Geographical Patterns in Cyanobacteria Distribution: Climate Influence at Regional Scale
Abstract
:1. Introduction
2. Results and Discussion
2.1. Regional Climate Characteristics
| Parameter | December–March | May–October | ||||
|---|---|---|---|---|---|---|
| Minimun | Mean | Maximun | Minimun | Mean | Maximun | |
| Mean Temperature (°C) | 3.7 | 6.6 | 9.2 | 12.2 | 15.9 | 20.1 |
| Mean Pluviometry (mm) | 49 | 84 | 119 | 25.5 | 61.1 | 137.3 |
| Mean Global Radiation (kW/m²) | 8.65 | 17.53 | 34.30 | 22.55 | 49.54 | 67.51 |

2.2. Regional Climate Projected Evolution
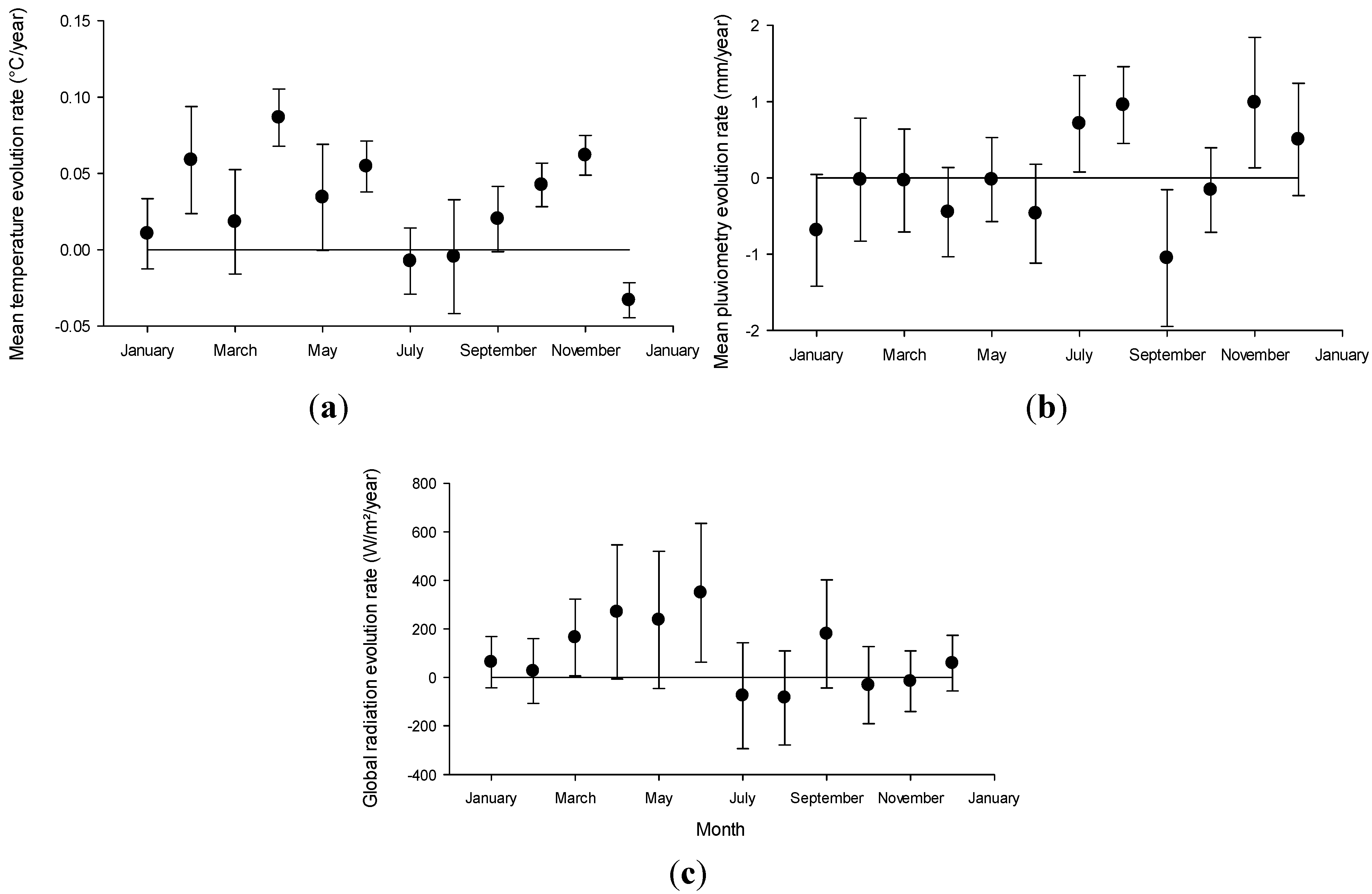
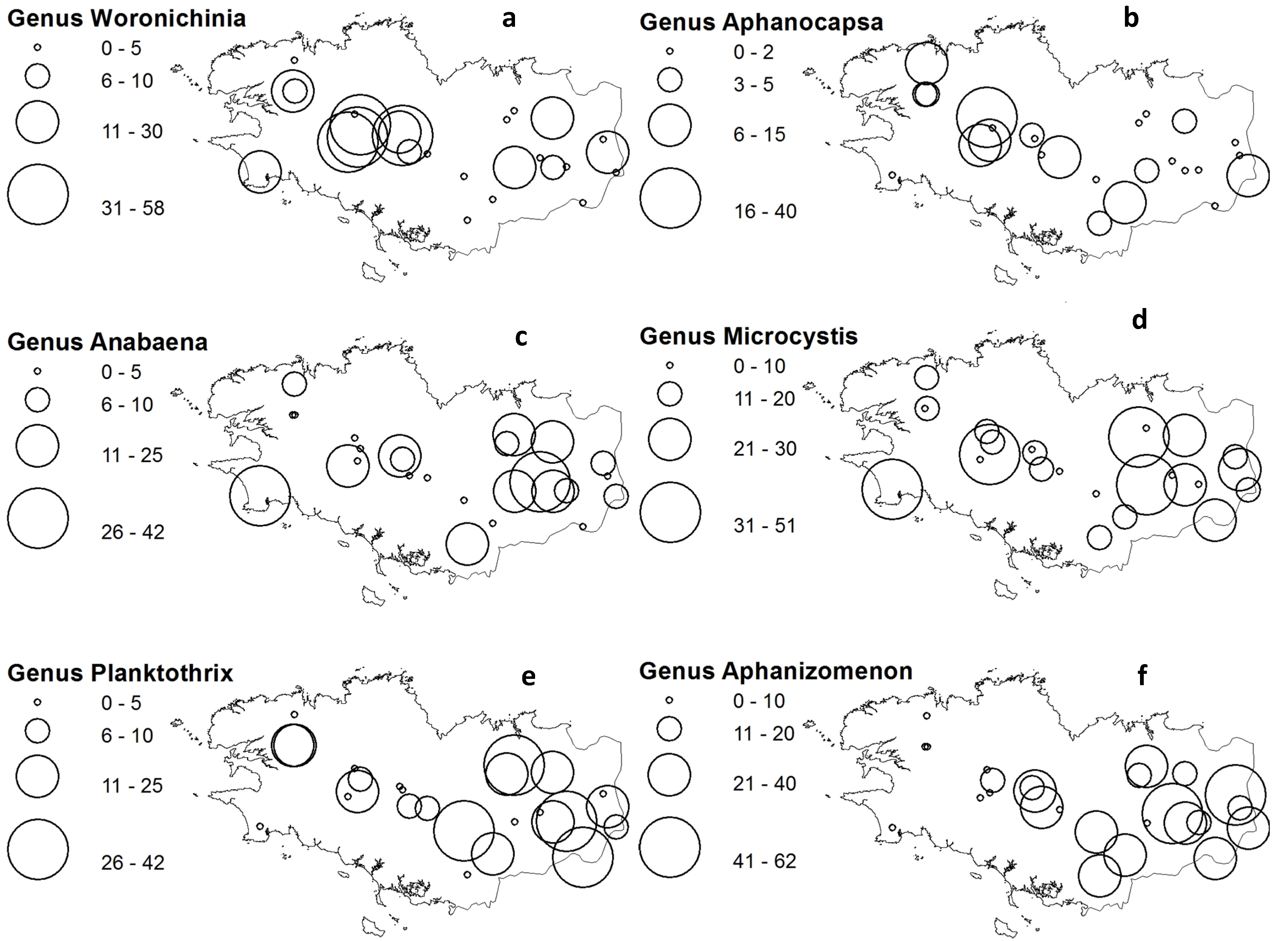
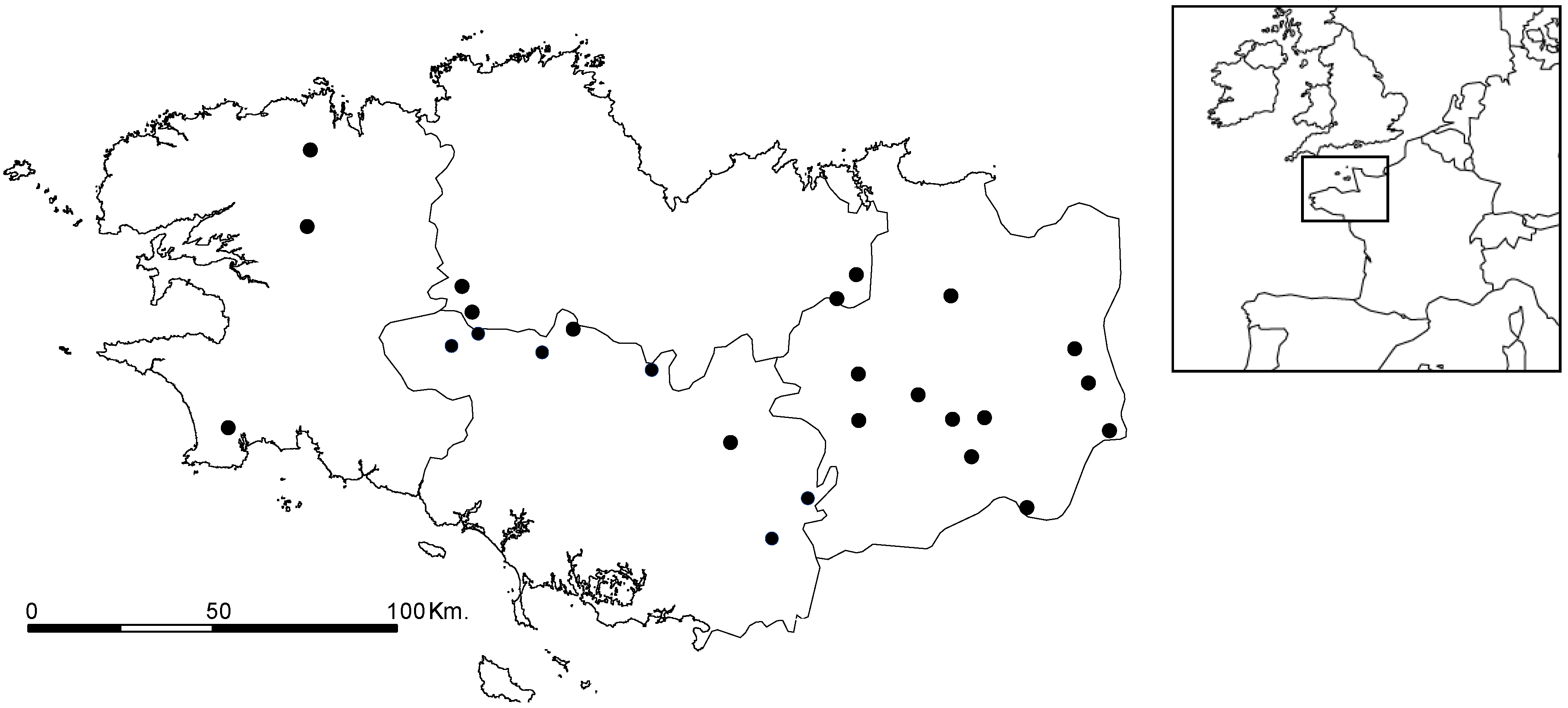
2.3. Cyanobacteria Distribution
2.4. Microcystin Occurrences

2.5. Cyanobacteria-Climate Relations
2.6. Microcystin-Climate Relations

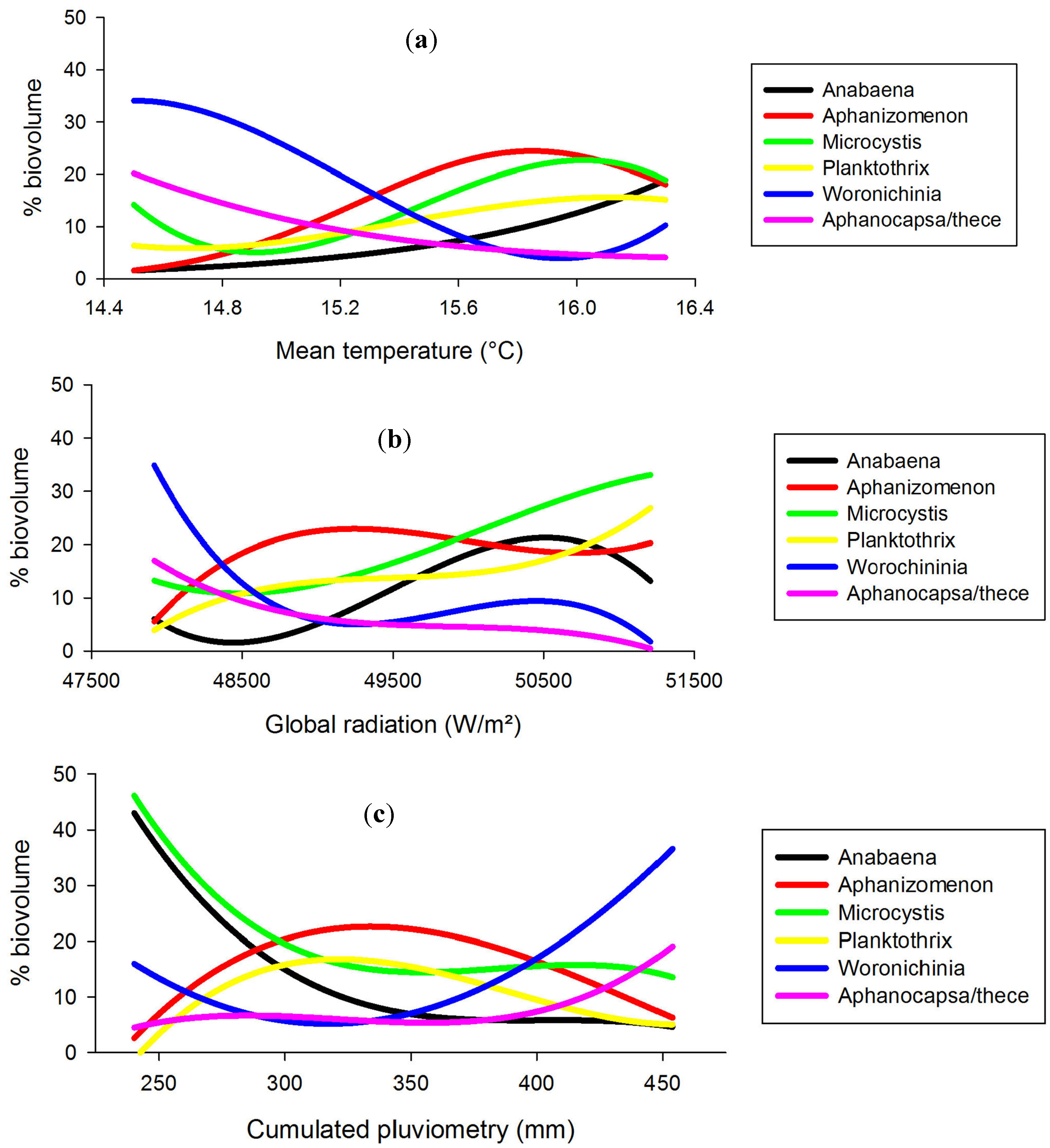
| Genus | MT | CGR | CP |
|---|---|---|---|
| °C | kW/m² | mm | |
| Aphanothece/capsa | <14 | <48 | >455 |
| Woronichinia | 14.4 | <48 | >455 |
| Aphanizomenon | 15.8 | 49.2 | 335 |
| Planktothrix | 16.1 | >51.2 | 320 |
| Microcystis | 16.1 | >51.2 | <240 |
| Anabaena | >16.5 | 50.5 | <240 |
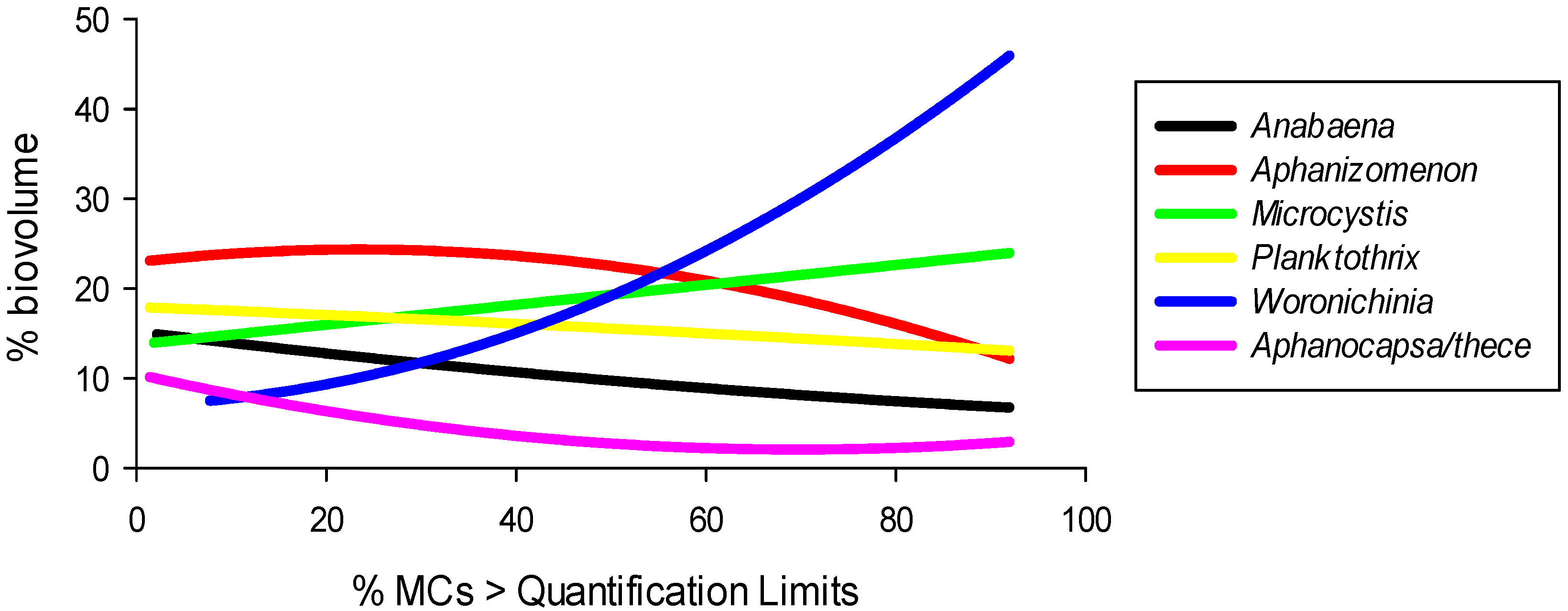
2.7. Cyanobacteria and Microcystin Domain Projections
3. Experimental Section
4. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- Chorus, I.; Bartram, J. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Spon Press: London, UK, 1999; p. 416. [Google Scholar]
- Falconer, I.R. Potential impact on human health of toxic cyanobacteria. Phycologia 1996, 35, 6–11. [Google Scholar] [CrossRef]
- Paerl, H.W.; Hall, N.S.; Calandrino, E. S. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ. 2011, 409, 1739–1745. [Google Scholar] [CrossRef]
