Aflatoxin B1 in Affecting Broiler’s Performance, Immunity, and Gastrointestinal Tract: A Review of History and Contemporary Issues
Abstract
:1. Introduction
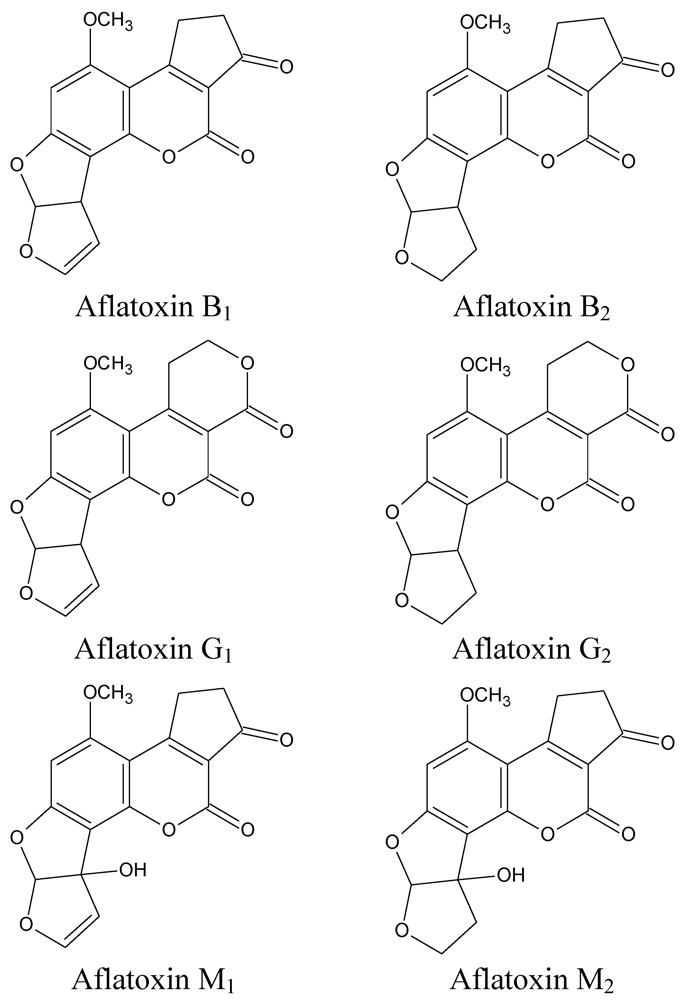
2. Metabolism of Aflatoxin B1
| Species | LD50 | Lesions in Liver | |||||
|---|---|---|---|---|---|---|---|
| Necrosis and Hemorrhage | Fibrosis | Regeneration of Nodules | Bile Duct Proliferation/Hyperplasia | Vacuolation and Fatty Infiltration | Enlarged Hepatic Cells | ||
| Rabbit | 0.4 | + | - | + | + | - | + |
| Duckling | 2.8 | + | - | + | + | + | + |
| Pig | 3.9 | + | + | + | + | + | + |
| Dog | 6.3 | + | + | + | + | + | + |
| Guinea pig | 10.6 | + | - | + | + | + | + |
| Sheep 2 | 12.5 | ||||||
| Mouse | 56.3 | - | - | - | - | + | + |
| Chicken | 72.0 | - | - | - | + | + | + |
| Rat | 73.3 | + | - | + | + | + | + |
2.1. Absorption and Excretion
2.2. Metabolism

3. Effects of Aflatoxin B1 on Performance and Serum Chemistry
| AFB1 (mg/kg) | Performance *,1,2,4 | Bruising 3 | Weight of Organs | Serum 1,3,4 | ||||
|---|---|---|---|---|---|---|---|---|
| Liver 1,4 | Spleen 1,4 | Bursa and Thymus 5 | Lipid | Protein | ||||
| ≤0.1 | ~ | ~ | ~ | ~ | ||||
| 0.5 | ↓ | ~ | ~ | ~ | ~ | |||
| 1.0 | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | ||
| 2.5 | ↓ | ↑ | ↑ | ↑ | ↓ | ↓ | ↓ | |
| ≥5.0 | ↓ | ↑ | ↑ | ↑ | ↓ | ↓ | ||
| AFB1 Level (ppm) | n * | Hematology and Serum Chemistry | Year of Study and Reference | |
|---|---|---|---|---|
| Bird Type, and Age (days) | Effects | No Effects | ||
| 0, 0.1 | 4 | ↓ AP | AST, γ-GT, TP, Chl, BUN, creatinine | 2010 [55] |
| ♂Ross308, 427–457 | (12) | |||
| 0, 0.3 | 12 | ↓ TP and Chl at 21 days | BUN, ALT, γ-GT, AST at 21 days. BUN, ALT, Hb at 35 days | 2000 [39] |
| Broilers, 1–35 | ↓ TP, Chl, γ-GT, AST at 35 days | |||
| 0, 0.8 | 7 | ↓ ALT | TP, albumin, globulin, Glc., AST, γ-GT, Ca, P | 2004 [40] |
| ♂Broilers, 14–49 | ||||
| 0, 1.0 | 4 | ↓ TP, albumin, Chl, Ca | Uric acid, γ-GT, P | 2008 [47] |
| ♂Cobb, 1–21 | (8) | |||
| 0, 1.0 | 10 | ↑ AP | TP, albumin, AST, γ-GT, uric acid, Chl, triglyceride | 2009 [17] |
| ♂Ross308, 1–42 | ||||
| 0, 1.0 | 5 | ↓ TP, albumin, globulin | BUN, Glc., AP, AST, γ-GT, CK, Na, K, Cl, Ca, P | 2010 [41] |
| broilers, 1–21 | (15) | |||
| 0, 2 | 5 | ↓ TP, albumin, globulin, AP, Glc, Ca, P | BUN, AST, γ-GT, CK, uric acid, Na, K, Cl | 2010 [41] |
| broilers, 1–21 | (15) | |||
| 0, 3 | 20 | ↓ TP, ALT | - | 2001 [42] |
| Hubb, 1–21 | ↑ AST | |||
| 0, 3.5 | 6 | ↓ TP, albumin, Chl, creatinine, Ca, MCV | AP, ALT, P, RBC, MCH, MCHC | 1997 [45] |
| broilers, 1–21 | (18) | |||
| 0, 4 | 6 | ↓ TP, BUN, Chl, PMCV, hematocrit % | - | 1997 [43] |
| ♂PetxHubb, 1–21 | (12) | |||
| 0, 4 | 5 | ↓ TP, albumin, globulin, Chl, Glc., Ca, P | - | 1998 [46] |
| ♂broilers, 1–21 | (15) | ↑ Na, Cl | ||
| 0, 5 | 6 | ↓ TP, albumin, Chl, uric acid, AP, Ca. | P | 1998 [56] |
| AAxPet, 1–21 | (12) | ↑ CK | ||
| 0, 5 | 6 | ↓ TP, albumin, Chl | - | 1998 [45] |
| broilers, 1–21 | (12) | ↑ BUN, CK | ||
4. Effects of Aflatoxin B1 on Adaptive Immunity
| AFB1 Level (ppm) | Vaccine Age | Effects | No Effects | Year of Study and Reference |
|---|---|---|---|---|
| Bird Type, Age (days) | ||||
| Humoral immunity: | ||||
| 0.1, 0.2, 0.4, 0.5, 1.0 | ? | - | Titers to ND and fowl cholera | 1985 [59] |
| Broiler, 14–49 | ||||
| 0.1, 0.2, 0.4, 0.8 | ? | - | Titers to ND and fowl cholera | 1985 [60] |
| broiler, 14-–49 | ||||
| 1 (AF) | 14 days | ↓ ND titers at 1 and 3 weeks post vaccination | ND titers at 2, 4, and 5 weeks post vaccination | 2003 [61] |
| Broiler, 7–49 | ||||
| 2.5 (AF) | 7 + 21 days | ↓ ND titers at 28 days age | - | 2000 [62] |
| Faobro, 1–21 | ||||
| 0, 0.6, 1.2, 2.5 | - | ↓ total complement activity at 2.5 ppm | total complement activity at 0.6 and 1.2 ppm | 1985 [63] |
| Broiler, 1–42 | ||||
| 5 | 1 + 21 days | ↑ secondary antibodies against IBD at 28 and 35 days | - | 1997 [64] |
| Broiler, 1–35 | ||||
| 0.2 | ? | ↓ antibody titers to ND, IB, and IBD | - | 1998 [65] |
| ♀Leghorn, 126–280 | ||||
| 2.5 | 21 days | - | ND, IB titers; at 35 days susceptibility to ND | 1978 [66] |
| ♂Leghorn, 1–28 | ||||
| 2.5 | 21 days | - | ND titers; susceptibility to ND at 35 days | 1978 [66] |
