Hyperhidrosis: Anatomy, Pathophysiology and Treatment with Emphasis on the Role of Botulinum Toxins
Abstract
:1. Introduction—Definition and Incidence
2. Objective
3. Method
4. Anatomy
5. Physiology
6. Treatments
| Class | Criteria | Level of evidence | Recommendation |
|---|---|---|---|
| I | Prospective, randomized, controlled, outcome masked, representative population with criteria A–E * | A: Two or more Class I studies | Established as effective, ineffective, or harmful |
| II | Prospective, matched cohort, representative population, masked outcome and meets A–E * OR RCT with one criteria in A–E * lacking | B: At least one Class I or two Class II | Probably effective, ineffective, or harmful and recommended |
| III | Controlled trial **, representative population, outcome independent of patient treatment | C: At least one Class II | Possibly effective, ineffective or harmful, may be used at discretion of clinician |
| IV | Uncontrolled study, case series, case report or expert opinion. | U | Data inadequate or conflicting |
6.1. Topical Agents
| Agent | Author and year | Type of hyperhidrosis | N | Study design | Class | Findings | Side effects |
|---|---|---|---|---|---|---|---|
| Topical Aluminium Chloride Hexahydrate 25% in Ethanol | Glent-Madsen et al., 1988 | AH | 30 | Randomized, double-blind, half-sided experiment | III | 25% aluminum chloride in ethanol alone was effective in all pts | Skin irritation |
| Topical Glycopyrrolate | Shaw et al., 1997 | Gustatory (Frey’s syndrome) | 13 | Double-blind, PBO-controlled, crossover study | II | All pts experienced significant improvement. Glycopyrrolate reduced the sweat response to a challenge by 82% (p < 0.01). The frequency of episodes of gustatory sweating also reduced by 51% (p < 0.01), with a nearly 100% reduction in the frequency of severe sweating (p < 0.01) | Eczematous reaction in one patient |
| Topical Glycopyrrolate | Hays 1978 | Gustatory (Frey’s syndrome) | 16 | Double blind clinical trial | III | Topical glycopyrrolate(0.5% and 1.0% ) abolished gustatory sweating for several days after single application. | No significant side effects |
| Topical 2% Diphemanil Methylsulfate (Prantal) | Laccourreye et al., 1990 | Gustatory (Frey’s syndrome) | 15 | Double blind study | II | Partial relief in 33.3% and total relief in 40%. Duration of relief varied from 2 to 4 days. | Dryness of the mouth noted in two pts. |
| Oral Menthatheline Bromide (Vagantin)(systemic anticholinergic) | Hund et al., 2004 | AH and PH | 41 | Randomized, PBO-controlled, double blind clinical trial | II | Mean axillary sweat production decreased in the treated arm from 89.2 ± 73.4 mg/min prior to therapy to 53.3 ± 48.7 mg/min during therapy (p = 0.02). No change in palmar sweat. | Dry mouth |
| Oral Menthatheline Bromide (Vagantin)(systemic anticholinergic) | Muller et al., 2012 | PH, AH or Plamo-Axillary | 339 | Multicenter, randomized, PBO controlled trial, blinding not accurately described | II | 50mg three times a day: improved DLQI, HDSS, and decreased mean axillary sweat production (p = 0.004). | Dry mouth frequently reported |