- Elliott, J.A. Is the future blue-green? A review of the current model predictions of how climate change could affect pelagic freshwater cyanobacteria. Water Res. 2012, 46, 1364–1371. [Google Scholar] [CrossRef] [Green Version]
- Reichwaldt, E.S.; Ghadouani, A. Effects of rainfall patterns on toxic cyanobacterial blooms in a changing climate: Between simplistic scenarios and complex dynamics. Water Res. 2012, 46, 1372–1393. [Google Scholar] [CrossRef]
- Kosten, S.; Huszar, V.L.M.; Bécares, E.; Costa, L.S.; Donk, E.; Hansson, L.A.; Scheffer, M. Warmer climates boost cyanobacterial dominance in shallow lakes. Global Change Biol. 2012, 18, 118–126. [Google Scholar] [CrossRef]
- Carey, C.C.; Ibelings, B.W.; Hoffmann, E.P.; Hamilton, D.P.; Brookes, J.D. Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res. 2012, 46, 1394–1407. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef]
- Zhang, M.; Duan, H.; Shi, X.; Yu, Y.; Kong, F. Contributions of meteorology to the phenology of cyanobacterial blooms: Implications for future climate change. Water Res. 2012, 46, 442–452. [Google Scholar] [CrossRef]
- Taranu, Z.E.; Zurawell, R.W.; Pick, F.; Gregory-Eaves, I. Predicting cyanobacterial dynamics in the face of global change: the importance of scale and environmental context. Global Change Biol. 2012, 18, 3477–3490. [Google Scholar] [CrossRef]
- Carvalho, L.; Kirika, A. Changes in shallow lake functioning: Response to climate change and nutrient reduction. Hydrobiologia 2003, 506–509, 789–796. [Google Scholar] [CrossRef]
