Phytochemicals in Cancer Prevention and Therapy: Truth or Dare?
Abstract
:1. Introduction
2. Isothiocyanates
| Toxin name | Chemical structure |
|---|---|
| Glucosinolate |  |
| Sulforaphane |  |
| Curcumin |  |
| Genistein | 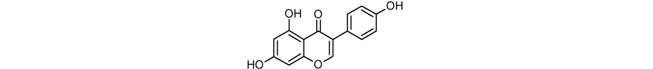 |
| Phenoxodiol (Synthetic genistein analog) | 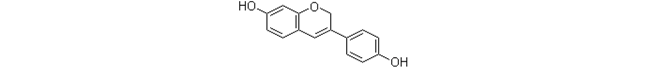 |
| Epigallocatechin gallate (ECGC) | 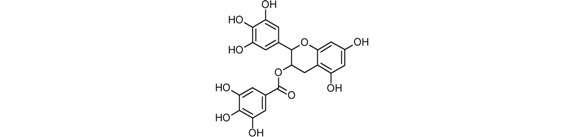 |
| Lycopene |  |
| Resveratrol | 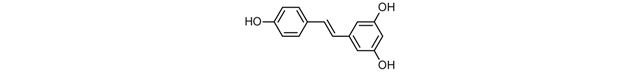 |
| Trial Lead Organizations | Therapy | Phase | Type of cancer | ||
|---|---|---|---|---|---|
| Polyphenol | Other treatments | ||||
| University of Texas - M.D. Anderson Cancer Center | Phenethyl isothiocyanate | Fludarabine | I | Active | Lymphoproliferative disorders |
| Mayo Clinic Cancer Center | 17-AAG 2 | Gemcitabine | II | Active | Recurrent Advanced Ovarian Epithelial or Primary Peritoneal Cavity Cancer |
| Mayo Clinic Cancer Center | 17-AAG 2 | Bortezomib | I | Active | Advanced solid tumors and lymphomas |
| Oregon Health and Science University - Knight Cancer Institute | Broccoli sprout extract | II | Active | Ductal carcinoma in situ and/or atypical ductal hyperplasia | |
| University of Texas - M. D. Anderson Cancer Center | Curcumin | II | Active | Advanced pancreatic cancer | |
| Rambam Medical Center | Curcumin | Gemcitabine | II | Active | Pancreatic cancer |
| Tel-Aviv Sourasky Medical Center | Curcumin | Gemcitabine and celecoxib | III | Active | Advance or inoperable pancreatic cancer |
| University of Texas - M. D. Anderson Cancer Center | Curcumin | Radiotherapy and capecitabine | II | Active | Rectal cancer |
| Tata Memorial Hospital | Curcumin | Ashwagandha extract3 | I/II | Active | Advanced osteosarcoma |
| Cleveland Clinic Taussig Cancer Center | Curcumin | Boswellia serrata4 | II | Active | Newly diagnosed or recurrent high-grade gliomas |
| University of Texas - M. D. Anderson Cancer Center | Curcumin | Bioperine | Completed | Multiple Myeloma | |
| Johns Hopkins University | Curcumin | II | Terminated | FAP5 | |
| Memorial Sloan-Kettering Cancer Center | Green Tea Extract | I | Completed | Advanced solid tumors | |
| Louisiana State University Health Sciences - Feist-Weiller Cancer Center | Green Tea Extract | II | Active | Breast cancer progression | |
| UCLA - Jonsson Comprehensive Cancer Center | Green Tea - Black tea | II | Active | Prostate adenocarcinoma | |
| University of Texas - M. D. Anderson Cancer Center | Polyphenon E 6 | I | Closed | Breast cancer | |
| University of Wisconsin - Paul P. Carbone Comprehensive Cancer Center | Polyphenon E 6 | II | Active | Bladder Cancer | |
| Barbara Ann Karmanos Cancer Institute | Polyphenon E 6 | II | Active | MGUS7 and Multiple Myeloma | |
| Mayo Clinic Cancer Center | Polyphenon E 6 | II | Active | Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma | |
| Louisiana State University Medical Center - Stanley S. Scott Cancer Center | Polyphenon E 6 | Erlotinib8 | I/II | Active | NSLC 9 |
| Aker Universitetssykehus HF | Syntetic Genistein (Bonistein) | II | Closed | Prostate cancer | |
| Northwestern University - Robert H. Lurie Comprehensive Cancer Center | Genistein | II | Closed | Prostate cancer | |
| Barbara Ann Karmanos Cancer Institute | Genistein | Gemcitabine | II | Completed | Breast cancer |
| University of North Carolina - Chapel Hill - Lineberger Comprehensive Cancer Center | Genistein | I | Closed | Prostate Cancer | |
| Robert H. Lurie Comprehensive Cancer Center at Northwestern University | Genistein | Interleukin-2 | II | Closed | Metastatic Melanoma or kidney cancer |
| University of Wisconsin Paul P. Carbone Comprehensive Cancer Center | Genistein | II | Closed | Bladder cancer | |
| Barbara Ann Karmanos Cancer Institute | Genistein | Gemcitabine, and Erlotinib | II | Closed | Pancreatic Cancer |
| University of Minnesota - Masonic Cancer Center | Genistein | I/II | Active | Osseous Metastases | |
| Parker Hughes Cancer Center | Genistein immunoconjugate | I | Closed | Recurrent B-cell Acute Lymphoblastic Leukemia or NHL 10 | |
| Barbara Ann Karmanos Cancer Institute | Soy isoflavones | II | Closed | Adenocarcinoma of the Prostate | |
| H. Lee Moffitt Cancer Center CCOP Research Bas | Soy isoflavones | NS | Closed | Breast cancer | |
| Barbara Ann Karmanos Cancer Institute | Soy isoflavones | Radiotherapy | II | Closed | Prostate cancer |
| Wake Forest University Comprehensive Cancer Center | Soy isoflavones | Cholecalciferol | II | Active | Prostate cancer |
| University of South Florida - H. Lee Moffitt Cancer Center and Research Institute | Purified isoflavones | II | Active | Prostate Cancer | |
| Novogen, Incorporated | Phenoxodiol 11 | I | Completed | Refractory solid tumors | |