| Oral Oxybutynin | Ghaleiha et al., 2012 | Hyperhidrosis secondary to Sertaline | 140 | double-blind, PBO-controlled | I | Improved HDSS in the drug compared to PBO group, p ≤ 0.05 | GI and GU symptoms, sedation, dry mouth, |
| Oral Oxybutynin at low doses | Nelson et al., 2012 | PH, AH, and plantar | 50 | Prospective, randomized, single blinded(patient blinded), PBO controlled | II | 5mg twice daily caused moderate to marked improvement in PH or AH, (70%) versus 27.3% in PBO (p < 0.001). Moderate or great improvement in plantar hyperhidrosis (>90%) compared to 13.4% in PBO (p < 0.01) | Dry mouth (frequent) in 47.8% |
6.2. Oral Agents
6.3. Iontophoresis
6.4. Botulinum Toxin—Studies and Methodology
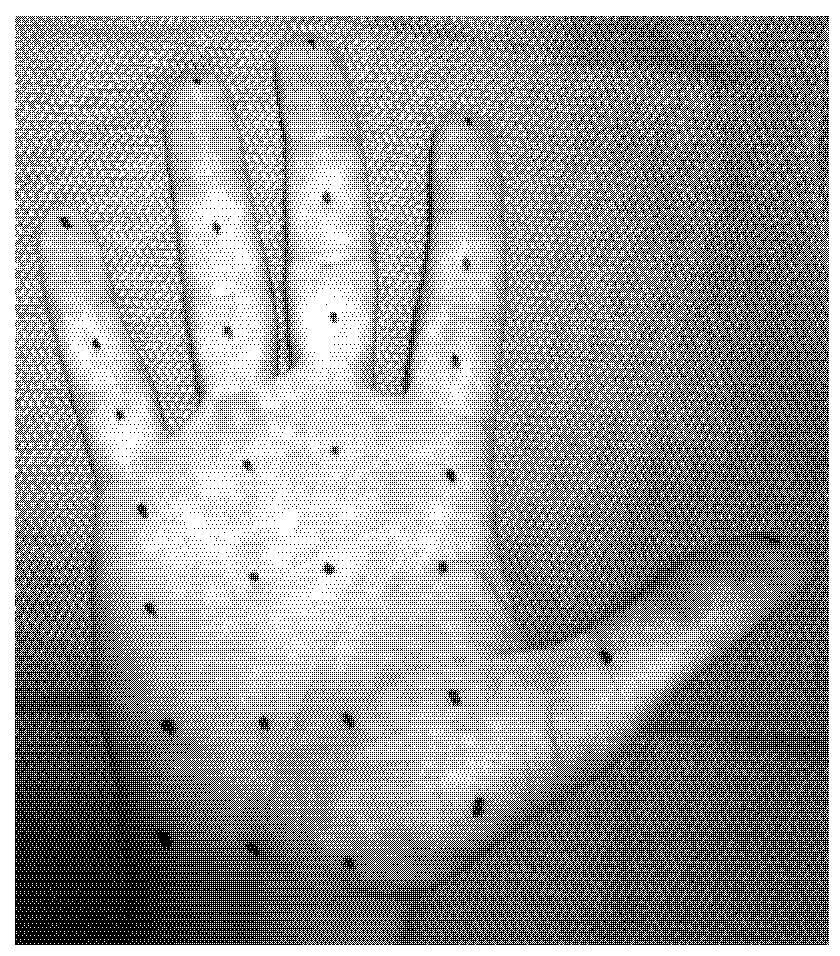

7. Axillary Hyperhidrosis (AH)
| Author & year | N | Class | Agent | Dose | Primary outcome | Result | Side effects |
|---|---|---|---|---|---|---|---|
| Schnider et al., 1999 [33] | 13 | II | A/Abo | 200 U | Sweat quantification (DNSS) and VAS at 3 w, 8 w and 13 w | DNSS: Mean difference between A/Abo and PBO 34.5% (p < 0.001) at 3 w, 36.9% (p < 0.001) at 8 w, and 28.4% (p < 0.001) at 13 w. For VAS, 56.5% (p < 0.001) at 3 w, 67.4% (p < 0.001) at 8 w, and 62.5% (p < 0.001) at 13 w. | Pruritus in A/Abo axilla (2 pts) lasting for 1 w. Mild constipation and increased palmar sweating (2 pts for 1 w) |
| Heckmann et al., 2001 [35] | 158 | I | A/Abo | 200 U | Reduction in sweat by gravimetry | Decrease in mean sweat production from 192 mg/min to 24 mg/min (p < 0.001). | No SAE |
| Naumann and Lowe 2001 [36] | 320 | I | A/Ona | 50 U/axilla | Percentage of responders (spontaneous axillary sweat production >50% reduction from baseline at 4 w, patient GATS, and SAE | 4 w—94% (227) of A/Ona group responded vs. 36% (28) of PBO. 16w—82% (198) A/Ona vs. 21% (16) in PBO. Higher patient satisfaction in A/Ona (p< 0.001). Adverse events: 27 (11%) in A/Ona, 4 (5%) in PBO (p > 0.05). | 13% common cold and 5% compensatory sweating in A/Ona group. |
| Odderson 2002 [37] | 18 | II | A/Ona | 100 U (50 U/ axilla) | Sweating per surface area (SPA) quantified monthly for 5 months. | A/Ona group showed average reduction in sweat production of 91.6% at 2 w (p < 0.05) and average reduction of 88.2% over 5 months | Mild compensatory hyperhidrosis between thighs in 1patient. |
| Baumann et al., 2005 [38] | 23 | III | B/Rima | 2500 U (0.5 mL) per axilla | Safety, efficacy and duration of action using participant assessment of axillary hyperhidrosis improvement, QOL, physician assessment score at Day 30 | Duration of action from 2.2–8.1 months (mean = 5 months). Day 30: Improvement in QOL score (p < 0.001) and physician assessment (p < 0.001). | Transient bruising, flu like symptoms, dry eyes, indigestion, menopausal bleeding in B/Rima group. |