- Malmaeus, J.M.; Blenckner, T.; Markensten, H.; Persson, I. Lake phosphorus dynamics and climate warming: A mechanistic model approach. Ecol. Model. 2006, 190, 1–14. [Google Scholar] [CrossRef]
- Mooij, W.M.; Janse, J.H.; De Senerponts Domis, L.N.; Hülsmann, S.; Ibelings, B.W. Predicting the effect of climate change on temperate shallow lakes with the ecosystem model PCLake. Hydrobiologia 2007, 584, 443–454. [Google Scholar] [CrossRef]
- Nõges, P.; Tuvikene, L.; Feldmann, T.; Tõnno, I.; Künnap, H.; Luup, H.; Nõges, T. The role of charophytes in increasing water transparency: A case study of two shallow lakes in Estonia. Hydrobiologia 2003, 506, 567–573. [Google Scholar]
- Wiedner, C.; Rücker, J.; Brüggemann, R.; Nixdorf, B. Climate change affects timing and size of populations of an invasive cyanobacterium in temperate regions. Oecologia 2007, 152, 473–484. [Google Scholar] [CrossRef]
- Liu, X.; Lu, X.; Chen, Y. The effects of temperature and nutrient ratios on Microcystis blooms in Lake Taihu, China: An 11-year investigation. Harmful Algae 2011, 10, 337–343. [Google Scholar] [CrossRef]
- Vasconcelos, V.; Morais, J.; Vale, M. Microcystins and cyanobacteria trends in a 14 year monitoring of a temperate eutrophic reservoir (Aguieira, Portugal). J. Environ. Monit. 2011, 13, 668–672. [Google Scholar] [CrossRef]
- De Nobel, W.T.; Matthijs, H.C.P.; Von Elert, E.; Mur, L.R. Comparison of the light-limited growth of the nitrogen-fixing cyanobacteria Anabaena and Aphanizomenon. New Phytol. 1998, 138, 579–587. [Google Scholar]
- Baker, P.D.; Bellifemine, D. Environmental influences on akinete germination of Anabaena circinalis and implications for management of cyanobacterial blooms. Hydrobiologia 2000, 427, 65–73. [Google Scholar] [CrossRef]