| Yale Cancer Center | Phenoxodiol 11 | Docetaxel | I/II | Active | Ovarian epithelial, fallopian tube or primary peritoneal cavity cancer |
| Marshall Edwards, Inc. | Phenoxodiol 11 | II | Active | Prostate cancer | |
| University of Texas - M. D. Anderson Cancer Center | Lycopene | II | Closed | Prostate cancer | |
| H. Lee Moffitt Cancer Center and Research Institute | Lycopene | Completed | Prostate cancer | ||
| University of Illinois Cancer Center | Lycopene | NS | Closed | Prostate cancer or benign prostatic hyperplasia | |
| North Central Cancer Treatment Group | Lycopene | II | Completed | Metastatic prostate cancer | |
| USC/Norris Comprehensive Cancer Center and Hospital | Lycopene | Supplements12 | II | Active | Recurrent prostate cancer |
| Toronto Western Hospital | Lycopene | Antioxidants13 | II | Active | Early stage prostate cancer |
| Toronto Western Hospital | Lycopene | Antioxidants13 | II | Active | Prostate cancer |
| Ohio State University Medical Center - Arthur G. James Cancer Hospital and Solove Research Institute | Tomato-Soy Juice | I/II | Active | Prostate cancer | |
| Chao Family Comprehensive Cancer Center at University of California Irvine Medical Center | Resveratrol | I/II | Closed | Colon Cancer | |
| University of Michigan - Comprehensive Cancer Center | Resveratrol | I | Active | Resectable colorectal cancer | |
| GlaxoSmithkline | SRT50114 | I | Active | Colorectal cancer and hepatic metastases | |
| GlaxoSmithkline | SRT50114 | Bortezomib | II | Active | Multiple Myeloma |
3. Curcumin
4. Genistein
5. (-)-Epigallocatechin-3-gallate (EGCG)
6. Lycopene
7. Resveratrol
8. Conclusions
Acknowledgements
References and Notes
- Russo, G.L. Ins and outs of dietary phytochemicals in cancer chemoprevention. Biochem. Pharmacol. 2007, 74, 533–544. [Google Scholar]
- Sporn, M.B.; Suh, N. Chemoprevention: An essential approach to controlling cancer. Nat. Rev. Cancer 2002, 2, 537–543. [Google Scholar]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar]
- Hairborne, J.B. The Flavonoids Advances in Research since 1986; Chapman & Hall: London, UK, 1993; pp. 1–676. [Google Scholar]
- Russo, M.; Tedesco, I.; Iacomino, G.; Palumbo, R.; Galano, G.; Russo, G.L. Dietary Phytochemicals in Chemoprevention of Cancer. Curr. Med. Chem.-Immun. Endoc. Metab. Agents 2005, 5, 61–72. [Google Scholar]
- D'Incalci, M.; Steward, W.P.; Gescher, A.J. Use of cancer chemopreventive phytochemicals as antineoplastic agents. Lancet Oncol. 2005, 6, 899–904. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [PubMed]
- Manach, C.; Hubert, J.; Llorach, R.; Scalbert, A. The complex links between dietary phytochemicals and human health deciphered by metabolomics. Mol. Nutr. Food Res. 2009, 53, 1303–1315. [Google Scholar]
- Ross, S.A. Nutritional genomic approaches to cancer prevention research. Exp. Oncol. 2007, 29, 250–256. [Google Scholar]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar]
- Shapiro, T.A.; Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol. Biomarkers Prev. 1998, 7, 1091–1100. [Google Scholar]
- Rouzaud, G.; Young, S.A.; Duncan, A.J. Hydrolysis of glucosinolates to isothiocyanates after ingestion of raw or microwaved cabbage by human volunteers. Cancer Epidemiol. Biomarkers Prev. 2004, 13, 125–131. [Google Scholar]
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Holst, B.; Rowland, I.; De Schrijver, R.; Hansen, M.; Gerhauser, C.; Mithen, R.; Dekker, M. Glucosinolates in Brassica vegetables: The influence of the food supply chain on intake, bioavailability and human health. Mol. Nutr. Food Res. 2009, 53, S219–S265. [Google Scholar]
- Shammas, M.A.; Neri, P.; Koley, H.; Batchu, R.B.; Bertheau, R.C.; Munshi, V.; Prabhala, R.; Fulciniti, M.; Tai, Y.T.; Treon, S.P.; Goyal, R.K.; Anderson, K.C.; Munshi, N.C. Specific killing of multiple myeloma cells by (-)-epigallocatechin-3-gallate extracted from green tea: biologic activity and therapeutic implications. Blood 2006, 108, 2804–2810. [Google Scholar]
- Al Janobi, A.A.; Mithen, R.F.; Gasper, A.V.; Shaw, P.N.; Middleton, R.J.; Ortori, C.A.; Barrett, D.A. Quantitative measurement of sulforaphane, iberin and their mercapturic acid pathway metabolites in human plasma and urine using liquid chromatography-tandem electrospray ionisation mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006, 844, 223–234. [Google Scholar]
- Hanlon, N.; Coldham, N.; Gielbert, A.; Sauer, M.J.; Ioannides, C. Repeated intake of broccoli does not lead to higher plasma levels of sulforaphane in human volunteers. Cancer Lett. 2009, 284, 15–20. [Google Scholar]
