Incidence of Fusarium Species and Mycotoxins in Silage Maize
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
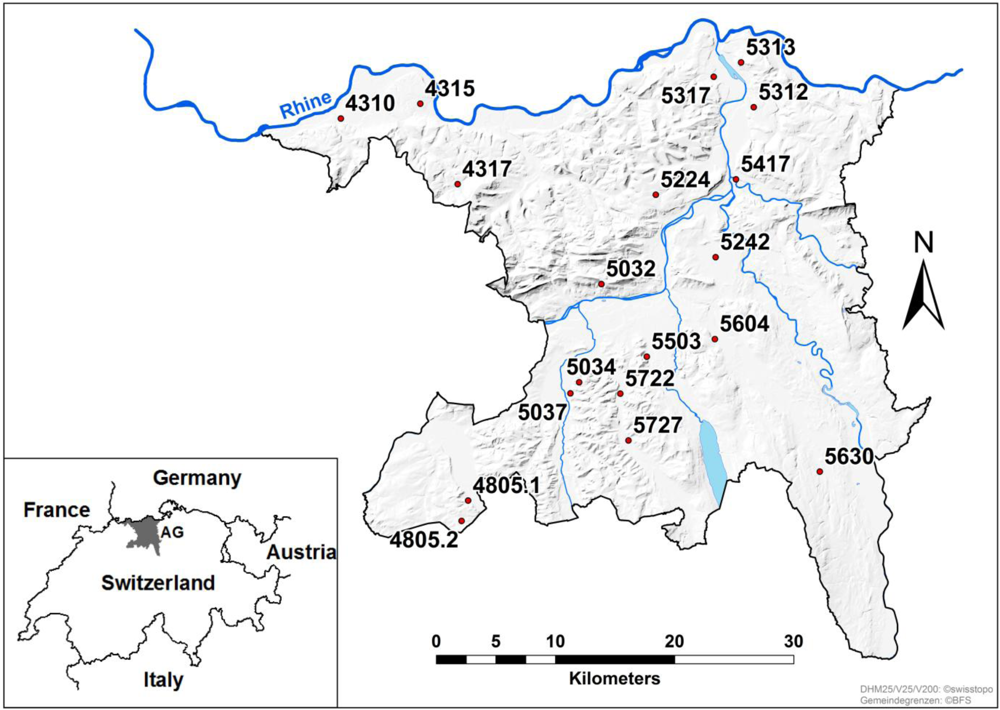
2.2. Pre-Harvest Disease Rating
2.3. Isolation and Identification of Fusarium Species from Maize Particles
2.4. Toxin Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Fusarium Species Spectrum on Silage Maize and Power of Prediction of Infection by the Pre-Harvest Disease Rating
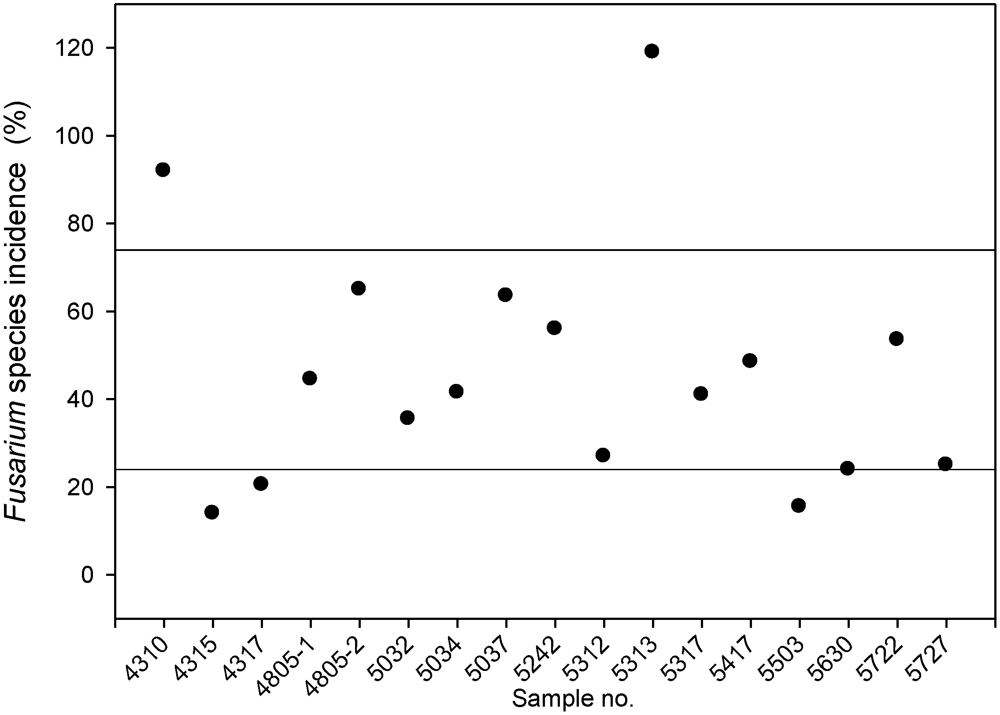
| Sample no. (postal codes) | F. sporotrichioides | F. verticillioides | F. graminearum | F. avenaceum | F. proliferatum | F. equiseti | F. poae | F. oxysporum | F. crookwellense | F. subglutinans | F. culmorum | F. tricinctum | F. spp.1 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4310 | 51 | 24 | 7 | 3 | 42 | 28 | 1 | 15 | 3 | 4 | 0 | 0 | 6 | 184 |
| 4315 | 1 | 6 | 4 | 6 | 3 | 0 | 3 | 2 | 1 | 0 | 0 | 0 | 2 | 28 |
| 4317 | 6 | 4 | 1 | 7 | 1 | 14 | 0 | 0 | 6 | 0 | 0 | 0 | 2 | 41 |
| 4805-1 | 12 | 24 | 2 | 1 | 1 | 25 | 1 | 13 | 5 | 0 | 0 | 0 | 5 | 89 |
| 4805-2 | 19 | 8 | 18 | 27 | 9 | 18 | 14 | 3 | 5 | 0 | 1 | 1 | 7 | 130 |
| 5032 | 27 | 2 | 13 | 15 | 3 | 1 | 1 | 2 | 1 | 3 | 0 | 1 | 2 | 71 |
| 5034 | 31 | 16 | 1 | 0 | 29 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 4 | 83 |
| 5037 | 20 | 21 | 23 | 5 | 21 | 10 | 3 | 7 | 2 | 1 | 2 | 0 | 12 | 127 |
| 5242 | 19 | 10 | 25 | 13 | 4 | 16 | 6 | 1 | 2 | 1 | 0 | 0 | 15 | 112 |
| 5312 | 6 | 7 | 6 | 6 | 3 | 10 | 2 | 0 | 4 | 2 | 0 | 0 | 8 | 54 |
| 5313 | 18 | 47 | 26 | 40 | 29 | 6 | 32 | 1 | 8 | 4 | 0 | 1 | 26 | 238 |
| 5317 | 4 | 9 | 36 | 16 | 0 | 5 | 0 | 0 | 0 | 2 | 2 | 0 | 8 | 82 |
| 5417 | 16 | 22 | 15 | 7 | 8 | 1 | 1 | 0 | 8 | 3 | 1 | 1 | 14 | 97 |