- Hadas, O.; Pinkas, R.; Malinsky-Rushansky, N.; Shalev-Alon, G.; Delphine, E.; Berner, T.; Sukenik, A.; Kaplan, A. Physiological variables determined under laboratory conditions may explain the bloom of Aphanizomenon ovalisporum in Lake Kinneret. Eur. J. Phycol. 2002, 37, 259–267. [Google Scholar] [CrossRef]
- McCausland, M.; Thompson, P.; Blackburn, S.I. Ecophysiological influence of light and mixing on Anabaena circinalis (Nostocales, Cyanobacteria). Eur. J. Phycol. 2005, 40, 9–20. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Nakahara, H. The formation and degradation of cyanobacterium Aphanizomenon flos-aquae blooms: The importance of pH, water temperature, and day length. Limnology 2005, 6, 1–6. [Google Scholar] [CrossRef]
- Imai, H.; Chang, K-H.; Kusaba, M.; Nakano, S-I. Temperature-dependent dominance of Microcystis (Cyanophyceae) species: M. aeruginosa and M. weisenbergii. J. Plankton Res. 2009, 31, 171–178. [Google Scholar]
- Rapala, J.; Sivonen, K. Assessment of environmental conditions that favor hepatotoxic and neurotoxic Anabaena spp. strains cultured under light limitation at different temperatures. Microb. Ecol. 1998, 36, 181–192. [Google Scholar] [CrossRef]
- Wiedner, C.; Visser, P.M.; Fastner, J.; Metcalf, J.S.; Codd, G.A.; Mur, L.R. Effects of light on the microcystin content of microcystis strain PCC 7806. Appl. Environ. Microbiol. 2003, 69, 1475–1481. [Google Scholar] [CrossRef]
- Tonk, L.; Visser, P.M.; Christiansen, G.; Dittmann, E.; Snelder, E.O.; Wiedner, C.; Huisman, J. The microcystin composition of the cyanobacterium Planktothrix agardhii changes toward a more toxic variant with increasing light intensity. Appl. Environ. Microbiol. 2005, 71, 5177–5181. [Google Scholar] [CrossRef]