| Lowe et al., 2007 [39] | 322 | II | A/Ona | 75 U OR 50 U/axilla | HDSS | 2-point improvement on the 4-point HDSS reported in 75% in the 75-U and 50-U A/Ona groups and in 25% of PBO (p < 0.001). Median duration of effect 197 days, 205 days, and 96 days in 75-U, 50-U, and PBO groups respectively. | No SAE reported |
7.1. Toxin versus Placebo (Table 3)
7.2. Comparative Studies
7.2.1. Toxin and Saline versus Toxin and Anesthetic
7.2.2. Comparing Two Toxins
7.2.3. Comparing Modes of Application
7.3. Overall Level of Evidence in the Placebo Controlled and Comparator Studies
| Toxin | Level of Evidence |
|---|---|
| A/Ona | Level B |
| A/Abo | Level B |
| A/Inco | Level C |
| B/Rima | Level U |
8. Palmar Hyperhidrosis
8.1. Toxin versus Placebo (Table 5)
| Author & Year | N | Class | Agent | Dose | Primary outcome | Result | Side effects |
|---|---|---|---|---|---|---|---|
| Schnider et al., 1997 [12] | 11 | II | A/Abo | 120 U | DNSS | Mean reduction in sweat production: 26%, 26%, and 31% at w 3, 8, 13 (p < 0.001) and improvement in VAS 38%, 40%, and 35% at w 3, 8, 13 respectively (p = 0.002) for A/Abo group. | Minor , reversible weakness of handgrip lasting between 2 and 5 w, and minor hematoma at injection site |
| Lowe et al., 2002 [25] | 19 | II | A/Ona | 100 U | Sweat production (gravimetric measurement) and physician’s and patient’s rating of severity. Safety evaluations via grip strength | Mean percentage change from baseline was significantly greater in the A/Ona-treated palms than in the PBO-treated palms at day 28 (p = 0.0037). Similar results found for Minor’s test. | Finger tingling and numbness in one A/Ona patient. One PBO patient with weakness of injected hand, one patient bilateral hand pain. |
| Baumann et al., 2005 [18] | 20 | II | B/Rima | 5000 U | Efficacy, duration, safety, and patient and investigator assessment | Patient assessed efficacy showed significant difference in favor of B/Rima through day 120. Physician assessment did not show difference at day 30. Mean duration of effect; 3.8 months. Onset of effect: within 1 w. | Transient dry mouth in 18. Indigestion, dry hands. Muscle weakness in 12. Decreased grip strength in 10. |
8.2. Comparative Studies
8.3. Overall Level of Evidence in Placebo-Controlled and Comparator Studies
| Toxin | Level of Evidence |
|---|---|
| A/Ona | Level B |
| A/Abo | Level B |
| A/Inco | Level U |
| B/Rima | Level C |
9. Gustatory Hyperhidrosis
10. Compensatory Hyperhidrosis
11. Surgical Therapy
12. Conclusion and Clinical Comments
13. A Technical Note from the Senior Author
Conflicts of Interest
References
- Naumann, M.K.; Hamm, H.; Lowe, N.J. Botox Hyperhidrosis Clinical Study Group. Effect of botulinum toxin type a on quality of life measures in patients with excessive axillary sweating: A randomized controlled trial. Br. J. Dermatol. 2002, 147, 1218–1226. [Google Scholar]
- Leung, A.K.C.; Chan, P.Y.H.; Choi, M.C.K. Hyperhidrosis. Int. J. Dermatol. 1999, 38, 561–567. [Google Scholar] [CrossRef]
- Adar, R. Palmar hyperhidrosis and its surgical treatment: A report of 100 cases. Ann. Surg. 1977, 186, 34. [Google Scholar] [CrossRef]