- Kuroiwa, Y.; Nishikawa, A.; Kitamura, Y.; Kanki, K.; Ishii, Y.; Umemura, T.; Hirose, M. Protective effects of benzyl isothiocyanate and sulforaphane but not resveratrol against initiation of pancreatic carcinogenesis in hamsters. Cancer Lett. 2006, 241, 275–280. [Google Scholar]
- Okazaki, K.; Umemura, T.; Imazawa, T.; Nishikawa, A.; Masegi, T.; Hirose, M. Enhancement of urinary bladder carcinogenesis by combined treatment with benzyl isothiocyanate and N-butyl-N-(4-hydroxybutyl)nitrosamine in rats after initiation. Cancer Sci. 2003, 94, 948–952. [Google Scholar]
- Michaud, D.S.; Spiegelman, D.; Clinton, S.K.; Rimm, E.B.; Willett, W.C.; Giovannucci, E.L. Fruit and vegetable intake and incidence of bladder cancer in a male prospective cohort. J. Natl. Cancer Inst. 1999, 91, 605–613. [Google Scholar]
- Choi, W.Y.; Choi, B.T.; Lee, W.H.; Choi, Y.H. Sulforaphane generates reactive oxygen species leading to mitochondrial perturbation for apoptosis in human leukemia U937 cells. Biomed. Pharmacother. 2008, 62, 637–644. [Google Scholar]
- Xu, K.; Thornalley, P.J. Studies on the mechanism of the inhibition of human leukaemia cell growth by dietary isothiocyanates and their cysteine adducts in vitro. Biochem. Pharmacol. 2000, 60, 221–231. [Google Scholar]
- Yang, Y.M.; Conaway, C.C.; Chiao, J.W.; Wang, C.X.; Amin, S.; Whysner, J.; Dai, W.; Reinhardt, J.; Chung, F.L. Inhibition of benzo(a)pyrene-induced lung tumorigenesis in A/J mice by dietary N-acetylcysteine conjugates of benzyl and phenethyl isothiocyanates during the postinitiation phase is associated with activation of mitogen-activated protein kinases and p53 activity and induction of apoptosis. Cancer Res. 2002, 62, 2–7. [Google Scholar]
- Tsou, M.F.; Peng, C.T.; Shih, M.C.; Yang, J.S.; Lu, C.C.; Chiang, J.H.; Wu, C.L.; Lin, J.P.; Lo, C.; Fan, M.J.; Chung, J.G. Benzyl isothiocyanate inhibits murine WEHI-3 leukemia cells in vitro and promotes phagocytosis in BALB/c mice in vivo. Leuk. Res. 2009, 33, 1505–1511. [Google Scholar]
- Fimognari, C.; Lenzi, M.; Cantelli-Forti, G.; Hrelia, P. Induction of differentiation in human promyelocytic cells by the isothiocyanate sulforaphane. In Vivo 2008, 22, 317–320. [Google Scholar]
- Hecht, S.S. Chemoprevention by isothiocyanates. J. Cell Biochem. Suppl. 1995, 22, 195–209. [Google Scholar]
- Zhang, Y.; Talalay, P. Anticarcinogenic activities of organic isothiocyanates: Chemistry and mechanisms. Cancer Res. 1994, 54, 1976s–1981s. [Google Scholar] [PubMed]
- Rao, C.V.; Wang, C.X.; Simi, B.; Lubet, R.; Kelloff, G.; Steele, V.; Reddy, B.S. Enhancement of experimental colon cancer by genistein. Cancer Res. 1997, 57, 3717–3722. [Google Scholar]
- Fox, E.; Razzouk, B.I.; Widemann, B.C.; Xiao, S.; O'Brien, M.; Goodspeed, W.; Reaman, G.H.; Blaney, S.M.; Murgo, A.J.; Balis, F.M.; Adamson, P.C. Phase 1 trial and pharmacokinetic study of arsenic trioxide in children and adolescents with refractory or relapsed acute leukemia, including acute promyelocytic leukemia or lymphoma. Blood 2008, 111, 566–573. [Google Scholar]
- Doudican, N.A.; Bowling, B.; Orlow, S.J. Enhancement of arsenic trioxide cytotoxicity by dietary isothiocyanates in human leukemic cells via a reactive oxygen species-dependent mechanism. Leuk. Res. 2009, 34, 229–234. [Google Scholar]
- Akagi, K.; Sano, M.; Ogawa, K.; Hirose, M.; Goshima, H.; Shirai, T. Involvement of toxicity as an early event in urinary bladder carcinogenesis induced by phenethyl isothiocyanate, benzyl isothiocyanate, and analogues in F344 rats. Toxicol. Pathol. 2003, 31, 388–396. [Google Scholar] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2'-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health. C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as "Curecumin": From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple biological activities of curcumin: A short review. Life Sci. 2006, 78, 2081–2087. [Google Scholar]
- Kunnumakkara, A.B.; Anand, P.; Aggarwal, B.B. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008, 269, 199–225. [Google Scholar]
- Araujo, C.C.; Leon, L.L. Biological activities of Curcuma longa L. Mem. Inst. Oswaldo Cruz. 2001, 96, 723–728. [Google Scholar]
- Mazzanti, G.; Menniti-Ippolito, F.; Moro, P.A.; Cassetti, F.; Raschetti, R.; Santuccio, C.; Mastrangelo, S. Hepatotoxicity from green tea: A review of the literature and two unpublished cases. Eur. J. Clin. Pharmacol. 2009, 65, 331–341. [Google Scholar]
- Lev-Ari, S.; Starr, A.; Vexler, A.; Karaush, V.; Loew, V.; Greif, J.; Fenig, E.; Aderka, D.; Ben-Yosef, R. Inhibition of pancreatic and lung adenocarcinoma cell survival by curcumin is associated with increased apoptosis, down-regulation of COX-2 and EGFR and inhibition of Erk1/2 activity. Anticancer Res. 2006, 26, 4423–4430. [Google Scholar]