| 5503 | 5 | 4 | 3 | 11 | 1 | 0 | 0 | 0 | 2 | 3 | 0 | 2 | 0 | 31 |
| 5630 | 0 | 39 | 4 | 0 | 2 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 48 |
| 5722 | 8 | 4 | 53 | 20 | 3 | 0 | 4 | 0 | 1 | 0 | 2 | 1 | 11 | 107 |
| 5727 | 14 | 6 | 8 | 2 | 12 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 6 | 50 |
| Total | 257 | 253 | 245 | 179 | 171 | 135 | 68 | 47 | 49 | 24 | 8 | 7 | 129 | 1572 |
| % | 16.3 | 16.1 | 15.6 | 11.4 | 10.9 | 8.6 | 4.3 | 3.0 | 3.1 | 1.5 | 0.5 | 0.4 | 8.2 | 100 |
| Mean | 15.1 | 14.9 | 14.4 | 10.5 | 10.1 | 7.9 | 4.0 | 2.8 | 2.9 | 1.4 | 0.5 | 0.4 | 7.6 | 92.5 |
| SEM2 | 3.7 | 3.6 | 3.5 | 2.6 | 2.4 | 1.9 | 1.0 | 0.7 | 0.7 | 0.3 | 0.1 | 0.1 | 1.8 | 22.4 |
| Rank | 1 | 2 | 3 | 4 | 5 | 6 | 8 | 9 | 9 | 10 | 11 | 12 | 7 |
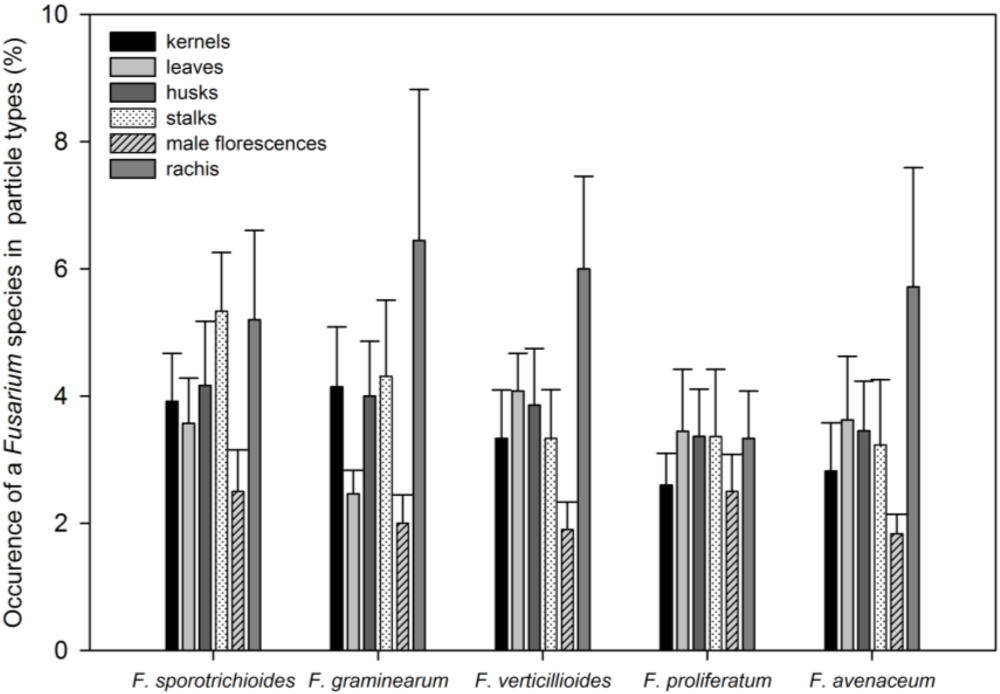
3.2. Toxin Spectra and Correlations between Fusarium Species and Toxins
| Toxin(µg kg−1) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample NO. | DON | NIV | AcDON | HT-2 | T-2 | ZON | ||||||
| 4310 | 930 | 190 | nd | 72 | 26 | d | ||||||
| 4315 | 780 | 200 | nd | nd | d | d | ||||||
| 4317 | 1130 | 560 | d | nd | 40 | nd | ||||||
| 4805-1 | 780 | 380 | nd | 130 | 42 | 97 | ||||||
| 4805-2 | 2190 | nd | nd | 120 | 84 | 430 | ||||||
| 5032 | 1080 | 700 | nd | 76 | 31 | 88 | ||||||
| 5034 | 860 | 690 | nd | nd | 14 | d | ||||||
| 5037 | 850 | nd | nd | nd | 16 | 94 | ||||||
| 5224 | 900 | nd | nd | 76 | nd | d | ||||||
| 5242 | 1030 | 690 | nd | nd | nd | nd | ||||||
| 5312 | 1590 | nd | nd | nd | nd | nd | ||||||
| 5313 | 2600 | nd | d | nd | nd | d | ||||||
| 5317 | 2990 | 760 | 135 | nd | nd | 260 | ||||||
| 5417 | 2240 | nd | 300 | nd | nd | 280 | ||||||
| 5503 | 810 | nd | nd | nd | nd | 150 | ||||||
| 5604 | 1650 | nd | nd | nd | nd | 230 | ||||||
