The Effects of Anthrax Lethal Toxin on Host Barrier Function
Abstract
:1. Introduction
2. Epithelial and Endothelial Barriers
3. Host Barriers Breached by Bacillus anthracis during Infection
4. Effect of Anthrax LT on Endothelial Barriers
4.1. Clinical Findings
4.2. Mechanisms of Action
5. Effect of Anthrax LT on Lung Epithelium
5.1. Clinical Findings
5.2. Mechanisms of Action
6. Effect of Anthrax LT on Intestinal Barriers
6.1. Clinical Findings
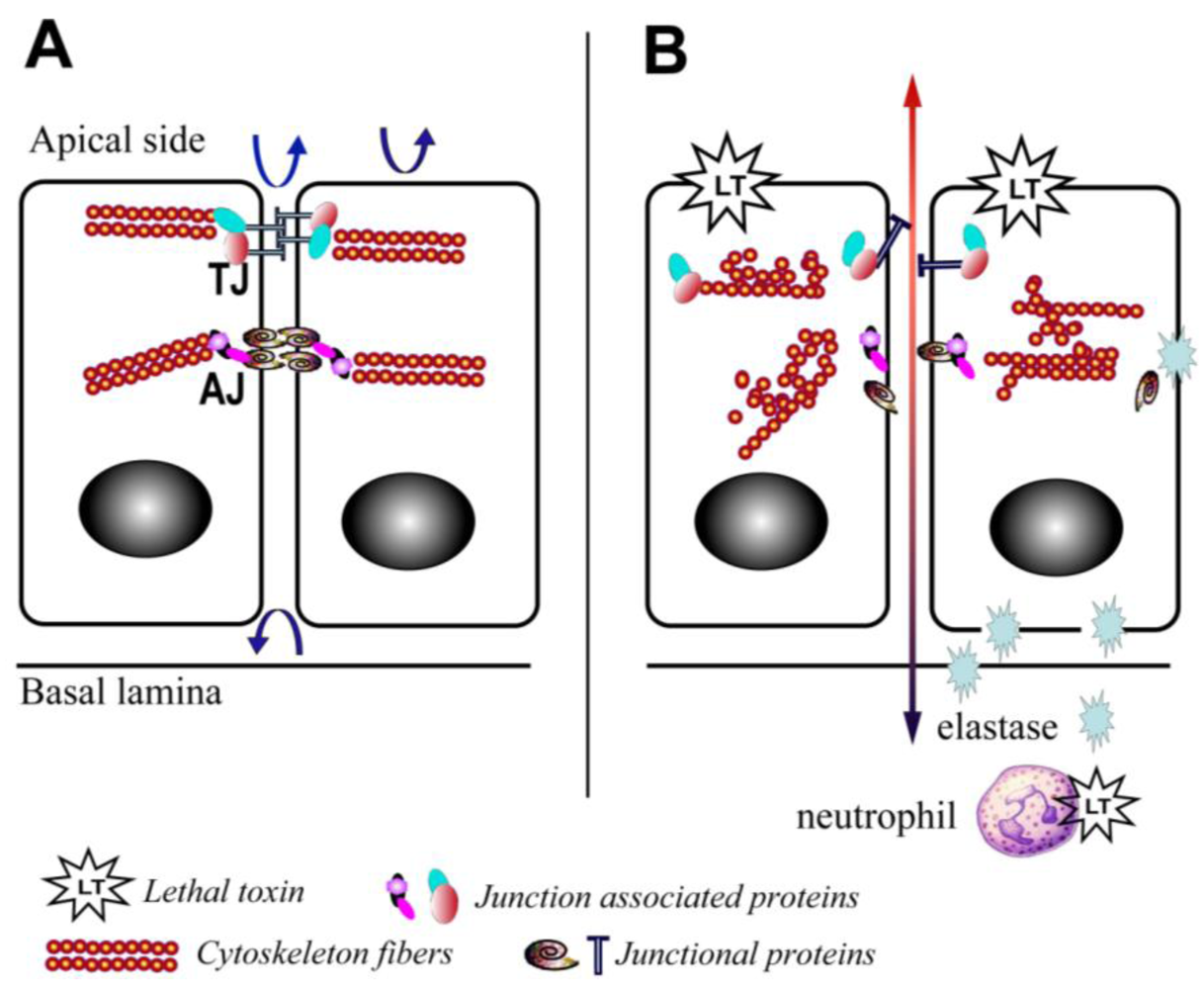
6.2. Mechanisms of Action
7. Effect of Anthrax LT on the Blood-Brain Barrier (BBB)
8. Final Remarks
Acknowledgments
Conflict of Interest
Disclaimer
References and Notes
- van der Goot, G.; Young, J.A. Receptors of anthrax toxin and cell entry. Mol. Aspects Med. 2009, 30, 406–412. [Google Scholar]
- Xu, L.; Frucht, D.M. Bacillus anthracis: A multi-faceted role for anthrax lethal toxin in thwarting host immune defenses. Int. J. Biochem. Cell. Biol. 2007, 39, 20–24. [Google Scholar]
- Moayeri, M.; Haines, D.; Young, H.A.; Leppla, S.H. Bacillus anthracis lethal toxin induces TNF-alpha-independent hypoxia-mediated toxicity in mice. J. Clin. Invest. 2003, 112, 670–682. [Google Scholar]
- Cordoba-Rodriguez, R.; Fang, H.; Lankford, C.S.; Frucht, D.M. Anthrax lethal toxin rapidly activates caspase-1/ICE and induces extracellular release of interleukin (IL)-1beta and IL-18. J. Biol. Chem. 2004, 279, 20563–20566. [Google Scholar]
- Boyden, E.D.; Dietrich, W.F. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 2006, 38, 240–244. [Google Scholar]
- Tournier, J.N.; Rossi Paccani, S.; Quesnel-Hellmann, A.; Baldari, C.T. Anthrax toxins: A weapon to systematically dismantle the host immune defenses. Mol. Aspects Med. 2009, 30, 456–466. [Google Scholar]
- Stanley, J.L.; Smith, H. Purification of factor I and recognition of a third factor of the anthrax toxin. J. Gen. Microbiol. 1961, 26, 49–63. [Google Scholar]
- Beall, F.A.; Taylor, M.J.; Thorne, C.B. Rapid lethal effect in rats of a third component found upon fractionating the toxin of Bacillus anthracis. J. Bacteriol. 1962, 83, 1274–1280. [Google Scholar]
- Fish, D.C.; Klein, F.; Lincoln, R.E.; Walker, J.S.; Dobbs, J.P. Pathophysiological changes in the rat associated with anthrax toxin. J. Infect. Dis. 1968, 118, 114–124. [Google Scholar]
- Fang, H.; Sun, C.; Xu, L.; Owen, R.J.; Auth, R.D.; Snoy, P.J.; Frucht, D.M. Neutrophil elastase mediates pathogenic effects of anthrax lethal toxin in the murine intestinal tract. J. Immunol. 2010, 185, 5463–5467. [Google Scholar]
- Gozes, Y.; Moayeri, M.; Wiggins, J.F.; Leppla, S.H. Anthrax lethal toxin induces ketotifen-sensitive intradermal vascular leakage in certain inbred mice. Infect. Immun. 2006, 74, 1266–1272. [Google Scholar]
