Thompson, T.M.; Wahl, M.; Pham, T.D.; Guichard, F.M.; et al. Public health response to puffer fish (tetrodotoxin) poisoning from mislabeled product. J. Food Prot. 2009, 72, 810–817. [Google Scholar]
- Centres for disease control and prevention. Morbidity and Mortality Weekly Report; Centres for disease control and prevention: Atlanta, GA, USA, 17 May 1996.
- Sims, J.K.; Ostman, D.C. Pufferfish poisoning: Emergency diagnosis and management of mild human tetrodotoxication. Ann. Emerg. Med. 1986, 15, 1094–1098. [Google Scholar] [CrossRef]
- Chau, R.; Kalaitzis, J.A.; Neilan, B.A. On the origins and biosynthesis of tetrodotoxin. Aquat. Toxicol. 2011, 104, 61–72. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Sugimoto, A.; Takai, A.; Yasumoto, T. Effects of specific modifications of several hydroxyls of tetrodotoxin on its affinity to rat brain membrane. J. Pharmacol. Exp. Ther. 1999, 289, 1688–1696. [Google Scholar]
- Kishi, Y.; Aratani, M.; Fukuyama, T.; Nakatsubo, F.; Goto, T.; Inoue, S.; Tanino, H.; Sugiura, S.; Kakoi, H. Synthetic studies on tetrodotoxin and related compounds. III. Stereospecific synthesis of an equivalent of acetylated tetrodamine. J. Am. Chem. Soc. 1972, 94, 9217–9219. [Google Scholar] [CrossRef]
- Nishikawa, T.; Urabe, D.; Yoshida, K.; Iwabuchi, T.; Asai, M.; Isobe, M. Stereocontrolled synthesis of 8,11-dideoxytetrodotoxin, unnatural analogue of puffer fish toxin. Org. Lett. 2002, 4, 2679–2682. [Google Scholar] [CrossRef]
- Hinman, A.; Du Bois, J. A stereoselective synthesis of (-)-tetrodotoxin. J. Am. Chem. Soc. 2003, 125, 11510–11511. [Google Scholar] [CrossRef]
- Nishikawa, T.; Urabe, D.; Yoshida, K.; Iwabuchi, T.; Asai, M.; Isobe, M. Total syntheses of 11-deoxytetrodotoxin and 8,11-dideoxytetrodotoxin. Pure Appl. Chem. 2003, 75, 251–257. [Google Scholar] [CrossRef]
- Ohyabu, N.; Nishikawa, T.; Isobe, M. First asymmetric total synthesis of tetrodotoxin. J. Am. Chem. Soc. 2003, 125, 8798–8805. [Google Scholar] [CrossRef]
- Nishikawa, T.; Urabe, D.; Isobe, M. An efficient total synthesis of optically active tetrodotoxin. Angew. Chem. Int. Ed. 2004, 43, 4782–4785. [Google Scholar] [CrossRef]
- Sato, K.; Akai, S.; Shoji, H.; Sugita, N.; Yoshida, S.; Nagai, Y.; Suzuki, K.; Nakamura, Y.; Kajihara, Y.; Funabashi, M.; et al. Stereoselective and efficient total synthesis of optically active tetrodotoxin from d-Glucose. J. Org. Chem. 2008, 73, 1234–1242. [Google Scholar] [CrossRef]
- Adachi, M.; Imazu, T.; Isobe, M.; Nishikawa, T. An improved synthesis of (-)-5,11-dideoxytetrodotoxin. J. Org. Chem. 2013, 78, 1699–1705. [Google Scholar] [CrossRef]
- Raybould, T.J.; Bignami, G.S.; Inouye, L.K.; Simpson, S.B.; Byrnes, J.B.; Grothaus, P.G.; Vann, D.C. A monoclonal antibody-based immunoassay for detecting tetrodotoxin in biological samples. J. Clin. Lab. Anal. 1992, 6, 65–72. [Google Scholar] [CrossRef]
- Cheun, B.S.; Loughran, M.; Hayashi, T.; Nagashima, Y.; Watanabe, E. Use of a channel biosensor for the assay of paralytic shellfish toxins. Toxicon 1998, 36, 1371–1381. [Google Scholar] [CrossRef]
- Campbell, K.; Barnes, P.; Haughey, S.A.; Higgins, C.; Kawatsu, K.; Vasconcelos, V.; Elliott, C.T. Development and single laboratory validation of an optical biosensor assay for tetrodotoxin detection as a tool to combat emerging risks in European seafood. Anal. Bioanal. Chem. 2013, 405, 7753–7763. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Yamagishi, Y.; Yasumoto, T. 5,6,11-Trideoxytetrodotoxin from the puffer fish, Fugu poecilonotus. Tetrahedron Lett. 1995, 36, 9329–9332. [Google Scholar] [CrossRef]
- Jang, J.-H.; Yotsu-Yamashita, M. 6,11-Dideoxytetrodotoxin from the puffer fish, Fugu pardalis. Toxicon 2007, 50, 947–951. [Google Scholar] [CrossRef]
- Malik, A.K.; Blasco, C.; Picó, Y. Liquid chromatography-mass spectrometry in food safety. J. Chromatogr. A 2010, 1217, 4018–4040. [Google Scholar] [CrossRef]