- Kunnumakkara, A.B.; Guha, S.; Krishnan, S.; Diagaradjane, P.; Gelovani, J.; Aggarwal, B.B. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007, 67, 3853–3861. [Google Scholar] [PubMed]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar]
- Bharti, A.C.; Donato, N.; Singh, S.; Aggarwal, B.B. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood 2003, 101, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Bharti, A.C.; Donato, N.; Aggarwal, B.B. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J. Immunol. 2003, 171, 3863–3871. [Google Scholar]
- Sung, B.; Kunnumakkara, A.B.; Sethi, G.; Anand, P.; Guha, S.; Aggarwal, B.B. Curcumin circumvents chemoresistance in vitro and potentiates the effect of thalidomide and bortezomib against human multiple myeloma in nude mice model. Mol. Cancer Ther. 2009, 8, 959–970. [Google Scholar]
- Vadhan, V.S.; Weber, D.; Giralt, S.; Alexanian, R.; Thomas, S.; Zhou, X.; Patel, P.; Bueso-Ramos, C.; Newman, R.; Aggarwal, B.B. Curcumin downregulates NF-kB and related genes in patients with multiple myeloma: Results of a phase1/2 study. Am. Soc. Hematol. 2007, in press.. [Google Scholar]
- Anand, P.; Sundaram, C.; Jhurani, S.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin and cancer: An "old-age" disease with an "age-old" solution. Cancer Lett. 2008, 267, 133–164. [Google Scholar]
- Chauhan, D.P. Chemotherapeutic potential of curcumin for colorectal cancer. Curr. Pharm. Des. 2002, 8, 1695–1706. [Google Scholar]
- Pereira, M.A.; Grubbs, C.J.; Barnes, L.H.; Li, H.; Olson, G.R.; Eto, I.; Juliana, M.; Whitaker, L.M.; Kelloff, G.J.; Steele, V.E.; Lubet, R.A. Effects of the phytochemicals, curcumin and quercetin, upon azoxymethane-induced colon cancer and 7,12-dimethylbenz[a]anthracene-induced mammary cancer in rats. Carcinogenesis 1996, 17, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, N.N.; Carothers, A.M.; Grunberger, D.; Bilinski, R.T.; Churchill, M.R.; Martucci, C.; Newmark, H.L.; Bertagnolli, M.M. Plant phenolics decrease intestinal tumors in an animal model of familial adenomatous polyposis. Carcinogenesis 2000, 21, 921–927. [Google Scholar]
- Garcea, G.; Berry, D.P.; Jones, D.J.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J. Consumption of the putative chemopreventive agent curcumin by cancer patients: Assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 120–125. [Google Scholar]
- Cruz-Correa, M.; Shoskes, D.A.; Sanchez, P.; Zhao, R.; Hylind, L.M.; Wexner, S.D.; Giardiello, F.M. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin. Gastroenterol. Hepatol. 2006, 4, 1035–1038. [Google Scholar]
- Syng-Ai, C.; Kumari, A.L.; Khar, A. Effect of curcumin on normal and tumor cells: Role of glutathione and bcl-2. Mol. Cancer Ther. 2004, 3, 1101–1108. [Google Scholar]
- Burgos-Moron, E.; Calderon-Montano, J.M.; Salvador, J.; Robles, A.; Lopez-Lazaro, M. The dark side of curcumin. Int. J. Cancer 2009, 126, 1771–1175. [Google Scholar]
- Lopez-Lazaro, M. Anticancer and carcinogenic properties of curcumin: Considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol. Nutr. Food Res. 2008, 52 (Suppl. 1), S103–S127. [Google Scholar] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar]
- Lampe, J.W.; Nishino, Y.; Ray, R.M.; Wu, C.; Li, W.; Lin, M.G.; Gao, D.L.; Hu, Y.; Shannon, J.; Stalsberg, H.; Porter, P.L.; Frankenfeld, C.L.; Wahala, K.; Thomas, D.B. Plasma isoflavones and fibrocystic breast conditions and breast cancer among women in Shanghai, China. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Mills, P.K.; Beeson, W.L.; Phillips, R.L.; Fraser, G.E. Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer 1989, 64, 598–604. [Google Scholar] [PubMed]
- Kurahashi, N.; Iwasaki, M.; Sasazuki, S.; Otani, T.; Inoue, M.; Tsugane, S. Soy product and isoflavone consumption in relation to prostate cancer in Japanese men. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 538–545. [Google Scholar]
- Banerjee, S.; Li, Y.; Wang, Z.; Sarkar, F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008, 269, 226–242. [Google Scholar]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar]
- Chang, E.C.; Charn, T.H.; Park, S.H.; Helferich, W.G.; Komm, B.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S. Estrogen Receptors alpha and beta as determinants of gene expression: influence of ligand, dose, and chromatin binding. Mol. Endocrinol. 2008, 22, 1032–1043. [Google Scholar] [PubMed]
- Akiyama, T.; Ishida, J.; Nakagawa, S.; Ogawara, H.; Watanabe, S.; Itoh, N.; Shibuya, M.; Fukami, Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987, 262, 5592–5595. [Google Scholar]