| 5630 | 950 | nd | nd | nd | nd | nd | ||||||
| 5722 | 1250 | nd | nd | nd | nd | 83 | ||||||
| 5727 | 1160 | nd | nd | nd | nd | 97 | ||||||
| Mean | 1356 | 521.3 | 217.5 | 94.8 | 36.14 | 180.9 | ||||||
| EU Guidance Level | 900 1–12000 2 | n.a. | n.a. | n.a. | n.a. | 100 1–3000 2 | ||||||

3.3. Prediction of DON Content by Cropping Factors with a Regression Model
| Step | Factors | AICc | R2 | |||
|---|---|---|---|---|---|---|
| 1 | Harvest date | 302.2 | 0.26 | |||
| 2 | Harvest date | Hybrid | 308.0 | 0.31 | ||
| Harvest date | O. nubilalis | 299.8 | 0.45 | |||
| Harvest date | Precrop | 308.2 | 0.44 | |||
| Harvest date | Pre-precrop | 285.8 | 0.55 | |||
| Harvest date | Soil cultivation | 305.1 | 0.27 | |||
| Harvest date | Seed bed Prep. | 325.3 | 0.50 | |||
| 3 | Harvest date | Pre-precrop | Hybrid | 295.9 | 0.56 | |
| Harvest date | Pre-precrop | O. nubilalis | 285.6 | 0.66 | ||
| Harvest date | Pre-precrop | Precrop | 301.9 | 0.58 | ||
| Harvest date | Pre-precrop | Soil cultivation | 286.2 | 0.65 | ||
| Harvest date | Pre-precrop | Seed bed Prep. | 328.0 | 0.73 | ||
| Harvest date | Pre-precrop | Seed treatm. | 274.6 | 0.61 | ||
| 4 | Harvest date | Pre-precrop | Seed treatm. | Hybrid | 286.5 | 0.64 |
| Harvest date | Pre-precrop | Seed treatm. | O. nubilalis | 277.9 | 0.67 | |
| Harvest date | Pre-precrop | Seed treatm. | Precrop | 281.7 | 0.64 | |
| Harvest date | Pre-precrop | Seed treatm. | Soil cultivation | 275.4 | 0.71 | |
| Harvest date | Pre-precrop | Seed treatm. | Seed bed prep. | 346.2 | 0.85 |

4. Conclusions
Acknowledgments
Conflict of Interest
References