- Chen, X.-W.; Liu, H.-X.; Jin, Y.-B.; Li, S.-F.; Bi, X.; Chung, S.; Zhang, S.-S.; Jiang, Y.-Y. Separation, identification and quantification of tetrodotoxin and its analogs by LC-MS without calibration of individual analogs. Toxicon 2011, 57, 938–943. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Jang, J.-H.; Cho, Y.; Konoki, K. Optimization of simultaneous analysis of tetrodotoxin, 4-epitetrodotoxin, 4,9-anhydrotetrodotoxin, and 5,6,11-trideoxytetrodotoxin by hydrophilic interaction liquid chromatography-tandem mass spectrometry. Forensic Toxicol. 2011, 29, 61–64. [Google Scholar] [CrossRef]
- Kao, C.Y.; Furman, F.A. Pharmacological studies on tetrodotoxin, a potent neurotoxin. J. Pharmacol. Exp. Ther. 1963, 140, 31–40. [Google Scholar]
- Xu, Q.-H.; Zhao, X.-N.; Wei, C.-H.; Rong, K.-T. Immunologic protection of anti-tetrodotoxin vaccines against lethal activities of oral tetrodotoxin challenge in mice. Int. Immunopharmacol. 2005, 5, 1213–1224. [Google Scholar] [CrossRef]
- Katikou, P.; Georgantelis, D.; Sinouris, N.; Petsi, A.; Fotaras, T. First report on toxicity assessment of the Lessepsian migrant pufferfish Lagocephalus sceleratus (Gmelin, 1789) from European waters (Aegean Sea, Greece). Toxicon 2009, 54, 50–55. [Google Scholar] [CrossRef]
- Guzmán, A.; de Henestrosa, A.R.F.; Marín, A.-P.; Ho, A.; Borroto, J.I.G.; Carasa, I.; Pritchard, L. Evaluation of the genotoxic potential of the natural neurotoxin Tetrodotoxin (TTX) in a battery of in vitro and in vivo genotoxicity assays. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2007, 634, 14–24. [Google Scholar] [CrossRef]
- Woodward, R.B. The structure of tetrodotoxin. Pure Appl. Chem. 1964, 9, 49–74. [Google Scholar] [CrossRef]
- Wang, J.; Araki, T.; Tatsuno, R.; Nina, S.; Ikeda, K.; Hamasaki, M.; Sakakura, Y.; Takatani, T.; Arakawa, O. Transfer profile of intramuscularly administered tetrodotoxin to artificial hybrid specimens of pufferfish, Takifugu rubripes and Takifugu niphobles. Toxicon 2011, 58, 565–569. [Google Scholar] [CrossRef]
- Fong, B.M.-W.; Tam, S.; Tsui, S.-H.; Leung, K.S.-Y. Development and validation of a high-throughput double solid phase extraction-liquid chromatography-tandem mass spectrometry method for the determination of tetrodotoxin in human urine and plasma. Talanta 2011, 83, 1030–1036. [Google Scholar] [CrossRef]
- Taylor, A.D.; Vaisocherová, H.; Deeds, J.; DeGrasse, S.; Jiang, S. Tetrodotoxin detection by a surface plasmon resonance sensor in pufferfish matrices and urine. J. Sens. 2011, 2011, 1–10. [Google Scholar]
- Man, C.N.; Noor, N.M.; Harn, G.L.; Lajis, R.; Mohamad, S. Screening of tetrodotoxin in puffers using gas chromatography-mass spectrometry. J. Chromatogr. A 2011, 1217, 7455–7459. [Google Scholar]
- Diener, M.; Christian, B.; Ahmed, M.; Luckas, B. Determination of tetrodotoxin and its analogs in the puffer fish Takifugu oblongus from Bangladesh by hydrophilic interaction chromatography and mass-spectrometric detection. Anal. Bioanal. Chem. 2007, 389, 1997–2002. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Ho, P.-H.; Hwang, C.-C.; Hwang, P.-A.; Cheng, C.-A.; Hwang, D.-F. Tetrodotoxin in several species of xanthid crabs in southern Taiwan. Food Chem. 2006, 95, 205–212. [Google Scholar] [CrossRef]
- Asakawa, M.; Toyoshima, T.; Ito, K.; Bessho, K.; Yamaguchi, C.; Tsunetsugu, S.; Shida, Y.; Kajihara, H.; Mawatari, S.F.; Noguchi, T.; et al. Paralytic toxicity in the ribbon worm Cephalothrix species (Nemertea) in Hiroshima Bay, Hiroshima Prefecture, Japan and the isolation of tetrodotoxin as a main component of its toxins. Toxicon 2003, 41, 747–753. [Google Scholar] [CrossRef]
- Asakawa, M.; Ito, K.; Kajihara, H. Highly toxic ribbon worm Cephalothrix simula containing tetrodotoxin in Hiroshima Bay, Hiroshima Prefecture, Japan. Toxins 2013, 5, 376–395. [Google Scholar] [CrossRef]