- Briand, E.; Yéprémian, C.; Humbert, J-F.; Quiblier, C. Competition between microcystin- and non-microcystin-producing Planktothrix agardhii (cyanobacteria) strains under different environmental conditions. Environ. Microbiol. 2008, 10, 3337–3348. [Google Scholar] [CrossRef]
- Preußel, K.; Wessel, G.; Fastner, J.; Chorus, I. Response of cylindrospermopsin production and release in Aphanizomenon flos-aquae (cyanobacteria) to varying light and temperature conditions. Harmful Algae 2009, 8, 645–650. [Google Scholar] [CrossRef]
- Qin, B.; Zhu, G.; Gao, G.; Zhang, Y.; Li, W.; Paerl, H.W.; Carmichael, W.W. A drinking water crisis in Lake Taihu, China: Linkage to climatic variability and lake management. Environ. Manag. 2010, 45, 105–112. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Brookes, J.D.; Carey, C.C. Resilience to blooms. Science 2011, 334, 46–47. [Google Scholar]
- Terray, L.; Boé, J. Quantifying 21st-century France climate change and related uncertainties. Geoscience 2013, 345, 136–149. [Google Scholar] [CrossRef]
- Baurès, E.; Pitois, F.; Jung, A.V.; Thomas, O. Oracle: Objectivizing cyanobacteria-associated risks in recreational waters. In WIT Transaction on Ecology and Environment; Wessex Institute of Technology Press: Southampton, UK, 2013; Volume 178, pp. 133–143. [Google Scholar]
- Pitois, F.; Thomas, O.; Thoraval, I.; Baurès, E. Learning from 8 years of regional cyanobacteria observation in Brittany in view of sanitary survey improvement. Environ. Int. 2014, 62, 113–118. [Google Scholar] [CrossRef]