- Sakla, M.S.; Shenouda, N.S.; Ansell, P.J.; Macdonald, R.S.; Lubahn, D.B. Genistein affects HER2 protein concentration, activation, and promoter regulation in BT-474 human breast cancer cells. Endocrine 2007, 32, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.N.; Muqim, N.; Bhuiyan, M.; Kucuk, O.; Pienta, K.J.; Sarkar, F.H. Inhibition of prostate specific antigen expression by genistein in prostate cancer cells. Int. J. Oncol. 2000, 16, 1091–1097. [Google Scholar]
- Ullmann, U.; Metzner, J.; Frank, T.; Cohn, W.; Riegger, C. Safety, tolerability, and pharmacokinetics of single ascending doses of synthetic genistein (Bonistein) in healthy volunteers. Adv. Ther. 2005, 22, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Silasi, D.A.; Alvero, A.B.; Rutherford, T.J.; Brown, D.; Mor, G. Phenoxodiol: Pharmacology and clinical experience in cancer monotherapy and in combination with chemotherapeutic drugs. Expert Opin. Pharmacother. 2009, 10, 1059–1067. [Google Scholar]
- Choueiri, T.K.; Mekhail, T.; Hutson, T.E.; Ganapathi, R.; Kelly, G.E.; Bukowski, R.M. Phase I trial of phenoxodiol delivered by continuous intravenous infusion in patients with solid cancer. Ann. Oncol. 2006, 17, 860–865. [Google Scholar]
- Onozawa, M.; Kawamori, T.; Baba, M.; Fukuda, K.; Toda, T.; Sato, H.; Ohtani, M.; Akaza, H.; Sugimura, T.; Wakabayashi, K. Effects of a soybean isoflavone mixture on carcinogenesis in prostate and seminal vesicles of F344 rats. Jpn. J. Cancer Res. 1999, 90, 393–398. [Google Scholar]
- Sharma, O.P.; Adlercreutz, H.; Strandberg, J.D.; Zirkin, B.R.; Coffey, D.S.; Ewing, L.L. Soy of dietary source plays a preventive role against the pathogenesis of prostatitis in rats. J. Steroid Biochem. Mol. Biol. 1992, 43, 557–564. [Google Scholar]
- Banerjee, S.; Zhang, Y.; Ali, S.; Bhuiyan, M.; Wang, Z.; Chiao, P.J.; Philip, P.A.; Abbruzzese, J.; Sarkar, F.H. Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Res. 2005, 65, 9064–9072. [Google Scholar]
- Banerjee, S.; Zhang, Y.; Wang, Z.; Che, M.; Chiao, P.J.; Abbruzzese, J.L.; Sarkar, F.H. In vitro and in vivo molecular evidence of genistein action in augmenting the efficacy of cisplatin in pancreatic cancer. Int. J. Cancer 2007, 120, 906–917. [Google Scholar]
- Begum, M.; Tashiro, H.; Katabuchi, H.; Suzuki, A.; Kurman, R.J.; Okamura, H. Neonatal estrogenic exposure suppresses PTEN-related endometrial carcinogenesis in recombinant mice. Lab. Invest. 2006, 86, 286–296. [Google Scholar]
- Yamasaki, M.; Fujita, S.; Ishiyama, E.; Mukai, A.; Madhyastha, H.; Sakakibara, Y.; Suiko, M.; Hatakeyama, K.; Nemoto, T.; Morishita, K.; Kataoka, H.; Tsubouchi, H.; Nishiyama, K. Soy-derived isoflavones inhibit the growth of adult T-cell leukemia cells in vitro and in vivo. Cancer Sci. 2007, 98, 1740–1746. [Google Scholar]
- Raffoul, J.J.; Banerjee, S.; Che, M.; Knoll, Z.E.; Doerge, D.R.; Abrams, J.; Kucuk, O.; Sarkar, F.H.; Hillman, G.G. Soy isoflavones enhance radiotherapy in a metastatic prostate cancer model. Int. J. Cancer 2007, 120, 2491–2498. [Google Scholar]
- Takimoto, C.H.; Glover, K.; Huang, X.; Hayes, S.A.; Gallot, L.; Quinn, M.; Jovanovic, B.D.; Shapiro, A.; Hernandez, L.; Goetz, A.; Llorens, V.; Lieberman, R.; Crowell, J.A.; Poisson, B.A.; Bergan, R.C. Phase I pharmacokinetic and pharmacodynamic analysis of unconjugated soy isoflavones administered to individuals with cancer. Cancer Epidemiol. Biomarkers Prev. 2003, 12, 1213–1221. [Google Scholar]
- Fischer, L.; Mahoney, C.; Jeffcoat, A.R.; Koch, M.A.; Thomas, B.E.; Valentine, J.L.; Stinchcombe, T.; Boan, J.; Crowell, J.A.; Zeisel, S.H. Clinical characteristics and pharmacokinetics of purified soy isoflavones: Multiple-dose administration to men with prostate neoplasia. Nutr. Cancer 2004, 48, 160–170. [Google Scholar]
- Miltyk, W.; Craciunescu, C.N.; Fischer, L.; Jeffcoat, R.A.; Koch, M.A.; Lopaczynski, W.; Mahoney, C.; Jeffcoat, R.A.; Crowell, J.; Paglieri, J.; Zeisel, S.H. Lack of significant genotoxicity of purified soy isoflavones (genistein, daidzein, and glycitein) in 20 patients with prostate cancer. Am. J. Clin. Nutr. 2003, 77, 875–882. [Google Scholar] [PubMed]
- Pendleton, J.M.; Tan, W.W.; Anai, S.; Chang, M.; Hou, W.; Shiverick, K.T.; Rosser, C.J. Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy. BMC Cancer 2008, 8, 132–142. [Google Scholar]
- Uckun, F.M.; Evans, W.E.; Forsyth, C.J.; Waddick, K.G.; Ahlgren, L.T.; Chelstrom, L.M.; Burkhardt, A.; Bolen, J.; Myers, D.E. Biotherapy of B-cell precursor leukemia by targeting genistein to CD19-associated tyrosine kinases. Science 1995, 267, 886–891. [Google Scholar]
- Uckun, F.M.; Narla, R.K.; Zeren, T.; Yanishevski, Y.; Myers, D.E.; Waurzyniak, B.; Ek, O.; Schneider, E.; Messinger, Y.; Chelstrom, L.M.; Gunther, R.; Evans, W. In vivo toxicity, pharmacokinetics, and anticancer activity of Genistein linked to recombinant human epidermal growth factor. Clin. Cancer Res. 1998, 4, 1125–1134. [Google Scholar]