- Del Sorbo, F.; Brancati, F.; de Joanna, G.; Valente, E.M.; Lauria, G.; Albanese, A. Primary focal hyperhidrosis in a new family not linked to known loci. Dermatology 2011, 223, 335–342. [Google Scholar] [CrossRef]
- Hornberger, J.; Grimes, K.; Naumann, M.; Glaser, D.A.; Lowe, N.J.; Naver, H.; Ahn, S.; Stolman, L.P. Recognition, diagnosis, and treatment of primary focal hyperhidrosis. J. Am. Acad. Dermatol. 2004, 51, 274–286. [Google Scholar] [CrossRef]
- Bachmann, M.; Myers, J.E.; Bezuidenhout, B.N. Acrylamide monomer and peripheral neuropathy in chemical workers. Am. J. Ind. Med. 1992, 21, 217–222. [Google Scholar] [CrossRef]
- Sato, K.; Kang, W.H.; Saga, K.; Sato, K.T. Biology of sweat glands and their disorders. I. Normal sweat gland function. J. Am. Acad. Dermatol. 1989, 20, 537–563. [Google Scholar] [CrossRef]
- Strutton, D.R.; Kowalski, J.W.; Glaser, D.A.; Stang, P.E. Us prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: Results from a national survey. J. Am. Acad. Dermatol. 2004, 51, 241–248. [Google Scholar] [CrossRef]
- French, J.; Gronseth, G. Lost in a jungle of evidence: We need a compass. Neurology 2008, 71, 1634–1638. [Google Scholar] [CrossRef]
- Sato, K.; Kang, W.H.; Saga, K.; Sato, K.T. Biology of sweat glands and their disorders. II. Disorders of sweat gland function. J. Am. Acad. Dermatol. 1989, 20, 713–726. [Google Scholar]
- Walling, H.W. Primary hyperhidrosis increases the risk of cutaneous infection: A case-control study of 387 patients. J. Am. Acad. Dermatol. 2009, 61, 242–246. [Google Scholar] [CrossRef]
- Schnider, P.; Binder, M.; Auff, E.; Kittler, H.; Berger, T.; Wolff, K. Double-blind trial of botulinum a toxin for the treatment of focal hyperhidrosis of the palms. Br. J. Dermatol. 1997, 136, 548–552. [Google Scholar] [CrossRef]
- Sato, K.; Sato, F. Effect of vip on sweat secretion and camp accumulation in isolated simian eccrine glands. Am. J. Physiol. 1987, 253, R935–R941. [Google Scholar]
- Rothman, S. Physiology and Biochemistry of the Skin; The University of Chicago Press: Chicago, IL, USA, 1954; pp. 168–180. [Google Scholar]
- Saadia, D.; Voustianiouk, A.; Wang, A.K.; Kaufmann, H. Botulinum toxin type a in primary palmar hyperhidrosis: Randomized, single-blind, two-dose study. Neurology 2001, 57, 2095–2099. [Google Scholar] [CrossRef]
- Shelley, W.B.; Hurley, H.J., Jr. Studies on topical antiperspirant control of axillary hyperhidrosis. Acta Derm. Venereol. 1975, 55, 241–260. [Google Scholar]
- Baumann, L.; Slezinger, A.; Halem, M.; Vujevich, J.; Mallin, K.; Charles, C.; Martin, L.K.; Black, L.; Bryde, J. Double-blind, randomized, placebo-controlled pilot study of the safety and efficacy of myobloc (botulinum toxin type b) for the treatment of palmar hyperhidrosis. Dermatol. Surg. 2005, 31, 263–270. [Google Scholar]
- Try, C.; Messikh, R.; Elkhyat, A.; Aubin, F.; Humbert, R.P. Use of oral oxybutynin at 7.5 mg per day in primary hyperhidrosis. Rev. Méd. Liege 2012, 67, 520–526. [Google Scholar]