| ♂Leghorn, 1–49 | ||||
| Cell mediated immunity: | ||||
| 0, 0.1, 0.2, 0.4, 0.8 | - | ↓ DHST from 0.2 ppm | - | 1985 [60] |
| Broiler, 14-49 | ||||
| 0.1, 0.2, 0.4, 0.5, 1.0 | - | ↓DHST at 0.4 ppm AFB1 + AFB2 | DHST on AFB1 alone | 1985 [59] |
| Broiler, 14–49 | ||||
| 1 | - | ↓ DHST | - | 2003 [61] |
| Broiler, 7–49 | ||||
| 0.3 | - | ↓ DHST at 30, 45, and 60 days age | - | 1988 [67] |
| Leghorn, 1–42 | ||||
| Item | 2nd Week Exposure | 4th Week Exposure | 5th Week Exposure |
|---|---|---|---|
| Titers against ND: | |||
| 0.07 mg AFB1/kg diet | 33% | 407% | −27% |
| 0.75 mg AFB1/kg diet | 127% * | 594% | −28% |
| Serum protein: | |||
| 0.07 mg AFB1/kg diet | 5% | −2% | 2.6% |
| 0.75 mg AFB1/kg diet | −32% ** | −32% * | −21% * |
5. Effects of Aflatoxin B1 on Gastrointestinal Tract
5.1. Aflatoxin B1 and Gut Morphology
| AFB1 Level (ppm) | n * | Characteristics of Gut | Year of Study and Reference | |
|---|---|---|---|---|
| Bird Type, Age (days) | Effects | No Effects | ||
| 0.07, 0.7 | (7) | ↓ Density of duodenum and jejunum | Weight of proventriculus and gizzard | 2011 [71] |
| ♂ Ross308, 7–29 | ↑ Length of duodenum and jejunum | |||
| 0.02 | 5 | ↓ Density of intestine | Gizzard weight; intestinal weight and length | 2010 [48] |
| ♂ Hybro, 21–49 | ||||
| 0.1 | 4 | - | Pancreas weight | 2010 [57] |
| ♂ Ross308, 427–457 | (3) | |||
| 0.3 | 12 | - | Gizzard weight | 2000 [39] |
| Broilers, 1–35 | ||||
| 1 | 2 | Necrosis/fibrosis in crop and proventriculus. Catarrhal enteritis in intestine | - | 2009 [72] |
| Broiler, 1–28 | (5) | |||
| 0.6, 1.2, 2.5 | 8 | linear effect: ↑ crypt length in distal jejunum | Number and density of goblet cell in jejunum | 2009 [74] |
| ♀ W36, 140–154 | ||||
| 3.5 | 6 | - | Gizzard weight | 1997 [44] |
| Broilers, 1–21 | (4) | |||
| 4 | 6 | - | Gizzard and pancreas weight | 1997 [43] |
| ♂PetxHubb, 1–21 | (3) | |||
| 4 | 5 | ↑ Proventriculus and pancreas weight | Microscopic evaluation of pancreas and whole GIT | 1998 [46] |
| ♂ Broilers, 1–21 | (3) | |||
| 5 | 6 | ↑ Gizzard and pancreas weight | Proventriculus weight | 1998 [56] |
| AA x Pet, 1–21 | (2) | |||
| 5 | 6 | ↑ Proventriculus and pancreas weight | - | 1998 [45] |
| Broilers, 1–21 | (2) | |||
| 0, 0.6, 1.2, 2.5, 5, 10 | 4 | - | Breaking strength and size of large intestine | 1980 [73] |
| ♂CobbxCobb, 1–21 | (10) | |||
5.2. Aflatoxin B1 and Active Transport of Nutrients
5.3. Aflatoxin B1, and Digestibility and Activity of Digestive Enzymes
5.4. Aflatoxin B1 and Intestinal Innate Immunity
5.5. Interaction of Aflatoxin B1 with Gut Microbes
6. Conclusions
References
- van Egmond, H.P.; Jonker, M.A. Worldwide regulations on aflatoxins-the situation in 2002. J. Toxicol. Toxin Rev. 2004, 23, 273–293. [Google Scholar]
- Bilgrami, K.S.; Choudhary, A.K. Mycotoxins in preharvest contamination of agricultural crops. In Mycotoxins in Agriculture and Food Safety; Sinha, K.K., Bhatnagar, D., Eds.; Marcel Dekker: New York, NY, USA, 1998; pp. 1–43. [Google Scholar]
- Galvano, F.; Ritieni, A.; Piva, G.; Pietri, A. Mycotoxins in the human food chain. In The Mycotoxin Blue Book; Diaz, D.E., Ed.; Nottingham University Press: Nottingham, UK, 2005; pp. 187–224. [Google Scholar]
- Blount, W.P. Turkey “X” disease. Turkeys 1961, 9, 52–54. [Google Scholar]
- Allcroft, R.; Carnaghan, R.B.A.; Sargeant, K.; O’Kelly, J. A toxic factor in Brazillian groundnut meal. Vet. Rec. 1961, 73, 428–429. [Google Scholar]
- Sargeant, K.; Sheridan, A.; O’Kelly, J.; Carnaghan, R.B. Toxicity associated with certain samples of groundnuts. Nature 1961, 192, 1096–1097. [Google Scholar]
- Patterson, D.S.P. Aflatoxin and related compounds: Introduction. In Mycotoxic Fungi, Mycotoxins, Mycotoxicoses, an Encyclopaedic Handbook, 1st; Wyllie, T.D., Morehouse, L.G., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1977; Volume 1, pp. 131–135. [Google Scholar]
- Van der Zijden, A.S.M.; Koelensmid, W.A.A.B.; Boldingh, J.; Barrett, C.B.; Ord, W.O.; Philp, J. Isolation in crystalline form of a toxin responsible for turkey X disease. Nature 1962, 195, 1060–1062. [Google Scholar]
- Nesbitt, B.F.; O’Kelly, J.; Sargeant, K.; Sheridan, A. Toxic metabolites of Aspergillus flavus. Nature 1962, 195, 1062–1063. [Google Scholar]