- Nakamura, M.; Yasumoto, T. Tetrodotoxin derivatives in puffer fish. Toxicon 1985, 23, 271–276. [Google Scholar] [CrossRef]
- Yotsu, M.; Yasumoto, T.; Kim, Y.H.; Naoki, H.; Kao, C.Y. The structure of chiriquitoxin from the Costa Rican frog Atelopus chiriquiensis. Tetrahedron Lett. 1990, 31, 3187–3190. [Google Scholar] [CrossRef]
- Hwang, D.F.; Noguchi, T.; Arakawa, O.; Abe, T.; Hashimoto, K. Toxicological studies on several species of puffer in Taiwan. Nippon Suisan Gakkaishi 1988, 54, 2001–2008. [Google Scholar]
- Goto, T.; Kishi, Y.; Takahashi, S.; Hirata, Y. Tetrodotoxin. Tetrahedron 1965, 21, 2059–2088. [Google Scholar] [CrossRef]
- Pavelka, L.A.; Fuhrman, F.A.; Mosher, H.S. 11- Nortetrodotoxin-6,6-diol and 11-nortetrodotoxin-6-ol. Heterocycles 1982, 17, 225–230. [Google Scholar] [CrossRef]
- Endoa, A.; Khora, S.S.; Murata, M.; Naoki, H.; Yasumoto, T. Isolation of 11-nortetrodotoxin-6(R)-OL and other tetrodotoxin derivatives from the puffer Fugu niphobles. Tetrahedron Lett. 1988, 29, 4127–4128. [Google Scholar] [CrossRef]
- Yotsu, M.; Hayashi, Y.; Khora, S.S.; Sato, S.i.; Yasumoto, T. Isolation and structural assignment of 11-nortetrodotoxin-6(S)-ol from the puffer Arothron nigropunctatus. Biosci. Biotechnol. Biochem. 1992, 56, 370–371. [Google Scholar] [CrossRef]
- Shoji, Y.; Yotsu-Yamashita, M.; Miyazawa, T.; Yasumoto, T. Electrospray ionization mass spectrometry of tetrodotoxin and its analogs: Liquid chromatography/mass spectrometry, tandem mass spectrometry, and liquid chromatography/tandem mass spectrometry. Anal. Biochem. 2001, 290, 10–17. [Google Scholar] [CrossRef]
- LATOXAN, France. Available online: http://www.latoxan.com/PHP/00000171.php (accessed on 14 February 2014).
- Acros Organics (BVBA), Belgium. Available online: http://wercs.acros.com/wercsdata/document.aspx?prd=ACR25647~~PDF~~MTR~~CLP1~~EN~~2010-12-05%2013:41:44~~Tetrodotoxin,%20citrate%20free (accessed on 14 February 2014).
- CMS Chemicals Ltd., UK. Available online: http://www.cms-chemicals.com/cms/website.nsf/%28allcategorycms%29/86C09EFA1D2EE78B802579FA00589A94 (accessed on 14 February 2014).
- Hangzhou Dayangchem Co., Zhejiang, China. Available online: http://dayangchem.guidechem.com/pro-show2145147.html (accessed on 14 February 2014).
- Acros Organics N.V., Belgium. Available online: https://fscimage.fishersci.com/msds/01139.htm (accessed on 14 February 2014).
- Sigma Aldrich Ltd. Available online: http://www.sigmaaldrich.com/catalog/product/sigma/t8024?lang=en®ion=IE (accessed on 14 February 2014).
- Biotium, USA. Available online: http://www.biotium.com/product/product_info/MSDS/MSDS%2000060.pdf (accessed on 14 February 2014).
- Alomone Labs. Available online: http://www.linscottsdirectory.com/products/alomone-labs/tetrodotoxin-citrate-free-14 (accessed on 14 February 2014).
- EMD Millipore (Calbiochem®), Germany. Available online: http://www.emdmillipore.com/life-science-research/tetrodotoxin-citrate-free-fugu-sp-/EMD_BIO-584415/p_bAqb.s1LILwAAAEWlWEfVhTm (accessed on 14 February 2014).
- Affix Scientific, USA. Available online: http://www.affixscientific.com/product/tetrodotoxin-citrate-fr/ (accessed on 14 February 2014).
- Abcam plc, UK. Available online: http://www.abcam.com/Tetrodotoxin-citrate-ab120055.html (accessed on 14 February 2014).
- Biorbyt Limited. Available online: http://www.biorbyt.com/tetrodotoxin-citrate-free (accessed on 14 February 2014).
- Carbosynth Limited, UK. Available online: http://www.carbosynth.com/carbosynth/website.nsf/%28w-productdisplay%29/86C09EFA1D2EE78B802579FA00589A94 (accessed on 14 February 2014).
- Biotium, G.S.-. USA. Available online: http://www.clonagen.com/clonagen/d10e987f-efe7-45f3-b1a9-2f9d3cb88753/tetrodotoxin_citrate_free_product.aspx (accessed on 14 February 2014).