- Warfel, J.M.; Steele, A.D.; D’Agnillo, F. Anthrax lethal toxin induces endothelial barrier dysfunction. Am. J. Pathol. 2005, 166, 1871–1881. [Google Scholar]
- Kirby, J.E. Anthrax lethal toxin induces human endothelial cell apoptosis. Infect. Immun. 2004, 72, 430–439. [Google Scholar]
- Mullin, J.M.; Agostino, N.; Rendon-Huerta, E.; Thornton, J.J. Keynote review: Epithelial and endothelial barriers in human disease. Drug Discov. Today 2005, 10, 395–408. [Google Scholar]
- Dejana, E. Endothelial cell-cell junctions: Happy together. Nat. Rev. Mol. Cell. Biol. 2004, 5, 261–270. [Google Scholar]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20, quiz 21-22. [Google Scholar]
- Breitkreutz, D.; Mirancea, N.; Nischt, R. Basement membranes in skin: Unique matrix structures with diverse functions? Histochem. Cell. Biol. 2009, 132, 1–10. [Google Scholar]
- Fromm, M.; Schulzke, J.D.; Hegel, U. Epithelial and subepithelial contributions to transmural electrical resistance of intact rat jejunum, in vitro. Pflugers Arch. 1985, 405, 400–402. [Google Scholar]
- Gitter, A.H.; Bendfeldt, K.; Schulzke, J.D.; Fromm, M. Trans/paracellular, surface/crypt, and epithelial/subepithelial resistances of mammalian colonic epithelia. Pflugers Arch. 2000, 439, 477–482. [Google Scholar]
- Turnbull, P.C. Introduction: Anthrax history, disease and ecology. Curr. Top. Microbiol. Immunol. 2002, 271, 1–19. [Google Scholar]
- Martin, G.J.; Friedlander, A.M. Bacillus anthracis (anthrax). In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 7th; Mandell, G.L., Bennett, J.E., Dolin, R., Eds.; Churchill Livingstone Elsevier: Philadelphia, PA, USA, 2010; Volume 2, pp. 2715–2725. [Google Scholar]
- Jernigan, D.B.; Raghunathan, P.L.; Bell, B.P.; Brechner, R.; Bresnitz, E.A.; Butler, J.C.; Cetron, M.; Cohen, M.; Doyle, T.; Fischer, M.; et al. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg. Infect. Dis. 2002, 8, 1019–1028. [Google Scholar] [PubMed]
- Inglesby, T.V.; O’Toole, T.; Henderson, D.A.; Bartlett, J.G.; Ascher, M.S.; Eitzen, E.; Friedlander, A.M.; Gerberding, J.; Hauer, J.; Hughes, J.; McDade, J.; Osterholm, M.T.; Parker, G.; Perl, T.M.; Russell, P.K.; Tonat, K. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 2002, 287, 2236–2252. [Google Scholar]
- Booth, M.G.; Hood, J.; Brooks, T.J.; Hart, A. Anthrax infection in drug users. Lancet 2010, 375, 1345–1346. [Google Scholar]
- Ringertz, S.H.; Hoiby, E.A.; Jensenius, M.; Maehlen, J.; Caugant, D.A.; Myklebust, A.; Fossum, K. Injectional anthrax in a heroin skin-popper. Lancet 2000, 356, 1574–1575. [Google Scholar]
- Dutz, W.; Saidi, F.; Kohout, E. Gastric anthrax with massive ascites. Gut 1970, 11, 352–354. [Google Scholar]
- Knudson, G.B. Treatment of anthrax in man: History and current concepts. Mil. Med. 1986, 151, 71–77. [Google Scholar]