- Asao, T.; Büchi, G.; Abdel-Kadar, M.M.; Chang, S.B.; Wick, E.L.; Wogan, G.N. Structures of aflatoxins B1 and G1. J. Am. Chem. Soc. 1965, 87, 882–886. [Google Scholar]
- Butler, W.H. Aflatoxin. In Mycotoxins; Purchase, I.F.H., Ed.; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1974; pp. 1–3. [Google Scholar]
- Diaz, D.E. The Mycotoxin Blue Book, 1st ed; Nottingham University Press: Nottingham, UK, 2005; pp. 25–56. [Google Scholar]
- Dersjant-Li, Y.; Verstegen, M.W.A.; Gerrits, W.J.J. The impact of low concentrations of aflatoxin, deoxynivalenol or fumonisin in diets on growing pigs and poultry. Nutr. Res. Rev. 2003, 16, 223–239. [Google Scholar]
- Diaz, G.J.; Calabrese, E.; Blain, R. Aflatoxicosis in chickens (Gallus gallus): An example of hormesis? Poult. Sci. 2008, 87, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Emafo, P.O. Species differences in the metabolism of aflatoxin B1. Afr. J. Med. Med. Sci. 1976, 5, 55–62. [Google Scholar]
- Miazzo, R.; Rosa, C.; de Queiroz, C.E.; Magnoli, C.; Chiacchiera, S.; Palacio, G.; Saenz, M.; Kikot, A.; Basaldella, E.; Dalcero, A. Efficacy of synthetic zeolite to reduce the toxicity of aflatoxin in broiler chicks. Poult. Sci. 2000, 79, 1–6. [Google Scholar]
- Denli, M.; Blandon, J.C.; Guynot, M.E.; Salado, S.; Perez, J.F. Effects of dietary AflaDetox on performance, serum biochemistry, histopathological changes, and aflatoxin residues in broilers exposed to aflatoxin B1. Poult. Sci. 2009, 88, 1444–1451. [Google Scholar]
- Fernández, A.; Ramos, J.J.; Sanz, M.D.C.; Saez, T.; de Luco, D.F. Alterations in the performance, haematology and clinical biochemistry of growing lambs fed with aflatoxin in the diet. J. Appl. Toxicol. 1996, 16, 85–91. [Google Scholar]
- Larsson, P.; Busk, L.; Tjälve, H. Hepatic and extrahepatic bioactivation and GSH conjugation of aflatoxin B1 in sheep. Carcinogenesis 1994, 15, 947–955. [Google Scholar]
- Hsieh, D.P.H.; Wong, J.J. Pharmacokinetics and excretion of aflatoxins. In The Toxicology of Aflatoxins. Human Health, Veterinary and Agricultural Significance; Eaton, D.L., Groopman, J.D., Eds.; Academic Press: San Diego, CA, USA, 1994; pp. 73–88. [Google Scholar]
- Ramos, A.J.; Hernández, E. In situ absorption of aflatoxins in rat small intestine. Mycopathologia 1996, 134, 27–30. [Google Scholar]
- Wogan, G.N.; Edwards, G.S.; Shank, R.C. Excretion and tissue distribution of radioactivity from aflatoxin B1-14-C in rats. Cancer Res. 1967, 27, 1729–1736. [Google Scholar]
- Coulombe, R.A., Jr.; Sharma, R.P. Clearance and excretion of intratracheally and orally administered aflatoxin B1 in the rat. Food Chem. Toxicol. 1985, 23, 827–830. [Google Scholar]
- Eaton, D.L.; Gallagher, E.P. Mechanisms of aflatoxin carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 1994, 34, 135–172. [Google Scholar]
- Wong, Z.A.; Hsieh, D.P.H. The comparative metabolism and toxicokinetics of aflatoxin B1 in the monkey, rat, and mouse. Toxicol. Appl. Pharmacol. 1980, 55, 115–125. [Google Scholar]
- Sawhney, D.S.; Vadehra, D.V.; Baker, R.C. The metabolism of 14C aflatoxins in laying hens. Poult. Sci. 1973, 52, 1302–1309. [Google Scholar]
- Mabee, M.S.; Chipley, J.R. Tissue distribution and metabolism of aflatoxin B 1-14 C in Broiler chickens. Appl. Microbiol. 1973, 25, 763–769. [Google Scholar]
- Wolzak, A.; Pearson, A.M.; Coleman, T.H. Aflatoxin carryover and clearance from tissues of laying hens. Food Chem. Toxicol. 1986, 24, 37–41. [Google Scholar]
- Hussain, Z.; Khan, M.Z.; Khan, A.; Javed, I.; Saleemi, M.K.; Mahmood, S.; Asi, M.R. Residues of aflatoxin B1 in broiler meat: Effect of age and dietary aflatoxin B1 levels. Food Chem. Toxicol. 2010, 48, 3304–3307. [Google Scholar]
- Fernandez, A.; Verde, M.T.; Gascon, M.; Ramos, J.J.; Gomez, J. Aflatoxin and its metabolites in tissues from laying hens and broiler chickens fed a contaminated diet. J. Sci. Food Agric. 1994, 65, 407–414. [Google Scholar]
- IARC, IARC Monograph on the Evaluation of Carcinogenic Risk to Humans, Some Naturally Occurring Substances: Food Items and Constituents, Heteroyclic Aromatic Amines and Mycotoxins; The International Agency for Research on Cancer: Lyon, France, 1993; 56.
