Influence of Fermentation and Drying Materials on the Contamination of Cocoa Beans by Ochratoxin A
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analytical Method
2.2. Collection of Samples
2.3. OTA Content in Different Cocoa Samples
2.4. Pod-Opening
| Range | Area | |||||
|---|---|---|---|---|---|---|
| Average | Min | Max | Abengourou ( n = 4) | Gagnoa ( n = 4) | San Pedro ( n = 9) | |
| OTA level (µg/kg) | 0.038 ± 0.025 (12/17) | <LD | 0.1 | 0.056 ± 0.04 a | 0.052 ± 0.023 a | 0.025 ± 0.026 a |
2.5. Fermentation
| Range | Fermentation material | |||||
|---|---|---|---|---|---|---|
| Average | Min | Max | Tarpaulins ( n = 17) | Banana leaves ( n = 17) | Boxes( n = 17) | |
| OTA level (µg/kg) | 0.275 ± 0.26 (29/51) | <LD | 2.1 | 0.366 ± 0.39 a | 0.23 ± 0.15 a | 0.228 ± 0.22 a |
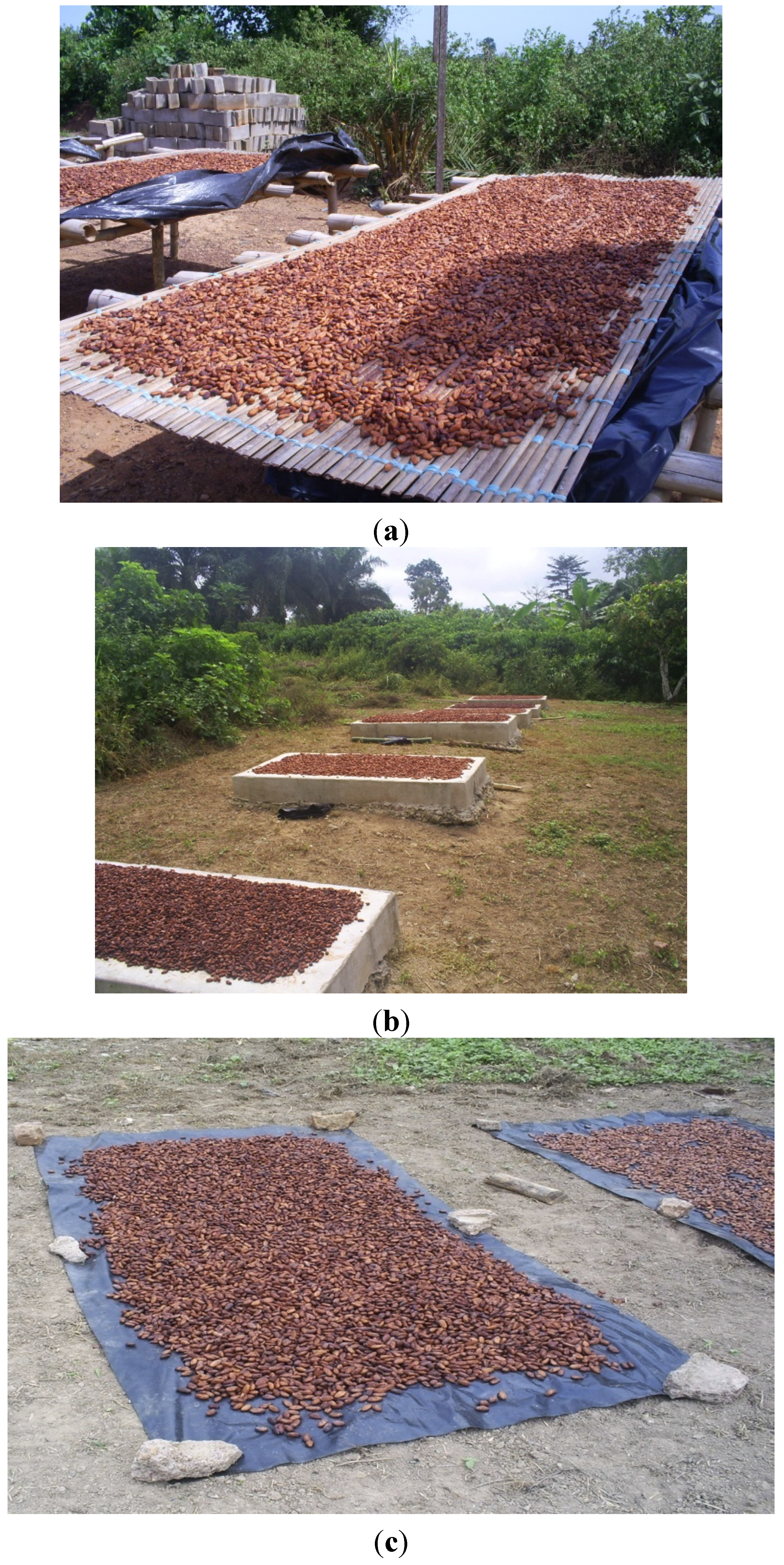
2.6. Drying
| Range | Drying platform | |||||
|---|---|---|---|---|---|---|
| Average | Min | Max | Rack | Black tarpaulin | Cement floor | |
| OTA level (µg/kg) | 0.569 ± 0.3 | <LD | 13.4 | 0.459 ± 0.04 a | 0.665 ± 0.023 a | 0.584 ± 0.026 a |
2.7. Storage
| Rack | Black tarpaulin | Cement floor | |
|---|---|---|---|
| OTA level (µg/kg) | 0.600 ± 0.025 a | 0.577 ± 0.032 a | 0.419 ± 0.29 a |
| Average level at the end of storage (µg/kg) | 0.558 ± 0.3 | ||
2.8. General Assessment of Contamination during Post-Harvest Operations
| Pod-opening | Fermentation | Drying | Storage | |
|---|---|---|---|---|
| OTA level (µg/kg) | 0.038 ± 0.02 a | 0.28 ± 0.26 a | 0.57 ± 0.3 b | 0.56 ± 0.3 b |
3. Experimental Section
3.1. Study Sites
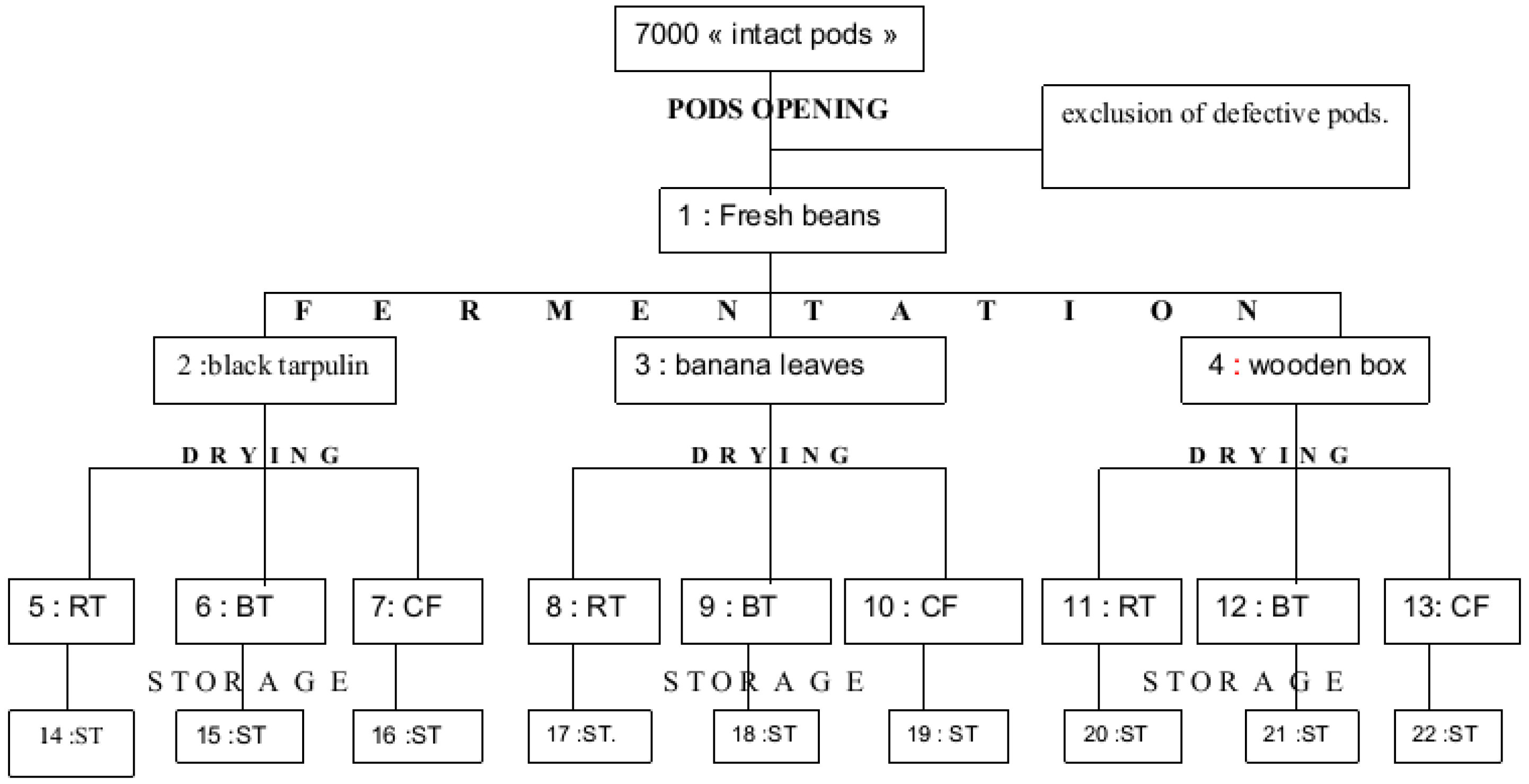
3.2. Preparation of Cocoa Samples—Bean Collection

3.3. Post-Harvesting Operations
3.3.1. Pod-Breaking
3.3.2. Fermentation



3.3.3. Drying
3.3.4. Storage
3.4. Treatment of Samples in the Laboratory

3.5. Determination of OTA—Extraction, Detection, and Quantification of OTA in Samples