- Coleman, M.E.; Thran, B.; Morse, S.S.; Hugh-Jones, M.; Massulik, S. Inhalation anthrax: Dose response and risk analysis. Biosecur. Bioterror. 2008, 6, 147–160. [Google Scholar]
- Peters, C.J.; Hartley, D.M. Anthrax inhalation and lethal human infection. Lancet 2002, 359, 710–711. [Google Scholar]
- Reissman, D.B.; Whitney, E.A.; Taylor, T.H., Jr.; Hayslett, J.A.; Dull, P.M.; Arias, I.; Ashford, D.A.; Bresnitz, E.A.; Tan, C.; Rosenstein, N.; Perkins, B.A. One-year health assessment of adult survivors of Bacillus anthracis infection. JAMA 2004, 291, 1994–1998. [Google Scholar]
- Brittingham, K.C.; Ruthel, G.; Panchal, R.G.; Fuller, C.L.; Ribot, W.J.; Hoover, T.A.; Young, H.A.; Anderson, A.O.; Bavari, S. Dendritic cells endocytose Bacillus anthracis spores: implications for anthrax pathogenesis. J. Immunol. 2005, 174, 5545–5552. [Google Scholar]
- Cleret, A.; Quesnel-Hellmann, A.; Vallon-Eberhard, A.; Verrier, B.; Jung, S.; Vidal, D.; Mathieu, J.; Tournier, J.N. Lung dendritic cells rapidly mediate anthrax spore entry through the pulmonary route. J. Immunol. 2007, 178, 7994–8001. [Google Scholar]
- Glomski, I.J.; Dumetz, F.; Jouvion, G.; Huerre, M.R.; Mock, M.; Goossens, P.L. Inhaled non-capsulated Bacillus anthracis in A/J mice: Nasopharynx and alveolar space as dual portals of entry, delayed dissemination, and specific organ targeting. Microbes Infect. 2008, 10, 1398–1404. [Google Scholar]
- Sanz, P.; Teel, L.D.; Alem, F.; Carvalho, H.M.; Darnell, S.C.; O’Brien, A.D. Detection of Bacillus anthracis spore germination in vivo by bioluminescence imaging. Infect. Immunol. 2008, 76, 1036–1047. [Google Scholar]
- Guidi-Rontani, C. The alveolar macrophage: The Trojan horse of Bacillus anthracis. Trends Microbiol. 2002, 10, 405–409. [Google Scholar]
- Abramova, F.A.; Grinberg, L.M.; Yampolskaya, O.V.; Walker, D.H. Pathology of inhalational anthrax in 42 cases from the Sverdlovsk outbreak of 1979. Proc. Natl. Acad. Sci. USA 1993, 90, 2291–2294. [Google Scholar]
- Grinberg, L.M.; Abramova, F.A.; Yampolskaya, O.V.; Walker, D.H.; Smith, J.H. Quantitative pathology of inhalational anthrax I: Quantitative microscopic findings. Mod. Pathol. 2001, 14, 482–495. [Google Scholar]
- Fritz, D.L.; Jaax, N.K.; Lawrence, W.B.; Davis, K.J.; Pitt, M.L.; Ezzell, J.W.; Friedlander, A.M. Pathology of experimental inhalation anthrax in the rhesus monkey. Lab. Invest. 1995, 73, 691–702. [Google Scholar]
- Rolando, M.; Munro, P.; Stefani, C.; Auberger, P.; Flatau, G.; Lemichez, E. Injection of Staphylococcus aureus EDIN by the Bacillus anthracis protective antigen machinery induces vascular permeability. Infect. Immunol. 2009, 77, 3596–3601. [Google Scholar]