- Eaton, D.L.; Gallagher, E.P. Mechanisms of aflatoxin carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 1994, 34, 135–172. [Google Scholar]
- Nigam, S.K.; Ghosh, S.K.; Malaviya, R. Aflatoxin, its metabolism and carcinogenesis-A historical review. J. Toxicol. Toxin Rev. 1994, 13, 179–203. [Google Scholar]
- IARC, IARC Monograph on the Evaluation of Carcinogenic Risk of Chemicals to Humans, Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; The International Agency for Research on Cancer: Lyon, France, 2002; 82.
- Do, J.H.; Choi, D.K. Aflatoxins: Detection, toxicity, and biosynthesis. Biotechnol. Bioprocess Eng. 2007, 12, 585–593. [Google Scholar]
- Micco, C.; Miraglia, M.; Onori, R.; Brera, C.; Mantovani, A.; Ioppolo, A.; Stasolla, D. Long-term administration of low doses of mycotoxins to poultry. 1. Residues of aflatoxin B1 and its metabolites in broilers and laying hens. Food Addit. Contam. 1988, 5, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Diaz, G.; Murcia, H.W.; Cepeda, S.M. Cytochrome P450 enzymes involved in the metabolism of aflatoxin B1 in chickens and quail. Poult. Sci. 2010, 89, 2461–2469. [Google Scholar]
- Devegowda, G.; Murthy, T.N.K. Mycotoxins: Their effects in poultry and some practical solutions. In The Mycotoxin Blue Book; Diaz, D.E., Ed.; Nottingham University Press: Nottingham, UK, 2005; pp. 25–56. [Google Scholar]
- Raju, M.V.L.N.; Devegowda, G. Influence of esterified-glucomannan on performance and organ morphology, serum biochemistry and haematology in broilers exposed to individual and combined mycotoxicosis (aflatoxin, ochratoxin and T-2 toxin). Br. Poult. Sci. 2000, 41, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, D.; Steidler, S.; Galletti, S.; Tameni, M.; Sonzogni, O.; Ravarotto, L. Efficacy of silymarin-phospholipid complex in reducing the toxicity of aflatoxin B1 in broiler chicks. Poult. Sci. 2004, 83, 1839–1843. [Google Scholar]
- Zhao, J.; Shirley, R.B.; Dibner, J.D.; Uraizee, F.; Officer, M.; Kitchell, M.; Vazquez-Anon, M.; Knight, C.D. Comparison of hydrated sodium calcium aluminosilicate and yeast cell wall on counteracting aflatoxicosis in broiler chicks. Poult. Sci. 2010, 89, 2147–2156. [Google Scholar]
- Valdivia, A.; Martinez, A.; Damian, F.; Quezada, T.; Ortiz, R.; Martinez, C.; Llamas, J.; Rodriguez, M.; Yamamoto, L.; Jaramillo, F.; et al. Efficacy of N-acetylcysteine to reduce the effects of aflatoxin B1 intoxication in broiler chickens. Poult. Sci. 2001, 80, 727–734. [Google Scholar] [PubMed]
- Edrington, T.; Kubena, L.; Harvey, R.; Rottinghaus, G. Influence of a superactivated charcoal on the toxic effects of aflatoxin or T-2 toxin in growing broilers. Poult. Sci. 1997, 76, 1205–1211. [Google Scholar]
- Kubena, L.; Harvey, R.; Buckley, S.; Edrington, T.; Rottinghaus, G. Individual and combined effects of moniliformin present in Fusarium fujikuroi culture material and aflatoxin in broiler chicks. Poult. Sci. 1997, 76, 265–270. [Google Scholar]
- Kubena, L.; Harvey, R.; Bailey, R.; Buckley, S.; Rottinghaus, G. Effects of a hydrated sodium calcium aluminosilicate (T-Bind) on mycotoxicosis in young broiler chickens. Poult. Sci. 1998, 77, 1502–1509. [Google Scholar]
- Ledoux, D.; Rottinghaus, G.; Bermudez, A.; Alonso-Debolt, M. Efficacy of a hydrated sodium calcium aluminosilicate to ameliorate the toxic effects of aflatoxin in broiler chicks. Poult. Sci. 1999, 78, 204–210. [Google Scholar]
- Gowda, N.K.S.; Ledoux, D.R.; Rottinghaus, G.E.; Bermudez, A.J.; Chen, Y.C. Efficacy of turmeric (curcuma longa), containing a known level of curcumin, and a hydrated sodium calcium aluminosilicate to ameliorate the adverse effects of aflatoxin in broiler chicks. Poult. Sci. 2008, 87, 1125–1130. [Google Scholar]
- Kana, J.R.; Teguia, A.; Tchoumboue, J. Effect of dietary plant charcoal from Canarium schweinfurthii Engl. and maize cob on aflatoxin B1 toxicosis in broiler chickens. Adv. Anim. Biosci. 2010, 1, 462–463. [Google Scholar] [CrossRef]