- Walling, H.W.; Swick, B.L. Treatment options for hyperhidrosis. Am. J. Clin. Dermatol. 2011, 12, 285–295. [Google Scholar] [CrossRef]
- Ghaleiha, A.; Jahangard, L.; Sherafat, Z.; Ahmadpanah, M.; Brand, S.; Holsboer-Trachsler, E.; Bajoghli, H.; Haghighi, M. Oxybutynin reduces sweating in depressed patients treated with sertraline: A double-blind, placebo-controlled, clinical study. Neuropsychiatr. Dis. Treat. 2012, 8, 407–412. [Google Scholar]
- Hund, M.; Sinkgraven, R.; Rzany, B. Randomized, placebo-controlled, double blind clinical trial for the evaluation of the efficacy and safety of oral methantheliniumbromide (vagantin) in the treatment of focal hyperhidrosis. J. Ger. Soc. Dermatol. 2004, 2, 343–349. [Google Scholar]
- Stolman, L.P. Treatment of excess sweating of the palms by iontophoresis. Arch. Dermatol. 1987, 123, 893–896. [Google Scholar] [CrossRef]
- Stolman, L.P. Hyperhidrosis: Medical and surgical treatment. Eplasty 2008, 8, e22. [Google Scholar]
- Davarian, S.; Kalantari, K.K.; Rezasoltani, A.; Rahimi, A. Effect and persistency of botulinum toxin iontophoresis in the treatment of palmar hyperhidrosis. Australas. J. Dermatol. 2008, 49, 75–79. [Google Scholar] [CrossRef]
- Montaser-Kouhsari, L.; Zartab, H.; Fanian, F.; Noorian, N.; Sadr, B.; Nassiri-Kashani, M.; Firooz, A. Comparison of intradermal injection with iontophoresis of abobotulinum toxin a for the treatment of primary axillary hyperhidrosis: A randomized, controlled trial. J. Dermatolog. Treat. 2013. [Google Scholar] [CrossRef]
- Kavanagh, G.M.; Shams, K. Botulinum toxin type a by iontophoresis for primary palmar hyperhidrosis. J. Am. Acad. Dermatol. 2006, 55, S115–S117. [Google Scholar] [CrossRef]
- Freeman, R.; Waldorf, H.A.; Dover, J.S. Autonomic neurodermatology (part ii): Disorders of sweating and flushing. Semin. Neurol. 1992, 12, 394–407. [Google Scholar] [CrossRef]
- Reinauer, S.; Neusser, A.; Schauf, G.; Holzle, E. Iontophoresis with alternating current and direct current offset (ac/dc iontophoresis): A new approach for the treatment of hyperhidrosis. Br. J. Dermatol. 1993, 129, 166–169. [Google Scholar] [CrossRef]
- Holzle, E. Tap water iontophoresis. Hautarzt Z. Dermatol. Venerol. Verwandte Geb. 2012, 63, 462–468. [Google Scholar] [CrossRef]
- Bushara, K.O.; Park, D.M.; Jones, J.C.; Schutta, H.S. Botulinum toxin—A possible new treatment for axillary hyperhidrosis. Clin. Exp. Dermatol. 1996, 21, 276–278. [Google Scholar] [CrossRef]
- Jankovic, J. Treatment of cervical dystonia with botulinum toxin. Move. Disord. 2004, 19, 109–115. [Google Scholar] [CrossRef]
- Mannava, S.; Mannava, K.A.; Nazir, O.F.; Plate, J.F.; Smith, B.P.; Koman, L.A.; Tuohy, C.J. Treatment of palmar hyperhidrosis with botulinum neurotoxin a. J. Hand Surg. 2013, 38, 398–400. [Google Scholar] [CrossRef]
- Lim, D.; Bekhor, P.; Webber, S.; Manohara, S.; Rodrigues, M.; Cartmill, A. BTX Injections for Excessive Sweating. Available online: http://www.sweatfree.com.au/sweat-treatments/botox-underarm-sweating2012 (accessed on 25 January 2013).