- Fulgueira, C.L.; Amigot, S.L.; Gaggiotti, M.; Romero, L.A.; Basilico, J.C. Forage quality: Techniques for testing. Fresh Prod. 2007, 1, 121–131. [Google Scholar]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Logrieco, A.; Mule, G.; Moretti, A.; Bottalico, A. Toxigenic Fusarium species and mycotoxins associated with maize ear rot in Europe. Eur. J. Plant Pathol. 2002, 108, 597–609. [Google Scholar] [CrossRef]
- Garon, D.; Richard, E.; Sage, L.; Bouchart, V.; Pottier, D.; Lebailly, P. Mycoflora and multimycotoxin detection in corn silage: Experimental study. J. Agric. Food Chem. 2006, 54, 3479–3484. [Google Scholar]
- Mansfield, M.A.; Kuldau, G.A. Microbiological and molecular determination of mycobiota in fresh and ensiled maize silage. Mycologia 2007, 99, 269–278. [Google Scholar]
- Wilkinson, J.M. Silage and animal health. Nat. Toxins 1999, 7, 221–232. [Google Scholar]
- Scudamore, K.A.; Livesey, C.T. Occurrence and significance of mycotoxins in forage crops and silage: A review. J. Sci. Food Agric. 1998, 77, 1–17. [Google Scholar]
- Dorn, B.; Forrer, H.-R.; Schürch, S.; Vogelgsang, S. Fusarium species complex on maize in Switzerland: Occurrence, prevalence, impact and mycotoxins in commercial hybrids under natural infection. Eur. J. Plant Pathol. 2009, 125, 51–61. [Google Scholar] [CrossRef]
- Richard, E.; Heutte, N.; Sage, L.; Pottier, D.; Bouchart, V.; Lebailly, P.; Garon, D. Toxigenic fungi and mycotoxins in mature corn silage. Food Chem. Toxicol. 2007, 45, 2420–2425. [Google Scholar]
- Oldenburg, E.; Ellner, F. Fusarium mycotoxins in forage maize—Detection and evaluation. Mycotoxin Res. 2005, 21, 105–107. [Google Scholar] [CrossRef]
- Scauflaire, J.; Mahieu, O.; Louvieaux, J.; Foucart, G.; Renard, F.; Munaut, F. Biodiversity of Fusarium species in ears and stalks of maize plants in Belgium. Eur. J. Plant Pathol. 2011.