- Yotsu-Yamashita, M.; Goto, A.; Nakagawa, T. Identification of 4-S-Cysteinyltetrodotoxin from the liver of the puffer fish, Fufu pardalis and formation of thiol adducts of tetrodotoxin from 4,9-anhydro-tetrodotoxin. Chem. Res. Toxicol. 2005, 18, 865–871. [Google Scholar] [CrossRef]
- Khora, S.S.; Yasumoto, T. Isolation of 11-oxotetrodotoxin from the puffer, Arothron nigropunctatus. Tetrahedron Lett. 1989, 30, 4393–4394. [Google Scholar] [CrossRef]
- Kono, M.; Matsui, T.; Furukawa, K.; Takase, T.; Yamamori, K.; Kaneda, H.; Aoki, D.; Jang, J.-H.; Yotsu-Yamashita, M. Examination of transformation among tetrodotoxin and its analogs in the living cultured juvenile puffer fish, kusafugu, Fugu niphobles by intramuscular administration. Toxicon 2008, 52, 714–720. [Google Scholar] [CrossRef]
- Nakashima, K.; Arakawa, O.; Taniyama, S.; Nonaka, M.; Takatani, T.; Yamamori, K.; Fuchi, Y.; Noguchi, T. Occurrence of saxitoxins as a major toxin in the ovary of a marine puffer Arothron firmamentum. Toxicon 2004, 43, 207–212. [Google Scholar] [CrossRef]
- Vázquez, N.; Yotsu-Yamashita, M.; Sierra-Beltrán, A.P.; Yasumoto, T.; Ochoa, J.L. Toxicities and distribution of tetrodotoxin in the tissues of puffer fish found in the coast of the Baja California Peninsula, Mexico. Toxicon 2000, 38, 729–734. [Google Scholar] [CrossRef]
- Katsutoshi, I.; Shinya, O.; Manabu, A.; Kentaro, B.; Shigeto, T.; Yasuo, S.; Susumu, O. Detection of tetrodotoxin (TTX) from two copepods infecting the grass puffer Takifugu niphobles: TTX attracting the parasites? Toxicon 2006, 48, 620–626. [Google Scholar] [CrossRef]
- Asakawa, M.; Gomez-Delan, G.; Tsuruda, S.; Shimomura, M.; Shida, Y.; Taniyama, S.; Barte-Quilantang, M.; Shindo, J. Toxicity assessment of the Xanthid Crab Demania cultripes from Cebu Island, Philippines. J. Toxicol. 2010, 2010, 1–7. [Google Scholar]
- Ho, P.-H.; Tsai, Y.-H.; Hwang, C.-C.; Hwang, P.-A.; Hwang, J.-H.; Hwang, D.-F. Paralytic toxins in four species of coral reef crabs from Kenting National Park in southern Taiwan. Food Control 2006, 17, 439–445. [Google Scholar] [CrossRef]
- Kanchanapongkul, J. Tetrodotoxin poisoning following ingestion of the toxic eggs of the horseshoe crab Carcinoscorpius rotundicauda, a case series from 1994 through 2006. Southeast Asian J. Trop. Med. Public Health 2008, 39, 303–306. [Google Scholar]
- Yasumoto, T.; Yotsu, M.; Endo, A.; Murata, M.; Naoki, H. lnterspecies distribution and biogenetic origin of tetrodotoxin and its derivatives. Pure Appl. Chem. 1989, 61, 505–508. [Google Scholar] [CrossRef]
- Narita, H.; Matsubara, S.; Miwa, N.; Akahane, S.; Murakami, M.; Goto, T.; Nara, M.; Noguchi, T.; Saito, T.; Shida, Y. Vibrio alginolyticus, a TTX-producing bacterium isolated from the starfish Astropecten polyacanthus. Nippon Suisan Gakk. 1987, 53, 617–621. [Google Scholar] [CrossRef]
- Nakagawa, T.; Jang, J.; Yotsu-Yamashita, M. Hydrophilic interaction liquid chromatography-electrospray ionization mass spectrometry of tetrodotoxin and its analogs. Anal. Biochem. 2006, 352, 142–144. [Google Scholar] [CrossRef]
- Yotsu, M.; Endo, A.; Yasumoto, T. An improved tetrodotoxin analyzer. Agric. Biol. Chem. 1989, 53, 893–895. [Google Scholar] [CrossRef]
- Umezawa, T.; Shinada, T.; Ohfune, Y. Synthesis of 13C-Labeled 5,6,11-Trideoxytetrodotoxin. Chem. Lett. 2010, 39, 1281–1282. [Google Scholar] [CrossRef]
- Williams, B.L. Behavioral and chemical ecology of marine organisms with respect to tetrodotoxin. Mar. Drugs 2010, 8, 381–398. [Google Scholar] [CrossRef]