- Schnider, P.; Binder, M.; Kittler, H.; Birner, P.; Starkel, D.; Wolff, K.; Auff, E. A randomized, double-blind, placebo-controlled trial of botulinum a toxin for severe axillary hyperhidrosis. Br. J. Dermatol. 1999, 140, 677–680. [Google Scholar] [CrossRef]
- Champion, R.H. Disorders of sweat glands. Textb. Dermatol. 1986, 1745–1762. [Google Scholar]
- Heckmann, M.; Ceballos-Baumann, A.O.; Plewig, G. Botulinum toxin a for axillary hyperhidrosis (excessive sweating). N. Engl. J. Med. 2001, 344, 488–493. [Google Scholar]
- Naumann, M.; Lowe, N.J. Botulinum toxin type a in treatment of bilateral primary axillary hyperhidrosis: Randomised, parallel group, double blind, placebo controlled trial. BMJ 2001, 323, 596–599. [Google Scholar] [CrossRef]
- Katara, A.N.; Domino, J.P.; Cheah, W.K.; So, J.B.; Ning, C.; Lomanto, D. Comparing t2 and t2-t3 ablation in thoracoscopic sympathectomy for palmar hyperhidrosis: A randomized control trial. Surg. Endosc. 2007, 21, 1768–1771. [Google Scholar]
- Odderson, I.R. Long-term quantitative benefits of botulinum toxin type a in the treatment of axillary hyperhidrosis. Dermatol. Surg. 2002, 28, 480–483. [Google Scholar] [CrossRef]
- Lowe, N.J.; Glaser, D.A.; Eadie, N.; Daggett, S.; Kowalski, J.W.; Lai, P.Y. Botulinum toxin type a in the treatment of primary axillary hyperhidrosis: A 52-week multicenter double-blind, randomized, placebo-controlled study of efficacy and safety. J. Am. Acad. Dermatol. 2007, 56, 604–611. [Google Scholar] [CrossRef]
- Vadoud-Seyedi, J.; Simonart, T. Treatment of axillary hyperhidrosis with botulinum toxin type a reconstituted in lidocaine or in normal saline: A randomized, side-by-side, double-blind study. Br. J. Dermatol. 2007, 156, 986–989. [Google Scholar] [CrossRef]
- Gulec, A.T. Dilution of botulinum toxin a in lidocaine vs. in normal saline for the treatment of primary axillary hyperhidrosis: A double-blind, randomized, comparative preliminary study. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 314–318. [Google Scholar]
- Talarico-Filho, S.; Mendonça DO Nascimento, M.; Sperandeo DE Macedo, F.; De Sanctis Pecora, C. A double-blind, randomized, comparative study of two type a botulinum toxins in the treatment of primary axillary hyperhidrosis. Dermatol. Surg. 2007, 33, S44–S50. [Google Scholar] [CrossRef]
- Dressler, D. Comparing botox and xeomin for axillar hyperhidrosis. J. Neural Transm. 2010, 117, 317–319. [Google Scholar] [CrossRef]
- Lowe, N.J.; Yamauchi, P.S.; Lask, G.P.; Patnaik, R.; Iyer, S. Efficacy and safety of botulinum toxin type a in the treatment of palmar hyperhidrosis: A double-blind, randomized, placebo-controlled study. Dermatolog. Surg. 2002, 28, 822–827. [Google Scholar]