- Khan, N.; Afaq, F.; Saleem, M.; Ahmad, N.; Mukhtar, H. Targeting multiple signaling pathways by green tea polyphenol (-)-epigallocatechin-3-gallate. Cancer Res. 2006, 66, 2500–2505. [Google Scholar]
- Carlson, J.R.; Bauer, B.A.; Vincent, A.; Limburg, P.J.; Wilson, T. Reading the tea leaves: Anticarcinogenic properties of (-)-epigallocatechin-3-gallate. Mayo Clin. Proc. 2007, 82, 725–732. [Google Scholar]
- Frei, B.; Higdon, J.V. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J. Nutr. 2003, 133, 3275S–3284S. [Google Scholar] [PubMed]
- Rietveld, A.; Wiseman, S. Antioxidant effects of tea: evidence from human clinical trials. J. Nutr. 2003, 133, 3285S–3292S. [Google Scholar] [PubMed]
- Kanadzu, M.; Lu, Y.; Morimoto, K. Dual function of (--)-epigallocatechin gallate (EGCG) in healthy human lymphocytes. Cancer Lett. 2006, 241, 250–255. [Google Scholar]
- Yang, C.S.; Chen, L.; Lee, M.J.; Balentine, D.; Kuo, M.C.; Schantz, S.P. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol. Biomarkers Prev. 1998, 7, 351–354. [Google Scholar]
- Lee, Y.K.; Bone, N.D.; Strege, A.K.; Shanafelt, T.D.; Jelinek, D.F.; Kay, N.E. VEGF receptor phosphorylation status and apoptosis is modulated by a green tea component, epigallocatechin-3-gallate (EGCG), in B-cell chronic lymphocytic leukemia. Blood 2004, 104, 788–794. [Google Scholar]
- Kuzuhara, T.; Suganuma, M.; Fujiki, H. Green tea catechin as a chemical chaperone in cancer prevention. Cancer Lett. 2008, 261, 12–20. [Google Scholar]
- Kuzuhara, T.; Sei, Y.; Yamaguchi, K.; Suganuma, M.; Fujiki, H. DNA and RNA as new binding targets of green tea catechins. J. Biol. Chem. 2006, 281, 17446–17456. [Google Scholar]
- Umeda, D.; Tachibana, H.; Yamada, K. Epigallocatechin-3-O-gallate disrupts stress fibers and the contractile ring by reducing myosin regulatory light chain phosphorylation mediated through the target molecule 67 kDa laminin receptor. Biochem. Biophys. Res. Commun. 2005, 333, 628–635. [Google Scholar]
- Umeda, D.; Yano, S.; Yamada, K.; Tachibana, H. Green tea polyphenol epigallocatechin-3-gallate signaling pathway through 67-kDa laminin receptor. J. Biol. Chem. 2008, 283, 3050–3058. [Google Scholar]
- Leone, M.; Zhai, D.; Sareth, S.; Kitada, S.; Reed, J.C.; Pellecchia, M. Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res. 2003, 63, 8118–8121. [Google Scholar]
- Golden, E.B.; Lam, P.Y.; Kardosh, A.; Gaffney, K.J.; Cadenas, E.; Louie, S.G.; Petasis, N.A.; Chen, T.C.; Schonthal, A.H. Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid-based proteasome inhibitors. Blood 2009, 113, 5927–5937. [Google Scholar]
- Katiyar, S.K.; Agarwal, R.; Wang, Z.Y.; Bhatia, A.K.; Mukhtar, H. (-)-Epigallocatechin-3-gallate in Camellia sinensis leaves from Himalayan region of Sikkim: inhibitory effects against biochemical events and tumor initiation in Sencar mouse skin. Nutr. Cancer 1992, 18, 73–83. [Google Scholar]
- Katiyar, S.K.; Bergamo, B.M.; Vyalil, P.K.; Elmets, C.A. Green tea polyphenols: DNA photodamage and photoimmunology. J. Photochem. Photobiol. B 2001, 65, 109–114. [Google Scholar]
- Scandlyn, M.J.; Stuart, E.C.; Somers-Edgar, T.J.; Menzies, A.R.; Rosengren, R.J. A new role for tamoxifen in oestrogen receptor-negative breast cancer when it is combined with epigallocatechin gallate. Br. J. Cancer 2008, 99, 1056–1063. [Google Scholar]
- Scaltriti, M.; Belloni, L.; Caporali, A.; Davalli, P.; Remondini, D.; Rizzi, F.; Astancolle, S.; Corti, A.; Bettuzzi, S. Molecular classification of green tea catechin-sensitive and green tea catechin-resistant prostate cancer in the TRAMP mice model by quantitative real-time PCR gene profiling. Carcinogenesis 2006, 27, 1047–1053. [Google Scholar]
- Bettuzzi, S.; Rizzi, F.; Belloni, L. Clinical relevance of the inhibitory effect of green tea catechins (GtCs) on prostate cancer progression in combination with molecular profiling of catechin-resistant tumors: an integrated view. Pol. J. Vet. Sci. 2007, 10, 57–60. [Google Scholar]
- Chow, H.H.; Cai, Y.; Alberts, D.S.; Hakim, I.; Dorr, R.; Shahi, F.; Crowell, J.A.; Yang, C.S.; Hara, Y. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol. Biomarkers Prev. 2001, 10, 53–58. [Google Scholar]
- Pisters, K.M.; Newman, R.A.; Coldman, B.; Shin, D.M.; Khuri, F.R.; Hong, W.K.; Glisson, B.S.; Lee, J.S. Phase I trial of oral green tea extract in adult patients with solid tumors. J. Clin. Oncol. 2001, 19, 1830–1838. [Google Scholar]
- Jatoi, A.; Ellison, N.; Burch, P.A.; Sloan, J.A.; Dakhil, S.R.; Novotny, P.; Tan, W.; Fitch, T.R.; Rowland, K.M.; Young, C.Y.; Flynn, P.J. A phase II trial of green tea in the treatment of patients with androgen independent metastatic prostate carcinoma. Cancer 2003, 97, 1442–1446. [Google Scholar]