- Hanifin, C.T. The chemical and evolutionary ecology of tetrodotoxin (TTX) toxicity in terrestrial vertebrates. Mar. Drugs 2010, 8, 577–593. [Google Scholar] [CrossRef]
- Childress, J.J.; Fisher, C.R.; Brooks, J.M.; Kennicutt, M.C.; Bidigare, R.; Anderson, A.E. A methanotrophic marine molluscan (Bivalvia, Mytilidae) symbiosis: Mussels fueled by gas. Science 1986, 233, 1306–1308. [Google Scholar]
- Ott, J.A.; Bright, M.; Schiemer, F. The ecology of a novel symbiosis between a marine peritrich ciliate and chemoautotrophic bacteria. Mar. Ecol. 1998, 19, 229–243. [Google Scholar] [CrossRef]
- Dubilier, N.; Bergin, C.; Lott, C. Symbiotic diversity in marine animals: The art of harnessing chemosynthesis. Nat. Rev. Microbiol. 2008, 6, 725–740. [Google Scholar] [CrossRef]
- Matsumura, K. Production of tetrodotoxin in puffer fish embryos. Environ. Toxicol. Pharmacol. 1998, 6, 217–219. [Google Scholar] [CrossRef]
- Cheng, C.A.; Lin, S.J.; Hwang, D.F. Paralytic toxins of the gastropod Natica lineata in Pingtung Prefecture. Food Sci. 1996, 26, 845–853. [Google Scholar]
- Caldwell, J.P. The evolution of myrmecophagy and its correlates in poison frogs (Family Dendrobatidae). J. Zool. 1996, 240, 75–101. [Google Scholar] [CrossRef]
- Hille, B. The permeability of the sodium channel to organic cations in myelinated nerve. J. Gen. Physiol. 1971, 58, 599–619. [Google Scholar] [CrossRef]
- Moran, O.; Picollo, A.; Conti, F. Tonic and phasic guanidinium toxin-block of skeletal muscle Na channels expressed in Mammalian cells. Biophys. J. 2003, 84, 2999–3006. [Google Scholar] [CrossRef]
- Matsui, T.; Yamamori, K.; Furukawa, K.; Kono, M. Purification and some properties of a tetrodotoxin binding protein from the blood plasma of kusafugu, Takifugu niphobles. Toxicon 2000, 38, 463–468. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Sugimoto, A.; Terakawa, T.; Shoji, Y.; Miyazawa, T.; Yasumoto, T. Purification, characterization, and cDNA cloning of a novel soluble saxitoxin and tetrodotoxin binding protein from plasma of the puffer fish, Fugu pardalis. Eur. J. Biochem. 2001, 268, 5937–5946. [Google Scholar] [CrossRef]
- Kaneko, Y.; Matsumoto, G.; Hanyu, Y. TTX resistivity of Na/ channel in newt retinal neuron. Biochem. Biophys. Res. Commun. 1997, 240, 651–656. [Google Scholar] [CrossRef]
- Maruta, S.; Yamaoka, K.; Yotsu-Yamashita, M. Two critical residues in p-loop regions of puffer fish Na+ channels on TTX sensitivity. Toxicon 2008, 51, 381–387. [Google Scholar] [CrossRef]
- Nagashima, Y.; Yamamoto, K.; Shimakura, K.; Shiomi, K. A tetrodotoxin-binding protein in the hemolymph of shore crab Hemigrapsus sanguineus: Purification and properties. Toxicon 2002, 40, 753–760. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Shoji, Y.; Terakawa, T.; Yamada, S.; Miyazawa, T.; Yasumoto, T. Mutual binding inhibition of tetrodotoxin and saxitoxin to their binding protein from the plasma of the puffer fish, Fugu pardalis. Biosci. Biotechnol. Biochem. 2002, 66, 2520–2524. [Google Scholar] [CrossRef]
- Hwang, P.-A.; Tsai, Y.-H.; Lin, H.-P.; Hwang, D.-F. Tetrodotoxin-binding proteins isolated from five species of toxic gastropods. Food Chem. 2007, 103, 1153–1158. [Google Scholar] [CrossRef]
- Fukuda, A.; Tani, A. Records of puffer poisonings report 3. Nippon Igaku Oyobi Kenko Hoken 1941, 3528, 7–13. [Google Scholar]
- Leung, K.S.-Y.; Fong, B.M.-W.; Tsoi, Y.-K. Analytical challenges: Determination of tetrodotoxin in human urine and plasma by LC-MS/MS. Mar. Drugs 2011, 9, 2291–2303. [Google Scholar] [CrossRef]
- ExPASy, S.B.R.P. Sodium/potassium-exchanging ATPase. Available online: http://enzyme.expasy.org/EC/3.6.3.9 (accessed on 14 February 2014).