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Suarez-Quiroz, M.; Gonzalez-Rios, O.; Barel, M.; Guyot, B.; Schorr-Galindo, S.; Guiraud, J. Effect of the post-harvest processing procedure on OTA occurrence in artificially contaminated coffee. Int. J. Food Microbiol. 2005, 103, 339–345. [Google Scholar]
- Kuiper-Goodman, T.; Scott, P.M. Risk assessment of the mycotoxin Ochratoxin A. Biom. Biomed. Environ. Sci. 1989, 2, 179–248. [Google Scholar]
- Jorgensen, K. Survey of pork, poultry, coffee, beer and pulses for Ochratoxin A. Food Addit. Contam. 1998, 15, 550–554. [Google Scholar] [CrossRef]
- Sangare-Tigori, B.; Moukha, S.; Kouadio, J.H.; Dano, D.S.; Betbeder, A.M.; Achour, A.; Creppy, E.E. Ochratoxin A in human blood in Abidjan, Côte d’Ivoire. Toxicon 2006, 47, 894–900. [Google Scholar]
- Chiavaro, E.; Lepiani, A.; Colla, F.; Bettoni, P.; Pari, E.; Spotti, E. Ochratoxin A determination in ham by immunoafïinitty clean up and quick fluorimetric method. Food Addit. Contam. 2002, 19, 575–581. [Google Scholar]
- Miraglia, M.; Brera, C. Assessment of Dietary Intake of Ochratoxin A by the Population of EU Member States; Report of Experts Participating in Task 3.2.7. Istituto Superiore di Sanità: Rome, Italy, 2002. [Google Scholar]
- Burdaspal, P.A.; Legarda, T.M. Ochratoxin A in samples of different types of chocolate and cacao powder, marketed in Spain and fifteen foreign countries. Alimentaria 2003, 347, 143–153. [Google Scholar]
- Krogh, P.; Axelsen, N.H.; Elling, F.; Gyrd-Hansen, N.; Hald, B.; Hyldegaard-Hensen, J.; Larsen, A.E.; Madsen, A.; Mortensen, H.P.; Möller, T.; et al. Experimental porcine nephropathy: Changes of renal function and structure per orally induced by christalline Ochratoxin A. Acta Pathol. Microbiol. Scand. A 1974, 246, 1–21. [Google Scholar]
- EFSA. Opinion of the scientific panel on contaminants in food chain on a request from the commission related to ochratoxin A in food. EFSA J. 2006, 365, 1–56. [Google Scholar]