- Reynolds, C.S. The Ecology of Freshwater Phytoplankton; Cambridge University Press: Cambridge, UK, 1984; p. 384. [Google Scholar]
- Reynolds, C.S. Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006; p. 535. [Google Scholar]
- Dolman, A.M.; Rücker, J.; Pick, F.R.; Fastner, J.; Rohrlack, T.; Mischke, U.; Wiedner, C. Cyanobacteria and cyanotoxins: The influence of nitrogen versus phosphorus. PLoS ONE 2012, 7, e38757. [Google Scholar]
- Carvalho, L.; Miller nee Ferguson, C.; Scott, E.M.; Codd, G.; Davies, P.S.; Tyler, A.N. Cyanobacterial blooms: Statistical models describing risk factors for national-scale lake assessment and lake management. Sci. Total Environ. 2011, 409, 5353–5358. [Google Scholar] [Green Version]
- Geitler, L. Cyanophyceae von Europa, Kryptogamen Flora von Deutschland, Osterreich und der Schweiz; Koeltz Scientific Books: Koeningstein, Germany, 1932; p. 1196. [Google Scholar]
- Komarek, J.; Anagnostidis, K. Cyanoprokaryota 19/1. Teil: Chroococcales; Ettl, H., Gärtner, G., Heynig, H., Mollenhauer, D., Eds.; Süßwasserflora von Mitteleuropa, Spektrum Academischer Verlag: Heidelberg, Germany, 1999; p. 548. [Google Scholar]
- Komarek, J.; Anagnostidis, K. Cyanoprokaryota 19/2. Teil: Oscillatoriales; Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Süßwasserflora von Mitteleuropa, Spektrum Academischer Verlag: Heidelberg, Germany, 2005; p. 759. [Google Scholar]
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitt. Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar]
- Environmental Protection Agency (EPA). LG 401—Standard Operating Procedure for Phytoplankton Analysis; EPA: Chicago, IL, USA, 2010; p. 44. [Google Scholar]