- Meyer, T.W.; Hostetter, T.H. Uremia. N. Engl. J. Med. 2007, 357, 1316–1325. [Google Scholar] [CrossRef]
- Nakashima, R.; Nakata, Y.; Kameoka, M.; Hayashi, N.; Watanabe, K.; Yagi, K. Case of tetrodotoxin intoxication in a uremic patient. Chudoku Kenkyu 2007, 20, 141–145. [Google Scholar]
- Chew, S.K.; Chew, L.S.; Wang, K.W.; Mah, P.K.; Tan, B.Y. Antocholinesterase drugs in the treatement of tetrodotoxin poisoning. Lancet 1984, 324. [Google Scholar] [CrossRef]
- Clark, R.F.; Williams, S.R.; Nordt, S.P.; Manoguerra, A.S. A review of selected sea-food poisoning. Undersea Hyperb. Med. 1999, 26, 175–184. [Google Scholar]
- Vale, J.A. Position statement: Gastric lavage. American academy of clinical toxicology; European association of poisons centres and clinical toxicologists. J. Toxicol. Clin. Toxicol. 1997, 35, 711–719. [Google Scholar] [CrossRef]
- Kaufman, B.; Wright, D.C.; Ballou, W.R.; Monheit, D. Protection against tetrodotoxin and saxitoxin intoxication by a cross-protective rabbit anti-tetrodotoxin antiserum. Toxicon 1991, 29, 581–587. [Google Scholar] [CrossRef]
- Rivera, V.R.; Poli, M.A.; Bignami, G.S. Prophylaxis and treatment with a monoclonal antibody of tetrodotoxin poisoning in mice. Toxicon 1995, 33, 1231–1237. [Google Scholar] [CrossRef]
- Joshi, S.K.; Joseph, P.M.; Hernandez, G.; Baker, S.; Shieh, C.-C.; Neelands, T.; Zhang, X.-F.; Niforatos, W.; Kage, K.; Han, P.; et al. Involvement of the TTX-resistant sodium channel Nav 1.8 in inflammatory and neuropathic, but not post-operative, pain states. Pain 2006, 123, 75–82. [Google Scholar] [CrossRef]
- Marcil, J.; Walczak, J.-S.; Guindon, J.; Ngoc, A.H.; Lu, S.; Beaulieu, P. Antinociceptive effects of tetrodotoxin (TTX) in rodents. Br. J. Anaesth. 2006, 96, 761–768. [Google Scholar] [CrossRef]
- Hagen, N.A.; du Souich, P.; Lapointe, B.; Ong-Lam, M.; Dubuc, B.; Walde, D.; Love, R.; Ngoc, A.H. Tetrodotoxin for moderate to severe cancer pain: A randomized, double blind, parallel design multicenter study. J. Pain Symptom Manag. 2008, 35, 420–429. [Google Scholar] [CrossRef]
- Shi, J.; Liu, T.T.; Wang, X.; Epstein, D.H.; Zhao, L.Y.; Zhang, X.L.; Lu, L. Tetrodotoxin reduces cue-induced drug craving and anxiety in abstinent heroin addicts. Pharmacol. Biochem. Behav. 2009, 92, 603–607. [Google Scholar] [CrossRef]
- Por, F.D.E. Lessepsian Migration, the Influx of Red Sea Biota into the Mediterranean by Way of the Suez Canal; Springer-Verlag: Berlin, Germany, 1978. [Google Scholar]
- Akyol, O.; Ünal, V.; Ceyhan, T.; Bilecenoglu, M. First confirmed record of Lagocephalus sceleratus (Gmelin, 1789) in the Mediterranean Sea. J. Fish Biol. 2005, 66, 1183–1186. [Google Scholar] [CrossRef]
- Golani, D.; Levy, Y. New records and rare occurrences of fish species from the Mediterranean coast of Israel. Zool. Middle East 2005, 36, 27–32. [Google Scholar] [CrossRef]
- Corsini, M.; Margies, P.; Kondilatos, G.; Economidis, P.S. Three new exotic fish records from the SE Aegean Greek waters. Sci. Mar. 2006, 70, 319–323. [Google Scholar]
- Peristeraki, P.; Lazarakis, G.; Tserpes, G. First Results on the maturity of the Lessepsian migrant Lagocephalus sceleratus (gmelin 1789) in the eastern meditettanean sea. Rapp. Comm. int. Mer Médit 2010, 39, 628. [Google Scholar]
- Jribi, I.; Bradai, M.N. First record of the Lessepsian migrant species Lagocephalus sceleratus (Gmelin, 1789) (Actinopterygii: Tetraodontidae) in the Central Mediterranean. BioInvasions Rec. 2012, 1, 49–52. [Google Scholar] [CrossRef]
- Milazzo, M.; Azzurro, E.; Badalamenti, F. On the occurrence of the silverstripe blaasop Lagocephalus sceleratus (Gmelin, 1789) along the Libyan coast. BioInvasions Rec. 2012, 1, 125–127. [Google Scholar] [CrossRef] [Green Version]
- Nader, M.R.; Indary, S.; Boustany, L.E. The Puffer Fish Lagocephalus Sceleratus (Gmelin, 1789) in the Eastern Mediterranean; EastMed Technical Documents 2012, GCP/INT/041/EC–GRE–ITA; FAO: Rome, Italy, 2012. [Google Scholar]