- International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; IARC Working Group, WHO: Lyon, France, 1993; Volume 56. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives (1995: Rome, Italy); World Health Organization; Food and Agriculture Organization of the United Nations. Evaluation of Certain Food Additives and Contaminants. Forty-fourth Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 1995. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives. Safety Evaluation of Certain Mycotoxins in Food/Prepared by the Fifty-Sixth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); FAO: Rome, Italy, 2001. [Google Scholar]
- O’Brien, M.; Nielsen, K.F.; O’Kiely, P.; Forristal, P.D.; Fuller, H.T.; Frisvad, J.C. Mycotoxins and other secondary metabolites produced in vitro by Penicillium paneum Frisvad and Penicillium roqueforti Thom isolated from baled grass silage in Ireland. J. Agric. Food Chem. 2006, 54, 9268–9276. [Google Scholar]
- The Commission of the European Communities (EC). Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 4–24. [Google Scholar]
- The Commission of the European Communities (EC). Commission Regulation (EU) No 105/2010 of 5 February 2010 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards ochratoxin A. Off. J. Eur. Union 2010, L035, 7–8. [Google Scholar]
- Bonvehi, S.J. Occurrence of ochratoxin A in cocoa products and chocolate. J. Agric. Food Chem. 2004, 52, 6347–6352. [Google Scholar]
- Amézqueta, S.; Gonzalez-Penas, E.; Murillo, M.; Lopez de Cerain, A. Occurrence of ochratoxin A in cocoa beans: Effect of shelling. Food Addit. Contam. 2005, 22, 590–595. [Google Scholar]
- Dembele, A.; Gerard, F.; Manda, P.; Nemlin, J.G. Résultats des Études de Recherché du Devis Programme OTA No. DP/IVC/2005/16: Contamination du Café et du Cacao par l’Ochratoxine A (OTA) en Côte d’Ivoire; Rapport de Synthèse 2007; Ministère de l’Agriculture, Direction Générale des Productions et de la Diversification Agricoles: Abidjan, Côte d’Ivoire, 2007. [Google Scholar]