- McLarty, J.; Bigelow, R.L.; Smith, M.; Elmajian, D.; Ankem, M.; Cardelli, J.A. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev. Res. (Phila Pa) 2009, 2, 673–682. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Lee, Y.K.; Call, T.G.; Nowakowski, G.S.; Dingli, D.; Zent, C.S.; Kay, N.E. Clinical effects of oral green tea extracts in four patients with low grade B-cell malignancies. Leuk. Res. 2006, 30, 707–712. [Google Scholar]
- Shanafelt, T.D.; Call, T.G.; Zent, C.S.; LaPlant, B.; Bowen, D.A.; Roos, M.; Secreto, C.R.; Ghosh, A.K.; Kabat, B.F.; Lee, M.J.; Yang, C.S.; Jelinek, D.F.; Erlichman, C.; Kay, N.E. Phase I trial of daily oral Polyphenon E in patients with asymptomatic Rai stage 0 to II chronic lymphocytic leukemia. J. Clin. Oncol. 2009, 27, 3808–3814. [Google Scholar]
- van Breemen, R.B.; Pajkovic, N. Multitargeted therapy of cancer by lycopene. Cancer Lett. 2008, 269, 339–351. [Google Scholar]
- Fang, L.; Pajkovic, N.; Wang, Y.; Gu, C.; van Breemen, R.B. Quantitative analysis of lycopene isomers in human plasma using high-performance liquid chromatography-tandem mass spectrometry. Anal. Chem. 2003, 75, 812–817. [Google Scholar]
- Henry, L.K.; Puspitasari-Nienaber, N.L.; Jaren-Galan, M.; van Breemen, R.B.; Catignani, G.L.; Schwartz, S.J. Effects of ozone and oxygen on the degradation of carotenoids in an aqueous model system. J. Agric. Food Chem. 2000, 48, 5008–5013. [Google Scholar]
- Clinton, S.K. Lycopene: Chemistry, biology, and implications for human health and disease. Nutr. Rev. 1998, 56, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Ukai, N.; Lu, Y.; Etoh, H. Photosensitized oxygenation of lycopene. Biosci. Biotechnol. Biochem. 1994, 58, 1718–1719. [Google Scholar]
- Krinsky, N.I. Mechanism of action of biological antioxidants. Proc. Soc. Exp. Biol. Med. 1992, 200, 248–254. [Google Scholar]
- Erdman, J.W., Jr.; Ford, N.A.; Lindshield, B.L. Are the health attributes of lycopene related to its antioxidant function? Arch. Biochem. Biophys. 2009, 483, 229–235. [Google Scholar]
- Boileau, T.W.; Boileau, A.C.; Erdman, J.W., Jr. Bioavailability of all-trans and cis-isomers of lycopene. Exp. Biol. Med. (Maywood) 2002, 227, 914–919. [Google Scholar]
- Clinton, S.K.; Emenhiser, C.; Schwartz, S.J.; Bostwick, D.G.; Williams, A.W.; Moore, B.J.; Erdman, J.W., Jr. cis-trans Lycopene isomers, carotenoids, and retinol in the human prostate. Cancer Epidemiol. Biomarkers Prev. 1996, 5, 823–833. [Google Scholar]
- Gustin, D.M.; Rodvold, K.A.; Sosman, J.A.; Diwadkar-Navsariwala, V.; Stacewicz-Sapuntzakis, M.; Viana, M.; Crowell, J.A.; Murray, J.; Tiller, P.; Bowen, P.E. Single-dose pharmacokinetic study of lycopene delivered in a well-defined food-based lycopene delivery system (tomato paste-oil mixture) in healthy adult male subjects. Cancer Epidemiol. Biomarkers Prev. 2004, 13, 850–860. [Google Scholar]
- van Breemen, R.B.; Xu, X.; Viana, M.A.; Chen, L.; Stacewicz-Sapuntzakis, M.; Duncan, C.; Bowen, P.E.; Sharifi, R. Liquid chromatography-mass spectrometry of cis- and all-trans-lycopene in human serum and prostate tissue after dietary supplementation with tomato sauce. J. Agric. Food Chem. 2002, 50, 2214–2219. [Google Scholar]
- Syed, D.N.; Khan, N.; Afaq, F.; Mukhtar, H. Chemoprevention of prostate cancer through dietary agents: Progress and promise. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 2193–2203. [Google Scholar]
- Burgess, L.C.; Rice, E.; Fischer, T.; Seekins, J.R.; Burgess, T.P.; Sticka, S.J.; Klatt, K. Lycopene has limited effect on cell proliferation in only two of seven human cell lines (both cancerous and noncancerous) in an in vitro system with doses across the physiological range. Toxicol. In Vitro 2008, 22, 1297–1300. [Google Scholar]
- Giovannucci, E. Does prostate-specific antigen screening influence the results of studies of tomatoes, lycopene, and prostate cancer risk? J. Natl. Cancer Inst. 2007, 99, 1060–1062. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Jin, T.; Zeng, X.; Wang, J.S. Lycopene inhibits the growth of human androgen-independent prostate cancer cells in vitro and in BALB/c nude mice. J. Nutr. 2005, 135, 287–290. [Google Scholar]
- Limpens, J.; Schroder, F.H.; de Ridder, C.M.; Bolder, C.A.; Wildhagen, M.F.; Obermuller-Jevic, U.C.; Kramer, K.; van Weerden, W.M. Combined lycopene and vitamin E treatment suppresses the growth of PC-346C human prostate cancer cells in nude mice. J. Nutr. 2006, 136, 1287–1293. [Google Scholar]
- Siler, U.; Herzog, A.; Spitzer, V.; Seifert, N.; Denelavas, A.; Hunziker, P.B.; Barella, L.; Hunziker, W.; Lein, M.; Goralczyk, R.; Wertz, K. Lycopene effects on rat normal prostate and prostate tumor tissue. J. Nutr. 2005, 135, 2050S–2052S. [Google Scholar] [PubMed]