- Lawrence, J.F.; Niedzwiadek, B.; Menard, C.; Lau, B.P.; Lewis, D.; Kuper-Goodman, T.; Holmes, C. Comparison of liquid chromatography/mass spectrometry, ELISA, and phosphatase assay for the determination of microcystins in blue-green algae products. J. AOAC Int. 2001, 84, 1035–1044. [Google Scholar]
- Mahmood, N.A.; Carmichael, W.W. The pharmacology of anatoxin-a(s), a neurotoxin produced by the freshwater cyanobacterium Anabaena flos-aquae NRC 525-17. Toxicon 1986, 24, 425–434. [Google Scholar] [CrossRef]
- Ferreira, F.M.; Franco Soler, J.M.; Fidalgo, M.L.; Fernández-Vila, P. PSP toxins from Aphanizomenon flos-aquae (cyanobacteria) collected in the Crestuma-Lever reservoir (Douro river, Northern Portugal). Toxicon 2001, 39, 757–761. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Li, D.; Shen, Y.; Li, G.; Liu, Y. First report of aphantoxins in China-waterblooms of toxigenic Aphanizomenon flos-aquae in lake Dianchi. Ecotoxicol. Environ. Safety 2006, 65, 84–92. [Google Scholar] [CrossRef]
- Preuβel, K.; Stüken, A.; Wiedner, C.; Chorus, I.; Fastner, J. First report on cylindrospermopsin producing Aphanizomenon flos-aquae (Cyanobacteria) isolated from two German lakes. Toxicon 2006, 47, 156–162. [Google Scholar] [CrossRef]
- Osswald, J.; Rellán, S.; Gago-Martinez, A.; Vasconcelos, V.M. Production of anatoxin-a by cyanobacterial strains isolated from Portuguese fresh water systems. Ecotoxicology 2009, 18, 1110–1115. [Google Scholar] [CrossRef]
- Li, R.; Carmichael, W.; Pereira, P. Morphological and 16S rRNA gene evidence for reclassification of the paralytic shellfish toxin producing Aphanizomenon flos-aquae LMECYA31 as Aphanizomenon issatschenkoi. J. Phycol. 2003, 39, 814–818. [Google Scholar] [CrossRef]