- Çelik, I.; Oğuz, H.; Demet, Ö.; Dönmez, H.H.; Boydak, M.; Sur, E. Efficacy of polyvinylpolypyrrolidone in reducing the immunotoxicity of aflatoxin in growing broilers. Br. Poult. Sci. 2000, 41, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Verma, J.; Johri, T.S.; Swain, B.K.; Ameena, S. Effect of graded levels of aflatoxin, ochratoxin and their combinations on the performance and immune response of broilers. Br. Poult. Sci. 2004, 45, 512–518. [Google Scholar]
- Havenstein, G.B.; Ferket, P.R.; Scheideler, S.E.; Larson, B.T. Growth, livability, and feed conversion of 1991 vs. 1957 broilers when fed “typical” 1957 and 1991 broiler diets. Poult. Sci. 1994, 73, 1785–1794. [Google Scholar] [PubMed]
- Havenstein, G.B.; Ferket, P.R.; Qureshi, M.A. Growth, livability, and feed conversion of 1991 vs. 1957 broilers when fed “typical” 1957 and 2001 broiler diets. Poult. Sci. 2003, 82, 1500–1508. [Google Scholar] [PubMed]
- Dozier, W.A., III; Kidd, M.T.; Corzo, A. Dietary amino acid responses of broiler chickens. J. Appl. Poult. Res. 2008, 17, 157–167. [Google Scholar] [CrossRef]
- Surai, P.F.; Dvorska, J.E. Effects of mycotoxins on antioxidant status and immunity. In The Mycotoxin Blue Book; Diaz, D.E., Ed.; Nottingham University Press: Nottingham, UK, 2005; pp. 93–137. [Google Scholar]
- Matur, E.; Ergul, E.; Akyazi, I.; Eraslan, E.; Cirakli, Z.T. The effects of Saccharomyces cerevisiae extract on the weight of some organs, liver, and pancreatic digestive enzyme activity in breeder hens fed diets contaminated with aflatoxins. Poult. Sci. 2010, 89, 2213–2220. [Google Scholar]
- Bailey, R.; Kubena, L.; Harvey, R.; Buckley, S.; Rottinghaus, G. Efficacy of various inorganic sorbents to reduce the toxicity of aflatoxin and T-2 toxin in broiler chickens. Poult. Sci. 1998, 77, 1623–1630. [Google Scholar]
- Yunus, A.W.; Nasir, M.K.; Farooq, U.; Böhm, J. Prevalence of poultry diseases in district Chakwal and their interactrion with mycotoxicosis: 1. Effects of age and flock size. J. Anim. Plant Sci. 2008, 18, 107–113. [Google Scholar]
- Yunus, A.W.; Nasir, M.K.; Aziz, T.; Böhm, J. Prevalence of poultry diseases in district Chakwal and their interactrion with mycotoxicosis: 2. Effects of season and feed. J. Anim. Plant Sci. 2009, 19, 1–5. [Google Scholar]
- Giambrone, J.J.; Diener, U.L.; Davis, N.D.; Panangala, V.S.; Hoerr, F.J. Effects of purified aflatoxin on broiler chickens. Poult. Sci. 1985, 64, 852–858. [Google Scholar]
- Giambrone, J.J.; Diener, U.L.; Davis, N.D.; Panangala, V.S.; Hoerr, F.J. Effects of aflatoxin on young turkeys and broiler chickens. Poult. Sci. 1985, 64, 1678–1684. [Google Scholar]
- Shivachandra, S.B.; Sah, R.L.; Singh, S.D.; Kataria, J.M.; Manimaran, K. Immunosuppression in broiler chicks fed aflatoxin and inoculated with fowl adenovirus serotype-4 (FAV-4) associated with hydropericardium syndrome. Vet. Res. Commum. 2003, 27, 39–51. [Google Scholar]
- Ibrahim, I.K.; Shareef, A.M.; Al-Joubory, K.M.T. Ameliorative effects of sodium bentonite on phagocytosis and Newcastle disease antibody formation in broiler chickens during aflatoxicosis. Res. Vet. Sci. 2000, 69, 119–122. [Google Scholar]
- Stewart, R.G.; Skeeles, J.K.; Wyatt, R.D.; Brown, J.; Page, R.K.; Russell, I.D.; Lukert, P.D. The effect of aflatoxin on complement activity in broiler chickens. Poult. Sci. 1985, 64, 616–619. [Google Scholar]
- Okotie-Eboh, G.O.; Kubena, L.F.; Chinnah, A.D.; Bailey, C.A. Effects of β-Carotene and Canthaxanthin on Aflatoxicosis in Broilers. Poult. Sci. 1997, 76, 1337–1341. [Google Scholar]
- Azzam, A.H.; Gabal, M.A. Aflatoxin and immunity in layer hens. Avian Pathol. 1998, 27, 570–577. [Google Scholar]
- Giambrone, J.J.; Partadiredja, M.; Eidson, C.S.; Kleven, S.H.; Wyatt, R.D. Interaction of aflatoxin with infectious bursal disease virus infection in young chickens. Avian Dis. 1978, 22, 431–439. [Google Scholar]
- Kadian, S.K.; Monga, D.P.; Goel, M.C. Effect of aflatoxin B1 on the delayed type hypersensitivity and phagocytic activity of reticuloendothelial system in chickens. Mycopathologia 1988, 104, 33–36. [Google Scholar]
- Corrier, D.E. Mycotoxins: Mechanism of immunosuppression. Vet. Immunol. Immunopathol. 1991, 30, 73–87. [Google Scholar]