- Cui, X.; Moayeri, M.; Li, Y.; Li, X.; Haley, M.; Fitz, Y.; Correa-Araujo, R.; Banks, S.M.; Leppla, S.H.; Eichacker, P.Q. Lethality during continuous anthrax lethal toxin infusion is associated with circulatory shock but not inflammatory cytokine or nitric oxide release in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, 699–709. [Google Scholar]
- Rolando, M.; Stefani, C.; Flatau, G.; Auberger, P.; Mettouchi, A.; Mhlanga, M.; Rapp, U.; Galmiche, A.; Lemichez, E. Transcriptome dysregulation by anthrax lethal toxin plays a key role in induction of human endothelial cell cytotoxicity. Cell. Microbiol. 2010, 12, 891–905. [Google Scholar]
- Moayeri, M.; Leppla, S.H. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol. Aspects Med. 2009, 30, 439–455. [Google Scholar]
- Guichard, A.; McGillivray, S.M.; Cruz-Moreno, B.; van Sorge, N.M.; Nizet, V.; Bier, E. Anthrax toxins cooperatively inhibit endocytic recycling by the Rab11/Sec15 exocyst. Nature 2010, 467, 854–858. [Google Scholar]
- Warfel, J.M.; D’Agnillo, F. Anthrax lethal toxin enhances IkappaB kinase activation and differentially regulates pro-inflammatory genes in human endothelium. J. Biol. Chem. 2009, 284, 25761–25771. [Google Scholar]
- Warfel, J.M.; D’Agnillo, F. Anthrax lethal toxin enhances TNF-induced endothelial VCAM-1 expression via an IFN regulatory factor-1-dependent mechanism. J. Immunol. 2008, 180, 7516–7524. [Google Scholar]
- Steele, A.D.; Warfel, J.M.; D’Agnillo, F. Anthrax lethal toxin enhances cytokine-induced VCAM-1 expression on human endothelial cells. Biochem. Biophys. Res. Commun. 2005, 337, 1249–1256. [Google Scholar]
- Steiner, O.; Coisne, C.; Cecchelli, R.; Boscacci, R.; Deutsch, U.; Engelhardt, B.; Lyck, R. Differential roles for endothelial ICAM-1, ICAM-2, and VCAM-1 in shear-resistant T cell arrest, polarization, and directed crawling on blood-brain barrier endothelium. J. Immunol. 2010, 185, 4846–4855. [Google Scholar]
- Sundy, J.S.; Haynes, B.F. Cytokines and adhesion molecules in the pathogenesis of vasculitis. Curr. Rheumatol. Rep. 2000, 2, 402–410. [Google Scholar]
- Jernigan, J.A.; Stephens, D.S.; Ashford, D.A.; Omenaca, C.; Topiel, M.S.; Galbraith, M.; Tapper, M.; Fisk, T.L.; Zaki, S.; Popovic, T.; Meyer, R.F.; Quinn, C.P.; Harper, S.A.; Fridkin, S.K.; Sejvar, J.J.; Shepard, C.W.; McConnell, M.; Guarner, J.; Shieh, W.J.; Malecki, J.M.; Gerberding, J.L.; Hughes, J.M.; Perkins, B.A. Bioterrorism-related inhalational anthrax: The first 10 cases reported in the United States. Emerg. Infect. Dis. 2001, 7, 933–944. [Google Scholar]
- Kuo, S.R.; Willingham, M.C.; Bour, S.H.; Andreas, E.A.; Park, S.K.; Jackson, C.; Duesbery, N.S.; Leppla, S.H.; Tang, W.J.; Frankel, A.E. Anthrax toxin-induced shock in rats is associated with pulmonary edema and hemorrhage. Microb. Pathog. 2008, 44, 467–472. [Google Scholar]