- Parry, D.W.; Jenkinson, P.; McLeod, L. Fusarium ear blight (scab) in small grain cereals—a review. Plant Pathol. 1995, 44, 207–238. [Google Scholar]
- Görtz, A.; Zuehlke, S.; Spiteller, M.; Steiner, U.; Dehne, H.; Waalwijk, C.; de Vries, I.; Oerke, E. Fusarium species and mycotoxin profiles on commercial maize hybrids in Germany. Eur. J. Plant Pathol. 2010, 128, 101–111. [Google Scholar]
- Reid, L.M.; Nicol, R.W.; Ouellet, T.; Savard, M.; Miller, J.D.; Young, J.C.; Stewart, D.W.; Schaafsma, A.W. Interaction of Fusarium graminearum and F. moniliforme in maize ears: Disease progress, fungal biomass, and mycotoxin accumulation. Phytopathology 1999, 89, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual, 1st ed; Wiley-Blackwell: Hoboken, NJ, USA, 2006. [Google Scholar]
- European Commission. Commission regulation (EC) setting maximum levels for certain contaminants in foodstuffs. No 1881/2006. Off. J. Eur. Union. 2006, L364, pp. 25–31. Available online: http://www.minagric.gr/en/data/Reg%28EC%29%201882_2006.pdf (accessed on 20 July 2011).
- European Commission. Commission regulation (EC) amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. No 1126/2007. Off. J. Eur. Union. 2007, L255, pp. 14–17. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:255:0014:0017:EN:PDF (accessed on 20 July 2011).
- European Commission. Commission recommendation on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding No 2006/576/EC. Off. J. Eur. Union. 2006, L229, pp. 7–9. Available online: http://eur-lex.europa.eu/LexUriServ/site/en/oj/2006/l_229/l_22920060823en00070009.pdf (accessed on 20 July 2011).
- Zöllner, P.; Jodlbauer, J.; Kleinova, M.; Kahlbacher, H.; Kuhn, T.; Hochsteiner, W.; Lindner, W. Concentration levels of zearalenone and its metabolites in urine, muscle tissue, and liver samples of pigs fed with mycotoxin-contaminated oats. J. Agric. Food Chem. 2002, 50, 2494–2501. [Google Scholar] [CrossRef] [PubMed]
- Fink-Gremmels, J. Mycotoxins in cattle feeds and carry-over to dairy milk: A review. Food Addit. Contam. Part A 2008, 25, 172–180. [Google Scholar]
- Collection of Occurrence Data of Fusarium Toxins in Food and Assessment of Dietary Intake by the Population of EU Member State. In SCOOP Task 3.2.10; European Commission: Brussels, Belgium, April 2003.