- Baumann, L.; Slezinger, A.; Halem, M.; Vujevich, J.; Martin, L.K.; Black, L.; Bryde, J. Pilot study of the safety and efficacy of myobloc (botulinum toxin type b) for treatment of axillary hyperhidrosis. Int. J. Dermatol. 2005, 44, 418–424. [Google Scholar] [CrossRef]
- Simonetta Moreau, M.; Cauhepe, C.; Magues, J.P.; Senard, J.M. A double-blind, randomized, comparative study of dysport vs. Botox in primary palmar hyperhidrosis. Br. J. Dermatol. 2003, 149, 1041–1045. [Google Scholar] [CrossRef]
- Laage-Hellman, J.E. Gustatory sweating and flushing after conservative parotidectomy. Acta Oto Laryngol. 1957, 48, 234–252. [Google Scholar] [CrossRef]
- Eckardt, A.; Kuettner, C. Treatment of gustatory sweating (frey’s syndrome) with botulinum toxin a. Head Neck 2003, 25, 624–628. [Google Scholar] [CrossRef]
- Haxton, H.A. Gustatory sweating. Brain 1948, 71, 16–25. [Google Scholar] [CrossRef]
- Naumann, M.; Zellner, M.; Toyka, K.V.; Reiners, K. Treatment of gustatory sweating with botulinum toxin. Ann. Neurol. 1997, 42, 973–975. [Google Scholar] [CrossRef]
- Santana-Rodriguez, N.; Clavo-Varas, B.; Ponce-Gonzalez, M.A.; Jarabo-Sarceda, J.R.; Perez-Alonso, D.; Ruiz-Caballero, J.A.; Olmo-Quintana, V.; Atallah Yordi, N.; Fiuza-Perez, M.D. Primary frontal hyperhidrosis successfully treated with low doses of botulinum toxin a as a useful alternative to surgical treatment. J. Dermatolog. Treat. 2012, 23, 49–51. [Google Scholar] [CrossRef]
- Furlan, A.D.; Mailis, A.; Papagapiou, M. Are we paying a high price for surgical sympathectomy? A systematic literature review of late complications. J. Pain 2000, 1, 245–257. [Google Scholar] [CrossRef]
- Riolo, J.; Gumucio, C.A.; Young, A.E.; Young, V.L. Surgical management of palmar hyperhidrosis. South. Med. J. 1990, 83, 1138–1143. [Google Scholar]
- Stolman, L.P. Treatment of hyperhidrosis. Dermatol. Clin. 1998, 16, 863–869. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lakraj, A.-A.D.; Moghimi, N.; Jabbari, B. Hyperhidrosis: Anatomy, Pathophysiology and Treatment with Emphasis on the Role of Botulinum Toxins. Toxins 2013, 5, 821-840. https://doi.org/10.3390/toxins5040821
Lakraj A-AD, Moghimi N, Jabbari B. Hyperhidrosis: Anatomy, Pathophysiology and Treatment with Emphasis on the Role of Botulinum Toxins. Toxins. 2013; 5(4):821-840. https://doi.org/10.3390/toxins5040821
Chicago/Turabian StyleLakraj, Amanda-Amrita D., Narges Moghimi, and Bahman Jabbari. 2013. "Hyperhidrosis: Anatomy, Pathophysiology and Treatment with Emphasis on the Role of Botulinum Toxins" Toxins 5, no. 4: 821-840. https://doi.org/10.3390/toxins5040821