- Maniatis, N.A.; Kotanidou, A.; Catravas, J.D.; Orfanos, S.E. Endothelial pathomechanisms in acute lung injury. Vascul. Pharmacol. 2008, 49, 119–133. [Google Scholar]
- Lehmann, M.; Noack, D.; Wood, M.; Perego, M.; Knaus, U.G. Lung epithelial injury by B. anthracis lethal toxin is caused by MKK-dependent loss of cytoskeletal integrity. PLoS One 2009, 4, e4755. [Google Scholar] [PubMed]
- Popova, T.; Espina, V.; Bailey, C.; Liotta, L.; Petricoin, E.; Popov, S. Anthrax infection inhibits the AKT signaling involved in the E-cadherin-mediated adhesion of lung epithelial cells. FEMS Immunol. Med. Microbiol. 2009, 56, 129–142. [Google Scholar]
- Firoved, A.M.; Miller, G.F.; Moayeri, M.; Kakkar, R.; Shen, Y.; Wiggins, J.F.; McNally, E.M.; Tang, W.J.; Leppla, S.H. Bacillus anthracis edema toxin causes extensive tissue lesions and rapid lethality in mice. Am. J. Pathol. 2005, 167, 1309–1320. [Google Scholar]
- Potten, C.S. Epithelial cell growth and differentiation. II. Intestinal apoptosis. Am. J. Physiol. 1997, 273, 253–257. [Google Scholar]
- Radtke, F.; Clevers, H. Self-renewal and cancer of the gut: Two sides of a coin. Science 2005, 307, 1904–1909. [Google Scholar]
- Moore, R.; Carlson, S.; Madara, J.L. Rapid barrier restitution in an in vitro model of intestinal epithelial injury. Lab. Invest. 1989, 60, 237–244. [Google Scholar]
- Nusrat, A.; Delp, C.; Madara, J.L. Intestinal epithelial restitution. Characterization of a cell culture model and mapping of cytoskeletal elements in migrating cells. J. Clin. Invest. 1992, 89, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.C.; Jorgensen, S.C.; Popova, T.G.; Bailey, C.L.; Popov, S.G. Neutrophil elastase and syndecan shedding contribute to antithrombin depletion in murine anthrax. FEMS Immunol. Med. Microbiol. 2008, 54, 309–318. [Google Scholar]
- Hirahashi, J.; Mekala, D.; van Ziffle, J.; Xiao, L.; Saffaripour, S.; Wagner, D.D.; Shapiro, S.D.; Lowell, C.; Mayadas, T.N. Mac-1 signaling via Src-family and Syk kinases results in elastase-dependent thrombohemorrhagic vasculopathy. Immunity 2006, 25, 271–283. [Google Scholar]
- Xu, L.; Fang, H.; Frucht, D.M. Anthrax lethal toxin increases superoxide production in murine neutrophils via differential effects on MAPK signaling pathways. J. Immunol. 2008, 180, 4139–4147. [Google Scholar]
- Crawford, M.A.; Aylott, C.V.; Bourdeau, R.W.; Bokoch, G.M. Bacillus anthracis toxins inhibit human neutrophil NADPH oxidase activity. J. Immunol. 2006, 176, 7557–7565. [Google Scholar]
- During, R.L.; Li, W.; Hao, B.; Koenig, J.M.; Stephens, D.S.; Quinn, C.P.; Southwick, F.S. Anthrax lethal toxin paralyzes neutrophil actin-based motility. J. Infect. Dis. 2005, 192, 837–845. [Google Scholar]