- Azzam, A.H.; Gabal, M.A. Interaction of aflatoxin in the feed and immunization against selected infectious diseases in poultry. I. Infectious bursal disease. Avian Pathol. 1997, 26, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Tung, H.-T.; Donaldson, W.E.; Hamilton, P.B. Effects of aflatoxin on some marker enzymes of lysosomes. Biochim. Biophys. Acta Gen. Subj. 1970, 222, 665–667. [Google Scholar]
- Yunus, A.W.; Ghareeb, K.; Abd-El-Fattah, A.A.M.; Twaruzek, M.; Böhm, B. Gross intestinal adaptations in relation to broiler performance during a chronic aflatoxin exposure. Poult. Sci. 2011, 90. in press. [Google Scholar]
- Kumar, R.; Balachandran, C. Histopathological changes in broiler chickens fed aflatoxin and cyclopiazonic acid. Vet. Arhiv. 2009, 79, 31–40. [Google Scholar]
- Warren, M.F.; Hamilton, P.B. Intestinal fragility during ochratoxicosis and aflatoxicosis in broiler chickens. Appl. Environ. Microbiol. 1980, 40, 641–645. [Google Scholar]
- Applegate, T.J.; Schatzmayr, G.; Pricket, K.; Troche, C.; Jiang, Z. Effect of aflatoxin culture on intestinal function and nutrient loss in laying hens. Poult. Sci. 2009, 88, 1235–1241. [Google Scholar]
- Ruff, M.D.; Wyatt, R.D. Intestinal absorption of L-methionine and glucose in chickens with aflatoxicosis. Toxicol. Appl. Pharmacol. 1976, 37, 257–262. [Google Scholar]
- Carrillo, M.C.; Monti, J.A.; Grosman, M.E.; Rodriguez Garay, E.A. Effect of pH on aflatoxin B1 transfer in the everted rat jejunum. Toxicol. Lett. 1985, 27, 35–44. [Google Scholar]
- Chotinski, D.; Profirov, I.; Voǐnova, R.; Borisova, L. SH group content and hydrolase activity of the small intestine mucosa in chickens fed a mixture containing aflatoxin B1. Vet. Med. Nauki 1987, 24, 48–51. [Google Scholar]
- Gursoy, N.; Sarac, B.; Durmus, N.; Parlak, A.; Yildirim, S.; Kaya, T.; Bagcivan, I. Changes in spontaneous contractions of rat ileum by aflatoxin in vitro. Food Chem. Toxicol. 2008, 46, 2124–2127. [Google Scholar]
- Winding, B.; Winding, H.; Bindslev, N. Second messengers and ion channels in acetylcholine-induced chloride secretion. Comp. Biochem. Physiol. C-Pharmacol. Toxicol. Endocrinol. 1992, 103, 195–205. [Google Scholar] [CrossRef]
- Yunus, A.W.; Awad, W.A.; Kröger, A.; Zentek, J.; Böhm, J. In vitro aflatoxin B1 exposure decreases response to carbamycholine in the hehunal epithelium of broilers. Poult. Sci. 2010, 89, 1372–1378. [Google Scholar]
- Nelson, T.S.; Johnson, Z.B.; Kirby, L.K.; Beasley, J.N. Digestion of dry matter and amino acids and energy utilization by chicks fed molded corn containing mycotoxins. Poult. Sci. 1982, 61, 584–585. [Google Scholar]
- Richardson, K.E.; Hamilton, P.B. Enhanced production of pancreatic digestive enzymes during aflatoxicosis in egg-type chickens. Poult. Sci. 1987, 66, 640–644. [Google Scholar]
- Osborne, D.J.; Hamilton, P.B. Decreased pancreatic digestive enzymes during aflatoxicosis. Poult. Sci. 1981, 60, 1818–1821. [Google Scholar]
- Verma, J.; Swain, B.K.; Johri, T.S. Effect of various levels of aflatoxin and ochratoxin A and combinations thereof on protein and energy utilisation in broilers. J. Sci. Food Agric. 2002, 82, 1412–1417. [Google Scholar]
- Verma, J.; Johri, T.S.; Swain, B.K. Effect of aflatoxin, ochratoxin and their combination on protein and energy utilisation in white leghorn laying hens. J. Sci. Food Agric. 2007, 87, 760–764. [Google Scholar]
- Müller, C.C.; Autenrieth, I.B.; Peschel, A. Innate defences of the intestinal epithelial barrier. Cell. Mol. Life Sci. 2005, 52, 1297–1307. [Google Scholar]
- Gaikwad, S.S.; Pillai, M.M. Effect of aflatoxin B1 in gastrointestine of mice. J. Ecophys. Occup. Health 2004, 4, 153–159. [Google Scholar]
- Fleming, S.E.; Youngman, L.D.; Ames, B.N. Intestinal cell proliferation is influenced by intakes of protein and energy, aflatoxin, and Whole-body radiation. Nutr. Cancer 1994, 22, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Watzl, B.; Neudecker, C.; Hänsch, G.M.; Rechkemmer, G.; Pool-Zobel, B.L. Short-term moderate aflatoxin B1 exposure has only minor effects on the gut-associated lymphoid tissue of Brown Norway rats. Toxicology 1999, 138, 93–102. [Google Scholar]
- García, J.C.D.R.; Ramos, C.M.; Pinton, P.; Elvira, S.M.; Oswald, I.P. Evaluation of the cytotoxicity of AFB1, FB1 and AFB1/FB1 in intestinal cell. Revista Iberoamericana de Micologia 2007, 24, 136–141. [Google Scholar]