- Dembele, A.; Coulibaly, A.; Traoré, S.K.; Mamadou, K.; Silue, N.; Abba, T. Détermination du niveau de contamination de l’ochratoxine A (OTA) dans les fèves de cacao à l’exportation. Tropicultura 2009, 27, 26–30. [Google Scholar]
- Dongo, L.; Bandyopadhyay, R.; Kumar, M.; Ojiambo, P.S. Occurrence of ochratoxin A in Nigerian ready for sale cocoa beans. Agric. J. 2008, 3, 4–9. [Google Scholar]
- Gilmour, M.; Lindblom, M. Management of Ochratoxin A in the Cocoa Supply Chain: A Summary of Work by the CAOBISCO/ECA/FCC Working Group on Ochratoxine A. In Mycotoxins: Detection Methods, Management, Public Health and Agricultural Trade; Leslie, R., Bandyopadhyay, R., Visconti, A., Eds.; CABI: Wallinford, CT, USA, 2008; pp. 231–243. [Google Scholar]
- Copetti, M.V.; Pereira, J.L.; Lamanaka, B.T.; Pitt, J.I.; Taniwaki, M.H. Ochratoxigenic fungi and ochratoxin A in cocoa during farm processing. Int. J. Food Microbiol. 2010, 143, 67–70. [Google Scholar] [CrossRef]
- Coulibaly, A.; Dembelé, A.; Biego, G.H.M.; Silué, N.; Touré, A.A. Evolution of the merchantability and the level of ochratoxin A of ivorian cocoa beans from production areas during the harvest season. Sustain. Agric. Res. 2012, 1, 178–187. [Google Scholar]
- Coulibaly, A.; Biego, G.H.M.; Dembelé, A.; Bohoussou, K.M.; Touré, A.A. Cocoa beans and cocoa derivatives from Côte d’Ivoire: Investigating ochratoxine A level and assessing dietary intake adults. Sustain. Agric. Res. 2013, 2, 173–180. [Google Scholar]
- Bastide, P.; Fourny, G.; Durand, N.; Petithuguenin, P.; Guyot, B.; Gilmour, M.; Lindblom, M. Identification of Ochratoxin A Sources during Cocoa Post-Harvest Processing: Influence of Harvest Quality and Climatic Factors. In Proceeding of 15th Intl Cocoa Research Conference, San Jose, Costa Rica, 9–17 October 2006.