- Vogelgsang, S.; Hecker, A.; Musa, T.; Dorn, B.; Forrer, H.R. On-farm experiments over five years in a grain maize-winter wheat rotation: Effect of maize residue treatments on Fusarium graminearum infection and deoxynivalenol contamination in wheat. Mycotoxin Res. 2011, 27, 81–96. [Google Scholar] [CrossRef]
- Blandino, M.; Reyneri, A.; Vanara, F. Effect of sowing time on toxigenic fungal infection and mycotoxin contamination of maize kernels. J. Phytopathol. 2009, 157, 7–14. [Google Scholar]
- Blandino, M.; Reyneri, A.; Vanara, F.; Tamietti, G.; Pietri, A. Influence of agricultural practices on Fusarium infection, fumonisin and deoxynivalenol contamination of maize kernels. World Mycotoxin J. 2009, 2, 409–418. [Google Scholar] [CrossRef]
- Schaafsma, A.W.; Tamburic-Ilincic, L.; Hooker, D.C. Effect of previous crop, tillage, field size, adjacent crop, and sampling direction on airborne propagules of Gibberella zeae/Fusarium graminearum, fusarium head blight severity, and deoxynivalenol accumulation in winter wheat. Can. J. Plant Pathol. 2005, 27, 217–224. [Google Scholar] [CrossRef]
- Oldenburg, E.; Brunotte, J.; Weinert, J. Strategies to reduce DON contamination of wheat with different soil tillage and variety systems. Mycotoxin Res. 2007, 23, 73–77. [Google Scholar]
- Lauren, D.R.; Di Menna, M.E.; Smith, W.A. Effects of temperature on fusaria and Fusarium mycotoxins during short-term storage of maize kernels (Zea mays). N. Z. J. Crop Hortic. Sci. 2004, 32, 77–84. [Google Scholar] [CrossRef]
- ArcMap, 9.3; Environmental Systems Research Institute: Zürich, Switzerland, 2008.
- Reid, L.M.; Hamilton, R.I.; Mather, D.E. Screening Maize for Resistence to Gibberella Ear Rot; Research Branch Agriculture and Agri-Food Canada: Ottawa, Canada, 1996; pp. 1–37. [Google Scholar]
- Papavizas, C.G. Evaluation of various media and antimicrobial agents for isolation of Fusarium from soil. Phytopathology 1967, 57, 848–852. [Google Scholar]
- Nirenberg, H. Differenzierung der erreger der Halmbruchkrankheit. 1 morphologie. J. Plant Dis. Prot. 1981, 88, 241–248. [Google Scholar]
- Dorn, B.; Forrer, H.R.; Jenny, E.; Wettstein, F.E.; Bucheli, T.D.; Vogelgsang, S. Fusarium species complex and mycotoxins in grain maize from maize hybrid trials and from grower’s fields. J. Appl. Microbiol. 2011. [Google Scholar]
- SigmaStat3.5®, 3.5; Systat Software, Inc.: Point Richmond, CA, USA, 2006.
- R, 2.10.0; The R Foundation for Statistical Computing: Vienna, Austria, 2010.
- Hurvich, C.M.; Tsai, C. Regression and time series model selection in small samples. Biometrika 1989, 76, 297–307. [Google Scholar]
- Mazerolle, M.J. AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c), R package version 1.1. Available online: http://crantastic.org/packages/AICcmodavg/versions/11253 (accessed on 20 July 2011).