- Huang, C.S.; Liao, J.W.; Hu, M.L. Lycopene inhibits experimental metastasis of human hepatoma SK-Hep-1 cells in athymic nude mice. J. Nutr. 2008, 138, 538–543. [Google Scholar]
- Chow, C.K. The relative efficacy of lycopene and beta-carotene in inhibiting experimental metastasis of human hepatoma SK-Hep-1 cells in athymic nude mice. J. Nutr. 2008, 138, 2289. [Google Scholar]
- Giovannucci, E. A review of epidemiologic studies of tomatoes, lycopene, and prostate cancer. Exp. Biol. Med. (Maywood) 2002, 227, 852–859. [Google Scholar] [PubMed]
- Kavanaugh, C.J.; Trumbo, P.R.; Ellwood, K.C. The U.S. Food and Drug Administration's evidence-based review for qualified health claims: Tomatoes, lycopene, and cancer. J. Natl. Cancer Inst. 2007, 99, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar]
- Kundu, J.K.; Surh, Y.J. Cancer chemopreventive and therapeutic potential of resveratrol: Mechanistic perspectives. Cancer Lett. 2008, 269, 243–261. [Google Scholar]
- Ulrich, S.; Wolter, F.; Stein, J.M. Molecular mechanisms of the chemopreventive effects of resveratrol and its analogs in carcinogenesis. Mol. Nutr. Food Res. 2005, 49, 452–461. [Google Scholar]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; Moon, R.C.; Pezzuto, J.M. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar]
- Bradamante, S.; Barenghi, L.; Villa, A. Cardiovascular protective effects of resveratrol. Cardiovasc. Drug Rev. 2004, 22, 169–188. [Google Scholar]
- Knutson, M.D.; Leeuwenburgh, C. Resveratrol and novel potent activators of SIRT1: Effects on aging and age-related diseases. Nutr. Rev. 2008, 66, 591–596. [Google Scholar]
- Ahmad, N.; Adhami, V.M.; Afaq, F.; Feyes, D.K.; Mukhtar, H. Resveratrol causes WAF-1/p21-mediated G(1)-phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin. Cancer Res. 2001, 7, 1466–1473. [Google Scholar]
- Delmas, D.; Rebe, C.; Micheau, O.; Athias, A.; Gambert, P.; Grazide, S.; Laurent, G.; Latruffe, N.; Solary, E. Redistribution of CD95, DR4 and DR5 in rafts accounts for the synergistic toxicity of resveratrol and death receptor ligands in colon carcinoma cells. Oncogene 2004, 23, 8979–8986. [Google Scholar]
- Fulda, S.; Debatin, K.M. Resveratrol modulation of signal transduction in apoptosis and cell survival: A mini-review. Cancer Detect. Prev. 2006, 30, 217–223. [Google Scholar]
- Cucciolla, V.; Borriello, A.; Oliva, A.; Galletti, P.; Zappia, V.; Della Ragione, F. Resveratrol: From basic science to the clinic. Cell Cycle 2007, 6, 2495–2510. [Google Scholar]
- Ziegler, C.C.; Rainwater, L.; Whelan, J.; McEntee, M.F. Dietary resveratrol does not affect intestinal tumorigenesis in Apc(Min/+) mice. J. Nutr. 2004, 134, 5–10. [Google Scholar]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; Pistell, P.J.; Poosala, S.; Becker, K.G.; Boss, O.; Gwinn, D.; Wang, M.; Ramaswamy, S.; Fishbein, K.W.; Spencer, R.G.; Lakatta, E.G.; Le Couteur, D.; Shaw, R.J.; Navas, P.; Puigserver, P.; Ingram, D.K.; de Cabo, R.; Sinclair, D.A. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; Geny, B.; Laakso, M.; Puigserver, P.; Auwerx, J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar]
- Drummond, D.C.; Noble, C.O.; Kirpotin, D.B.; Guo, Z.; Scott, G.K.; Benz, C.C. Clinical development of histone deacetylase inhibitors as anticancer agents. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 495–528. [Google Scholar]
- Wenzel, E.; Somoza, V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar]
- Bishayee, A. Cancer prevention and treatment with resveratrol: From rodent studies to clinical trials. Cancer Prev. Res. (Phila Pa) 2009, 2, 409–418. [Google Scholar]
- Levi, F.; Pasche, C.; Lucchini, F.; Ghidoni, R.; Ferraroni, M.; La Vecchia, C. Resveratrol and breast cancer risk. Eur. J. Cancer Prev. 2005, 14, 139–142. [Google Scholar]
- Chemoprevention Working Group. Prevention of cancer in the next millennium: Report of the Chemoprevention Working Group to the American Association for Cancer Research. Cancer Res. 1999, 59, 4743–4758. [Google Scholar] [PubMed]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Russo, M.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Phytochemicals in Cancer Prevention and Therapy: Truth or Dare? Toxins 2010, 2, 517-551. https://doi.org/10.3390/toxins2040517
Russo M, Spagnuolo C, Tedesco I, Russo GL. Phytochemicals in Cancer Prevention and Therapy: Truth or Dare? Toxins. 2010; 2(4):517-551. https://doi.org/10.3390/toxins2040517
Chicago/Turabian StyleRusso, Maria, Carmela Spagnuolo, Idolo Tedesco, and Gian Luigi Russo. 2010. "Phytochemicals in Cancer Prevention and Therapy: Truth or Dare?" Toxins 2, no. 4: 517-551. https://doi.org/10.3390/toxins2040517