- Tafuri, A.; Ferracane, R.; Ritieni, A. Ochratoxin A in Italian marketed cocoa products. Food Chem. 2004, 88, 487–494. [Google Scholar]
- Schwan, R.F.; Wheals, A.E. The microbiology of cocoa fermentation and its role in chocolate quality. Crit. Rev. Food Sci. Nutr. 2004, 44, 1–17. [Google Scholar]
- Mounjouenpou, P.; Gueule, D.; Ntoupka, M.; Durand, N.; Fontana-Tachon, A.; Guyot, B.; Guiraud, F.P. Influence of post-harvest processing on ochratoxin A content in cocoa and on consumer exposure in Cameroon. World Mycotoxin J. 2011, 4, 141–146. [Google Scholar] [CrossRef]
- Mounjouenpou, P.; Gueule, D.; Fontana-Tachon, A.; Guyot, B.; Tondje, P.R.; Guiraud, J.P. Filamentous fungi producing ochratoxin A during cocoa processing in Cameroon. Int. J. Food Microbiol. 2008, 128, 234–241. [Google Scholar]
- Karine, L. Enquête sur les Pratiques Culturales dans les Cacaoyères en Côte d’Ivoire; Projet PACCC/ICCO/Industrie sur L’amélioration de la qualité du cacao en Côte d’Ivoire: Abidjan, Côte d’Ivoire, 2001. [Google Scholar]
- Wood, G.A.R. From Harvest to Store. In Cocoa; Wood, G.A.R., Lass, R.A., Eds.; Longman Scientific and Technical: New York, NY, USA, 1985; pp. 444–504. [Google Scholar]
- Manda, P.; Dano, D.S.; Kouadio, J.H.; Diakité, A.; Sangaré-Tigori, B.; Ezoulin, M.J.M.; Soumahoro, A.; Dembele, A.; Fourny, G. Impact of industrial treatments on ochratoxin A content in artificially contaminated cocoa beans. Food Addit. Contam. Part A 2009, 26, 1081–1088. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dano, S.D.; Manda, P.; Dembélé, A.; Kouassi Abla, A.M.-J.; Bibaud, J.H.; Gouet, J.Z.; Ze Maria Sika, C.B. Influence of Fermentation and Drying Materials on the Contamination of Cocoa Beans by Ochratoxin A. Toxins 2013, 5, 2310-2323. https://doi.org/10.3390/toxins5122310
Dano SD, Manda P, Dembélé A, Kouassi Abla AM-J, Bibaud JH, Gouet JZ, Ze Maria Sika CB. Influence of Fermentation and Drying Materials on the Contamination of Cocoa Beans by Ochratoxin A. Toxins. 2013; 5(12):2310-2323. https://doi.org/10.3390/toxins5122310
Chicago/Turabian StyleDano, Sébastien Djédjé, Pierre Manda, Ardjourma Dembélé, Ange Marie-Joseph Kouassi Abla, Joel Henri Bibaud, Julien Zroh Gouet, and Charles Bruno Ze Maria Sika. 2013. "Influence of Fermentation and Drying Materials on the Contamination of Cocoa Beans by Ochratoxin A" Toxins 5, no. 12: 2310-2323. https://doi.org/10.3390/toxins5122310