- Liu, S.; Miller-Randolph, S.; Crown, D.; Moayeri, M.; Sastalla, I.; Okugawa, S.; Leppla, S.H. Anthrax toxin targeting of myeloid cells through the CMG2 receptor is essential for establishment of Bacillus anthracis infections in mice. Cell Host Microbe 2010, 8, 455–462. [Google Scholar]
- Gasbarrini, G.; Montalto, M. Structure and function of tight junctions. Role in intestinal barrier. Ital. J. Gastroenterol. Hepatol. 1999, 31, 481–488. [Google Scholar] [PubMed]
- Fang, H.; Xu, L.; Chen, T.Y.; Cyr, J.M.; Frucht, D.M. Anthrax lethal toxin has direct and potent inhibitory effects on B cell proliferation and immunoglobulin production. J. Immunol. 2006, 176, 6155–6161. [Google Scholar]
- Comer, J.E.; Chopra, A.K.; Peterson, J.W.; Konig, R. Direct inhibition of T-lymphocyte activation by anthrax toxins in vivo. Infect. Immunol. 2005, 73, 8275–8281. [Google Scholar]
- Fang, H.; Cordoba-Rodriguez, R.; Lankford, C.S.; Frucht, D.M. Anthrax lethal toxin blocks MAPK kinase-dependent IL-2 production in CD4+ T cells. J. Immunol. 2005, 174, 4966–4971. [Google Scholar]
- Paccani, S.R.; Tonello, F.; Ghittoni, R.; Natale, M.; Muraro, L.; D’Elios, M.M.; Tang, W.J.; Montecucco, C.; Baldari, C.T. Anthrax toxins suppress T lymphocyte activation by disrupting antigen receptor signaling. J. Exp. Med. 2005, 201, 325–331. [Google Scholar]
- Okugawa, S.; Moayeri, M.; Eckhaus, M.A.; Crown, D.; Miller-Randolph, S.; Liu, S.; Akira, S.; Leppla, S.H. MyD88-dependent signaling protects against anthrax lethal toxin-induced impairment of intestinal barrier function. Infect. Immunol. 2011, 79, 118–124. [Google Scholar]
- Owens, W.E.; Berg, R.D. Bacterial translocation from the gastrointestinal tract of athymic (nu/nu) mice. Infect. Immunol. 1980, 27, 461–467. [Google Scholar]
- Gautreaux, M.D.; Deitch, E.A.; Berg, R.D. Bacterial translocation from the gastrointestinal tract to various segments of the mesenteric lymph node complex. Infect. Immunol. 1994, 62, 2132–2134. [Google Scholar]
- Lanska, D.J. Anthrax meningoencephalitis. Neurology 2002, 59, 327–334. [Google Scholar]
- Vasconcelos, D.; Barnewall, R.; Babin, M.; Hunt, R.; Estep, J.; Nielsen, C.; Carnes, R.; Carney, J. Pathology of inhalation anthrax in cynomolgus monkeys (Macaca fascicularis). Lab. Invest. 2003, 83, 1201–1209. [Google Scholar]
- van Sorge, N.M.; Ebrahimi, C.M.; McGillivray, S.M.; Quach, D.; Sabet, M.; Guiney, D.G.; Doran, K.S. Anthrax toxins inhibit neutrophil signaling pathways in brain endothelium and contribute to the pathogenesis of meningitis. PLoS One 2008, 3, e2964. [Google Scholar]
- Bonventre, P.F.; Sueoka, W.; True, C.W.; Klein, F.; Lincoln, R. Attempts to implicate the central nervous system as a primary site of action for Bacillus anthracis lethal toxin. Fed. Proc. 1967, 26, 1549–1553. [Google Scholar]