- Wood, S.; Selwood, A.I.; Rueckert, A.; Holland, P.T.; Milne, J.R.; Smith, K.F.; Cary, C.S. First report of homoanatoxin-a and associated dog neurotoxicosis in New Zealand. Toxicon 2007, 50, 292–301. [Google Scholar] [CrossRef]
- Ballot, A.; Fastner, J.; Wiedner, C. Paralytic shellfish poisoning toxin-producing cyanobacterium Aphanizomenon gracile in Northeast Germany. Appl. Environ. Microbiol. 2010, 76, 1173–1180. [Google Scholar] [CrossRef]
- Pereira, P.; Li, R.; Carmichael, W.W.; Dias, E.; Franca, S. Taxonomy and production of paralytic shellfish toxins by the freshwater cyanobacterium Aphanizomenon gracile. Eur. J. Phycol. 2004, 39, 361–368. [Google Scholar] [CrossRef]
- Ledreux, A.; Thomazeau, S.; Catherine, A.; Duval, C.; Yéprémian, C.; Marie, A.; Bernard, C. Evidence for saxitoxins production by the cyanobacterium Aphanizomenon gracile in a French recreational water body. Harmful Algae 2010, 10, 88–97. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pitois, F.; Thoraval, I.; Baurès, E.; Thomas, O. Geographical Patterns in Cyanobacteria Distribution: Climate Influence at Regional Scale. Toxins 2014, 6, 509-522. https://doi.org/10.3390/toxins6020509
Pitois F, Thoraval I, Baurès E, Thomas O. Geographical Patterns in Cyanobacteria Distribution: Climate Influence at Regional Scale. Toxins. 2014; 6(2):509-522. https://doi.org/10.3390/toxins6020509
Chicago/Turabian StylePitois, Frédéric, Isabelle Thoraval, Estelle Baurès, and Olivier Thomas. 2014. "Geographical Patterns in Cyanobacteria Distribution: Climate Influence at Regional Scale" Toxins 6, no. 2: 509-522. https://doi.org/10.3390/toxins6020509