- Mosher, H.S.; Fuhrman, F.A.; Buchwald, H.D.; Fischer, H.G. Tarichatoxin-tetrodotoxin: A potent neurotoxin. Science 1964, 144, 1100–1110. [Google Scholar]
- Nagashima, Y.; Tanaka, N.; Shimakura, K.; Shiomi, K.; Shida, Y. Occurrence of tetrodotoxin-related substances in the nontoxic puffer Takifugu xanthopterus. Toxicon 2001, 39, 415–418. [Google Scholar] [CrossRef]
- Simon, K.D.; Mazlan, A.G.; Usup, G. Toxicity of puffer fishes (Lagocephalus wheeleri Abe, tabeta and kitahama, 1984 and Lagocephalus sceleratus Gmelin, 1789) from the east coast waters of peninsular Malaysia. J. Biol. Sci. 2009, 9, 482–487. [Google Scholar] [CrossRef]
- Noguchi, T.; Hashimoto, Y. Isolation of tetrodotoxin from a goby Gobius criniger. Toxicon 1973, 11, 305–307. [Google Scholar] [CrossRef]
- Thuesen, E.V.; Kogure, K.; Hashimoto, K.; Nemoto, T. Poison arrowworms: A tetrodotoxin venom in the marine phylum Chaetognatha. J. Exp. Mar. Biol. Ecol. 1988, 116, 249–256. [Google Scholar] [CrossRef]
- Ngy, L.; Tada, K.; Yu, C.-F.; Takatani, T.; Arakawa, O. Occurrence of paralytic shellfish toxins in Cambodian Mekong pufferfish Tetraodon turgidus: Selective toxin accumulation in the skin. Toxicon 2008, 51, 280–288. [Google Scholar] [CrossRef]
- Oliveira, J.S.; Fernandes, S.C.R.; Schwartz, C.A.; Bloch, C., Jr.; Taquita Melo, J.A.; Rodrigues Pires, O., Jr.; Carlos de Freitas, J. Toxicity and toxin identification in Colomesus asellus, an Amazonian (Brazil) freshwater puffer fish. Toxicon 2006, 48, 55–63. [Google Scholar] [CrossRef]
- Deeds, J.R.; White, K.D.; Etheridge, S.M.; Landsberg, J.H. Concentrations of saxitoxin and tetrodotoxin in three species of puffers from the Indian River Lagoon, Florida, the location for multiple cases of saxitoxin puffer poisoning from 2002 to 2004. Trans. Am. Fish. Soc. 2008, 137, 1317–1326. [Google Scholar] [CrossRef]
- Zaman, L.; Arakawa, O.; Shimosu, A.; Onoue, Y. Occurrence of paralytic shellfish poison in Bangladeshi freshwater puffers. Toxicon 1997, 35, 423–431. [Google Scholar] [CrossRef]
- Kungsuwan, A.; Arakawa, O.; Promdet, M.; Onoue, Y. Occurrence of paralytic shellfish poisons in thai freshwater puffers. Toxicon 1997, 35, 1341–1346. [Google Scholar] [CrossRef]
- Ito, K.; Asakawa, M.; Beppu, R.; Takayama, H.; Miyazawa, K. PSP-toxicification of the carnivorous gastropod Rapana venosa inhabiting the estuary of Nikoh River, Hiroshima Bay, Hiroshima Prefecture, Japan. Mar. Pollut. Bull. 2004, 48, 1116–1121. [Google Scholar] [CrossRef]
- Sato, S.; Kodama, M.; Ogata, T.; Saitanu, K.; Furuya, M.; Hirayama, K.; Kakinuma, K. Saxitoxin as a toxic principle of a freshwater puffer, Tetraodon fangi, in Thailand. Toxicon 1997, 35, 137–140. [Google Scholar] [CrossRef]
- Landsberg, J.H.; Hall, S.; Johannessen, J.N.; White, K.D.; Conrad, S.M.; Abbott, J.P.; Flewelling, L.J.; et al. Saxitoxin puffer fish poisoning in the united states, with the first report of Pyrodinium bahamense as the putative toxin source. Environ. Health Perspect. 2006, 114, 1502–1507. [Google Scholar] [CrossRef]
- Hille, B. Ionic Channels of Excitable Membranes; Sinauer: Sunderland, MA, USA, 1992. [Google Scholar]
- Chen, H.-M.; Lin, C.-H.; Wang, T.M. Effects of 4-aminopyridine on saxitoxin intoxication. Toxicol. Appl. Pharm. 1996, 141, 44–48. [Google Scholar]
- Chang, F.-C.T.; Spriggs, D.L.; Benton, B.J.; Keller, S.A.; Capacio, B.R. 4-Aminopyridine reverses saxitoxin (STX)- and tetrodotoxin (TTX)-induced cardiorespiratory depression in chronically instrumented guinea pigs. Fund. Appl. Toxicol. 1997, 38, 75–88. [Google Scholar] [CrossRef]
- Tsuda, K.; Ikuma, S.; Kawamura, M.; Tachiikawa, R.; Sakai, K. Tetrodotoxin VII on the structure of tetrodotoxin and its derivatives. Chem. Pharm. Bull. 1964, 12, 1357–1374. [Google Scholar] [CrossRef]