- Rao, J.R.; Sharma, N.N.; Iyer, P.K.; Sharma, A.K. Interaction between Eimeria uzura infection and aflatoxicosis in Japanese quail (Coturnix coturnix japonica). Vet. Parasitol. 1990, 35, 259–267. [Google Scholar]
- Rao, J.R.; Sharma, N.N.; Johri, T.S. Influence of dietary aflatoxin on Eimeria uzura infection in Japanese quail (Coturnix coturnix japonica). Vet. Parasitol. 1995, 56, 17–22. [Google Scholar]
- Ruff, M.D. Influence of dietary aflatoxin on the severity of Eimeria acervulina infection in broiler chickens. Avian Dis. 1978, 22, 471–480. [Google Scholar]
- Geiger, W.B.; Conn, J.E. The mechanism of the antibiotic action of clavacin and penicillic acid 1,2. J. Am. Chem. Soc. 1945, 67, 112–116. [Google Scholar]
- Rinderknecht, H.; Ward, J.L.; Bergel, F.; Morrison, A.L. Studies on antibiotics: 2. Bacteriological activity and possible mode of action of certain non-nitrogenous natural and synthetic antibiotics. Biochem. J. 1947, 41, 463–469. [Google Scholar]
- Brian, P.W.; Dawkins, A.W.; Grove, J.F.; Hemming, H.G.; Lowe, D.; Norris, G.L.F. Phytotoxic compounds produced by Fusarium equiseti. J. Exp. Bot. 1961, 12, 1–12. [Google Scholar]
- Ciegler, A. Patulin. In Mycotoxins in Human and Animal Health; Rodricks, J.V.C., Hesseltine, W., Mehlman, M.A., Eds.; Pathotox Publishers Inc.: Park Forest South, IL, USA, 1977; pp. 609–624. [Google Scholar]
- Wehner, F.C.; Thiel, P.G.; Van Rensburg, S.J.; Demasius, I.P.C. Mutagenicity to Salmonella typhimurium of some Aspergillus and Penicillium mycotoxins. Mutat. Res. 1978, 58, 193–203. [Google Scholar]
- Burmeister, H.R.; Hesseltine, C.W. Survey of the sensitivity of microorganisms to aflatoxin. Appl. Microbiol. 1966, 14, 403–404. [Google Scholar]
- Burmeister, H.R.; Hesseltine, C.W. Aflatoxin sensitivities of an L form and Bacillus megaterium. Bacteriol. Proc. 1967, 17, A97. [Google Scholar]
- Lillehoj, E.B.; Ciegler, A. Aflatoxin B1 binding and toxic effects on Bacillus megaterium. J. Gen. Microbiol. 1968, 54, 185–194. [Google Scholar]
- Beuchat, L.R.; Lechowich, R.V. Morphological alterations in Bacillus megaterium as produced by aflatoxin B1. Appl. Microbiol. 1971, 21, 124–131. [Google Scholar]
- Lillehoj, E.B.; Ciegler, A.; Hall, H.H. Fungistatic action of aflatoxin B1. Experientia 1967, 23, 187–188. [Google Scholar]
- Reiss, J. Untersuchungen über den Einfluss von Aflatoxin B1 auf die Morphologie und die cytochemisch fassbare Aktivität einiger Enzyme von Mucor hiemalis (Mucorales). Mycopathol. Mycol. Appl. 1970, 42, 225–231. [Google Scholar]
- Ong, T.M. Mutagenicity of aflatoxins in Neurospora crassa. Mutat. Res. 1970, 9, 615–618. [Google Scholar]
- Ong, T.M. Mutagenic activities of aflatoxin B1 and G1 in Neurospora crassa. Mol. Gen. Genet. 1971, 111, 159–170. [Google Scholar]
- Reiss, J. Inhibition of fungal sporulation by aflatoxin. Arch. Mikrobiol. 1971, 76, 219–222. [Google Scholar]
- Reiss, J. Hypha abnormalities in Thamnidium elegans Link due to aflatoxin B1. Z. Allg. Mikrobiol. 1971, 11, 637–638. [Google Scholar]
- Reiss, J. Effects of mycotoxins on higher plants, algae, fungi, and bacteria. In Mycotoxic Fungi Mycotoxins Mycotoxicoses. Mycotoxicoses of Man and Plants: Mycotoxin Control and Regulatory Practices, 1st; Wyllie, T.D., Morehouse, L.G., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1978; Volume 3, pp. 125–126. [Google Scholar]
- Ali-Vehmas, T.; Rizzo, A.; Westermarck, T.; Atroshi, F. Measurement of antibacterial activities of T-2 toxin, deoxynivalenol, ochratoxin A, aflatoxin B1 and fumonisin B1 Using microtitration tray-based turbidimetric techiques. Zentralbl. Veterinarmed. A 1998, 45, 453–458. [Google Scholar] [PubMed]
- Watson, D.H.; Lindsay, D.G. A critical review of biological methods for the detection of fungal toxins in foods and foodstuffs. J. Sci. Food Agric. 1982, 33, 59–67. [Google Scholar]
- Kranendonk, M.; Pintado, F.; Mesquita, P.; Laires, A.; Vermeulen, N.P.; Rueff, J. MX100, a new Escherichia coli tester strain for use in genotoxicity studies. Mutagenesis 1996, 11, 327–333. [Google Scholar]
- Mejia, G.E.; Ramos-Gomez, M.; Loarca-Pina, G. Antimutagenic activity of natural xanthophylls against aflatoxin B1 in Salmonella typhimurium. Environ. Mol. Mutagen. 1997, 30, 346–353. [Google Scholar]