- Bacon, C.W.; Hinton, D.M. Symptomless endophytic colonization of maize by Fusarium moniliforme. Can. J. Bot. 1996, 74, 144–148. [Google Scholar]
- Bacon, C.W.; Yates, I.E.; Hinton, D.M.; Meredith, F. Biological control of Fusarium moniliforme in maize. Environ. Health Perspect. 2001, 109 Suppl 2, 325–332. [Google Scholar]
- Munkvold, G.P.; Hellmich, R.L.; Showers, W.B. Reduced Fusarium ear rot and symptomless infection in kernels of maize genetically engineered for European corn borer resistance. Phytopathology 1997, 87, 1071–1077. [Google Scholar]
- Vogelgsang, S. Personal communication. Agroscope Reckenholz-Tänikon Research Station: Zurich, Switzerland, 2011. [Google Scholar]
- Sutton, J.C.; Baliko, W.; Liu, H.J. Fungal colonization and zearalenone accumulation in maize ears injured by birds. Can. J. Plant Sci. 1980, 60, 453–461. [Google Scholar]
- Reid, L.M.; Sinha, R.C. Maize maturity and the development of gibberella ear rot symptoms and deoxynivalenol after inoculation. Eur. J. Plant Pathol. 1998, 104, 147–154. [Google Scholar]
- Vogelgsang, S.; Jenny, E.; Hecker, A.; Bänziger, I.; Forrer, H.R. Fusaria and mycotoxins in wheat-monitoring of harvest samples from grower’s fields. Agrarforschung 2009, 16, 238–242. [Google Scholar]
- Weigand, S. Personal communication. Bavarian State Research Center for Agriculture: Weihenstephan, Germany, 2011. [Google Scholar]
- Speijers, G.J.A.; Speijers, M.H.M. Combined toxic effects of mycotoxins. Toxicol. Lett. 2004, 153, 91–98. [Google Scholar]
- Cooney, J.M.; Lauren, D.R.; Di Menna, M.E. Impact of competitive fungi on trichothecene production by Fusarium graminearum. J. Agric. Food Chem. 2001, 49, 522–526. [Google Scholar] [CrossRef]
- Ueno, Y. Trichothecenes-Chemical, Biological and Toxicological Aspects; Elsevier Science Ltd.: Amsterdam, The Netherlands, 1983. [Google Scholar]
- Berthiller, F.; Schuhmacher, R.; Adam, G.; Krska, R. Formation, determination and significance of masked and other conjugated mycotoxins. Anal. Bioanal. Chem. 2009, 395, 1243–1252. [Google Scholar]
- Gareis, M.; Bauer, J.; Thiem, J.; Plank, G.; Grabley, S.; Gedek, B. Cleavage of Zearalenone-glycoside, a “masked” mycotoxin, during digestion in swine. J. Vet. Med. Ser. B 1990, 37, 236–240. [Google Scholar] [CrossRef]
- Cotten, T.K.; Munkvold, G.P. Survival of Fusarium moniliforme, F. proliferatum, and F. subglutinans in maize stalk residue. Phytopathology 1998, 88, 550–555. [Google Scholar] [PubMed]
- BayerCropScience Mesurol® flüssig inkrustiertes Maissaatgu. Available online: http://www.bayercropscience.de/schnellinformation.cms?ProductId=b62d81ff-dacb-4e9c-b73c-9e3300f0c692 (accessed on 20 July 2011).
- Freedman, D.A. A note on screening regression equations. Am. Stat. 1983, 37, 152–155. [Google Scholar]
- Calcagno, V.; de Mazancourt, C. glmulti: An R package for easy automated model selection with (generalized) linear models. J. Stat. Softw. 2010, 34, 1–29. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Eckard, S.; Wettstein, F.E.; Forrer, H.-R.; Vogelgsang, S. Incidence of Fusarium Species and Mycotoxins in Silage Maize. Toxins 2011, 3, 949-967. https://doi.org/10.3390/toxins3080949
Eckard S, Wettstein FE, Forrer H-R, Vogelgsang S. Incidence of Fusarium Species and Mycotoxins in Silage Maize. Toxins. 2011; 3(8):949-967. https://doi.org/10.3390/toxins3080949
Chicago/Turabian StyleEckard, Sonja, Felix E. Wettstein, Hans-Rudolf Forrer, and Susanne Vogelgsang. 2011. "Incidence of Fusarium Species and Mycotoxins in Silage Maize" Toxins 3, no. 8: 949-967. https://doi.org/10.3390/toxins3080949
APA StyleEckard, S., Wettstein, F. E., Forrer, H.-R., & Vogelgsang, S. (2011). Incidence of Fusarium Species and Mycotoxins in Silage Maize. Toxins, 3(8), 949-967. https://doi.org/10.3390/toxins3080949