- Argaw, A.T.; Zhang, Y.; Snyder, B.J.; Zhao, M.L.; Kopp, N.; Lee, S.C.; Raine, C.S.; Brosnan, C.F.; John, G.R. IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J. Immunol. 2006, 177, 5574–5584. [Google Scholar]
- Kern, J.W.; Schneewind, O. BslA, a pXO1-encoded adhesin of Bacillus anthracis. Mol. Microbiol. 2008, 68, 504–515. [Google Scholar]
- Ebrahimi, C.M.; Kern, J.W.; Sheen, T.R.; Ebrahimi-Fardooee, M.A.; van Sorge, N.M.; Schneewind, O.; Doran, K.S. Penetration of the blood-brain barrier by Bacillus anthracis requires the pXO1-encoded BslA protein. J. Bacteriol. 2009, 191, 7165–7173. [Google Scholar]
- Sweeney, D.A.; Cui, X.; Solomon, S.B.; Vitberg, D.A.; Migone, T.S.; Scher, D.; Danner, R.L.; Natanson, C.; Subramanian, G.M.; Eichacker, P.Q. Anthrax lethal and edema toxins produce different patterns of cardiovascular and renal dysfunction and synergistically decrease survival in canines. J. Infect. Dis. 2010, 202, 1885–1896. [Google Scholar]
- Bouzianas, D.G. Medical countermeasures to protect humans from anthrax bioterrorism. Trends Microbiol. 2009, 17, 522–528. [Google Scholar]
- Schneemann, A.; Manchester, M. Anti-toxin antibodies in prophylaxis and treatment of inhalation anthrax. Future Microbiol. 2009, 4, 35–43. [Google Scholar]
- Arbibe, L.; Kim, D.W.; Batsche, E.; Pedron, T.; Mateescu, B.; Muchardt, C.; Parsot, C.; Sansonetti, P.J. An injected bacterial effector targets chromatin access for transcription factor NF-kappaB to alter transcription of host genes involved in immune responses. Nat. Immunol. 2007, 8, 47–56. [Google Scholar]
- Mittal, R.; Peak-Chew, S.Y.; McMahon, H.T. Acetylation of MEK2 and I kappa B kinase (IKK) activation loop residues by YopJ inhibits signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 18574–18579. [Google Scholar]
- Lin, S.L.; Le, T.X.; Cowen, D.S. SptP, a Salmonella typhimurium type III-secreted protein, inhibits the mitogen-activated protein kinase pathway by inhibiting Raf activation. Cell Microbiol. 2003, 5, 267–275. [Google Scholar]
- Shan, L.; He, P.; Sheen, J. Intercepting host MAPK signaling cascades by bacterial type III effectors. Cell Host Microbe 2007, 1, 167–174. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xie, T.; Auth, R.D.; Frucht, D.M. The Effects of Anthrax Lethal Toxin on Host Barrier Function. Toxins 2011, 3, 591-607. https://doi.org/10.3390/toxins3060591
Xie T, Auth RD, Frucht DM. The Effects of Anthrax Lethal Toxin on Host Barrier Function. Toxins. 2011; 3(6):591-607. https://doi.org/10.3390/toxins3060591
Chicago/Turabian StyleXie, Tao, Roger D. Auth, and David M. Frucht. 2011. "The Effects of Anthrax Lethal Toxin on Host Barrier Function" Toxins 3, no. 6: 591-607. https://doi.org/10.3390/toxins3060591