- Anraku, K.; Nonaka, K.; Yamaga, T.; Yamamoto, T.; Shin, M.-C.; Wakita, M.; Hamamoto, A.; Akaike, N. Removal of toxin (tetrodotoxin) from puffer ovary by traditional fermentation. Toxins 2013, 5, 193–202. [Google Scholar] [CrossRef]
- Doucette, G.J.; Powell, C.L.; Do, E.U.; Byon, C.Y.; Cleves, F.; McClain, S.G. Evaluation of 11-[3H]-tetrodotoxin use in a heterologous receptor binding assay for PSP toxins. Toxicon 2000, 38, 1465–1474. [Google Scholar] [CrossRef]
- Kawatsu, K.; Shibata, T.; Hamano, Y. Application of immunoaffinity chromatography for detection of tetrodotoxin from urine samples of poisoned patients. Toxicon 1999, 37, 325–333. [Google Scholar] [CrossRef]
- Tanu, M.B.; Mahmud, Y.; Takatani, T.; Kawatsu, K.; Hamano, Y.; Arakawa, O.; Noguchi, T. Localization of tetrodotoxin in the skin of a brackishwater puffer Tetraodon steindachneri on the basis of immunohistological study. Toxicon 2002, 40, 103–106. [Google Scholar] [CrossRef]
- Mahmud, Y.; Arakawa, O.; Ichinose, A.; Begum Tanu, M.; Takatani, T.; Tsuruda, K.; Kawatsu, K.; Hamano, Y.; Noguchi, T. Intracellular visualization of tetrodotoxin (TTX) in the skin of a puffer Tetraodon nigroviridis by immunoenzymatic technique. Toxicon 2003, 41, 605–611. [Google Scholar] [CrossRef]
- Monaliza, M.; Samsur, M. Toxicity and toxin properties study of puffer fish collected from sabah waters. Health Environ. J. 2011, 2, 14–17. [Google Scholar]
- Stokes, A.N.; Williams, B.L.; French, S.S. An improved competitive inhibition enzymatic immunoassay method for tetrodotoxin quantification. Biol. Proced. Online 2012, 14, 3. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M. Chemistry of puffer fish toxin. J. Toxicol. Toxin Rev. 2001, 20, 51–66. [Google Scholar] [CrossRef]
- Zaman, L.; Arakawa, O.; Shimosu, A.; Shida, Y.; Onoue, Y. Occurrence of a methyl derivative of saxitoxin in Bangladeshi freshwater puffers. Toxicon 1998, 36, 627–630. [Google Scholar] [CrossRef]
- Mohamad, S.; Yasunaga, Y.; Takefumi, S.; Tomohiro, T.; Osamu, A.; Tamao, N. Accumulation and depuration profiles of PSP toxins in the short-necked clam Tapes japonica fed with the toxic dinoflagellate Alexandrium catenella. Toxicon 2006, 48, 323–330. [Google Scholar] [CrossRef]
- Akaki, K.; Hatano, K. Determination of tetrodotoxin in puffer-fish tissues, and in serum and urine of intoxicated humans by liquid chromatography with tandem mass spectrometry. Shokuhin Eiseigaku Zasshi 2006, 47, 46–50. [Google Scholar] [CrossRef]
- Furey, A.; Moriarty, M.; Bane, V.; Kinsella, B.; Lehane, M. Ion suppression; a critical review on causes, evaluation, prevention and applications. Talanta 2013, 115, 104–122. [Google Scholar] [CrossRef]
- Hungerford, J.M. Committee on natural toxins and food allergens, U.S. food and drug administration marine and freshwater toxins. J. AOAC Int. 2006, 89, 248–269. [Google Scholar]
- Yakes, B.J.; Deeds, J.; White, K.; DeGrasse, S.L. Evaluation of surface plasmon resonance biosensors for detection of tetrodotoxin in food matrices and comparison to analytical methods. J. Agric. Food Chem. 2010, 59, 839–846. [Google Scholar]
- Wildlife, T.T. Rare Scorpion Fish no Fisherman’s Friend. The Times Newspaper—Wildlife, 11 May 2013. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bane, V.; Lehane, M.; Dikshit, M.; O'Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, Toxicity, Source, Distribution and Detection. Toxins 2014, 6, 693-755. https://doi.org/10.3390/toxins6020693
Bane V, Lehane M, Dikshit M, O'Riordan A, Furey A. Tetrodotoxin: Chemistry, Toxicity, Source, Distribution and Detection. Toxins. 2014; 6(2):693-755. https://doi.org/10.3390/toxins6020693
Chicago/Turabian StyleBane, Vaishali, Mary Lehane, Madhurima Dikshit, Alan O'Riordan, and Ambrose Furey. 2014. "Tetrodotoxin: Chemistry, Toxicity, Source, Distribution and Detection" Toxins 6, no. 2: 693-755. https://doi.org/10.3390/toxins6020693