- Miyazawa, M.; Okuno, Y.; Oshiro, K.; Kasahara, H.; Shimamura, H.; Nakamura, S.I.; Kameoka, H. Suppression of the SOS-inducing activity of Trp-P-1 and aflatoxin B1 by meso-dihydroguaiaretic acid from Machilus thunbergii in the Salmonella typhimurium TA1535/pSK1002 umu test. Biosci. Biotechnol. Biochem. 1998, 62, 1425–1427. [Google Scholar]
- Engler, K.H.; Coker, R.D.; Evans, I.H. Uptake of aflatoxin B1 and T-2 toxin by two mycotoxin bioassay microorganisms: Kluyveromyces marxianus and Bacillus megaterium. Arch. Microbiol. 2000, 174, 381–385. [Google Scholar]
- Gomes-Carneiro, M.R.; Dias, D.M.; Paumgartten, F.J. Study on the mutagenicity and antimutagenicity of beta-ionone in the Salmonella/microsome assay. Food Chem. Toxicol. 2006, 44, 522–527. [Google Scholar]
- Uwaifo, O.A. Reversion by vitamin K of aflatoxin B1 (AFB)-induced inhibition of oxygen uptake in three AFB-susceptible bacteria. Toxicol. Lett. 1983, 15, 57–60. [Google Scholar]
- Tiwari, R.P.; Dham, C.K.; Bhalla, T.C. Mechanism of action of aflatoxin B1 in Bacillus megaterium. Appl. Environ. Microbiol. 1985, 49, 904–907. [Google Scholar]
- Atroshi, F.; Rizzo, A.; Westermarck, T.; Ali-Vehmas, T. Effects of tamoxifen, melatonin, coenzyme Q10, and L-carnitine supplementation on bacterial growth in the presence of mycotoxins. Pharmacol. Res. 1998, 38, 289–295. [Google Scholar]
- Moricz, A.M.; Ott, P.G.; Szilagyi, M.; Otta, K.H.; Tyihak, E. Opposite effect of Cu(II) and Se(IV) ions on the antibacterial-toxic action of mycotoxins. Acta Biol. Hung. 2007, 58, 301–310. [Google Scholar]
- Tiwari, R.P.; Singh, G.; Vadehra, D.V. Drug resistance patterns and susceptibility to aflatoxin B1 of strains of Escherichia coli and Staphylococcus aureus. J. Med. Microbiol. 1986, 22, 115–118. [Google Scholar]
- Weekley, L.B.; Sherertz, P.C.; Kimbrough, T.D.; Llewellyn, G.C. Altered bacterial culture density following exposure to aflatoxins. J. Ind. Microbiol. Biotechnol. 1989, 4, 275–278. [Google Scholar]
- Kubena, L.F.; Bailey, R.H.; Byrd, J.A.; Young, C.R.; Corrier, D.E.; Stanker, L.H.; Rottinghaust, G.E. Cecal volatile fatty acids and broiler chick susceptibility to Salmonella typhimurium colonization as affected by aflatoxins and T-2 toxin. Poult. Sci. 2001, 80, 411–417. [Google Scholar]
- Larsen, A.B.; Cysewski, S.J.; Miller, J.M. Effect of aflatoxin on susceptibility of hamsters to Mycobacterium paratuberculosis. Am. J. Vet. Res. 1975, 36, 1545–1547. [Google Scholar]
- Abdelhamid, A.M.; El-Ayouty, S.A.; El-Saadany, H.H. The influence of contamination with separate mycotoxins (aflatoxins, ochratoxin A, citrinin, patulin, penicillic acid or sterigmatocystin) on the in vitro dry matter and organic matter digestibilities of some roughages (berseem hay and wheat straw). Arch. Anim. Nutr. 1992, 42, 179–185. [Google Scholar]
- Reiss, J. Influence of the mycotoxins aflatoxin B1, rubratoxin B, patulin and diacetoxyscirpenol on the fermentation activity of baker’s yeast. Mycopathologia 1973, 51, 337–345. [Google Scholar]
- Mohran, M.A.; Megalla, S.E.; Said, M.R. Effect of aflatoxin B-1 on the proteolytic activity of some lactic-acid bacteria. Mycopathologia 1984, 86, 99–101. [Google Scholar]
- Sutic, M.; Banina, A. Influence of aflatoxin B1 on gas production by lactic acid bacteria. J. Environ. Pathol. Toxicol. Oncol. 1990, 10, 149–153. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yunus, A.W.; Razzazi-Fazeli, E.; Bohm, J. Aflatoxin B1 in Affecting Broiler’s Performance, Immunity, and Gastrointestinal Tract: A Review of History and Contemporary Issues. Toxins 2011, 3, 566-590. https://doi.org/10.3390/toxins3060566
Yunus AW, Razzazi-Fazeli E, Bohm J. Aflatoxin B1 in Affecting Broiler’s Performance, Immunity, and Gastrointestinal Tract: A Review of History and Contemporary Issues. Toxins. 2011; 3(6):566-590. https://doi.org/10.3390/toxins3060566
Chicago/Turabian StyleYunus, Agha W., Ebrahim Razzazi-Fazeli, and Josef Bohm. 2011. "Aflatoxin B1 in Affecting Broiler’s Performance, Immunity, and Gastrointestinal Tract: A Review of History and Contemporary Issues" Toxins 3, no. 6: 566-590. https://doi.org/10.3390/toxins3060566




