Venom Proteins of the Parasitoid Wasp Nasonia vitripennis: Recent Discovery of an Untapped Pharmacopee
Abstract
:1. Introduction

2. Venom and Its Impact on Immune System
2.1. Immune suppression
2.1.1. Phenoloxidase cascade

2.1.2. Coagulation cascade
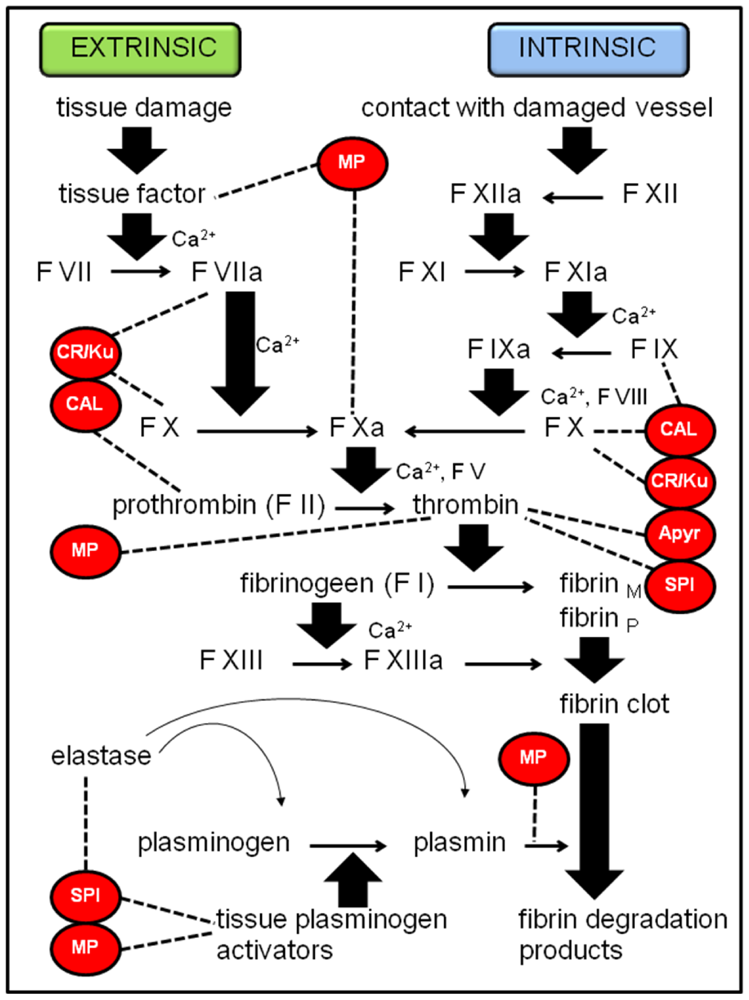
2.1.3. Other potential immune suppressing compounds
2.2. Immune stimulation
3. Venom and Developmental Arrest
4. Venom and Stimulation of Increments of Lipid Levels
5. Venom and Apoptosis
6. Venom and Nutritional Functions
7. “Unplaced” Venom Proteins
8. Conclusions and Future Prospects
References and Notes
- Rivers, D.B. Evaluation of host responses as means to assess ectoparasitic pteromalid wasp’s potential for controlling manure-breeding flies. Biol. Contr. 2004, 30, 181–192. [Google Scholar]
- Rivers, D.B.; Di Chira, J.; Tubman, A.; Yoder, J.A. Toxicity of crude venom from Nasonia vitripennis (Walker) (Hymenoptera: Pteromalidae) toward cultured cells and different developmental stages of mosquitoes and ticks. In Recent Advances in the Biochemistry, Toxicity and Mode of Action of Parasitic Wasp Venoms; Rivers, D.B., Yoder, J.A., Eds.; Research Signposts: Kerala, India, 2007; pp. 1–18. [Google Scholar]
- Ratcliffe, N.A.; King, P.E. Morphological, ultrastructural, histochemical and electrophoretic studies on the venom system of Nasonia vitripennis (Walker) (Hymenoptera: Pteromalidae). J. Morph. 1969, 127, 177–203. [Google Scholar] [CrossRef]
- Ratcliffe, N.A.; King, P.E. The “venom” system of Nasonia vitripennis (Walker) (Hymenoptera: pteromalidae). Proc. R. Ent. Soc. Lond. A 1967, 42, 49–61. [Google Scholar]
- Rivers, D.B.; Denlinger, D.L. Developmental fate of the flesh fly Sarcophaga bullata, envenomated by the pupal ectoparasitoid, Nasonia vitripennis. J. Insect Physiol. 1994, 40, 121–127. [Google Scholar]
- Rivers, D.B.; Denlinger, D.L. Venom-induced alteration in fly metabolism and its impact on larval development of the ectoparasitoid Nasonia vitripennis (Walker) (Hymenopter: Pteromalidae). J. Invert. Pathol. 1995, 66, 104–110. [Google Scholar]
- Rivers, D.B.; Genco, M.; Sanchez, R.A. In vitro analysis of venom from the wasp Nasonia vitripennis: susceptibility of different cell lines and venom-induced changes in plasma membrane permeability. In Vitro Cell. Dev. Biol. Animal 1999, 35, 102–110. [Google Scholar]
- Rivers, D.B.; Rocco, M.M.; Frayha, A.R. Venom from the ectoparasitic wasp Nasonia vitripennis increases Na+ influx and activates phospholipase C and phospholipase A2 dependent signal transduction pathways in cultured insect cells. Toxicon 2002, 40, 9–21. [Google Scholar] [PubMed]
- Rivers, D.B.; Ruggiero, L.; Hayes, M. The ectoparasitic wasp Nasonia vitripennis (Walker) (Hymenoptera: Pteromalidae) differentially affects cells mediating the immune response of its flesh fly host, Sarcophaga bullata Parker (Diptera: Sarcophagidae). J. Insect Phys. 2002, 48, 1053–1064. [Google Scholar]
- Rivers, D.B.; Ergin, E.; Uckan, F. Cell death in the host-parasitoid relationship. In New developments in cell apoptosis research; Corvin, A.J., Ed.; Nova Science Publishers: New York, NY, USA, 2006; pp. 69–96. [Google Scholar]
- Abt, M.; River, D.B. Characterization of phenoloxidase activity in venom from the ectoparasitoid Nasonia vitripennis (Walker) (Hymenoptera: Pteromalidae). J. Invert. Path. 2007, 94, 108–118. [Google Scholar]
- Rivers, D.B.; Brogan, A. Venom glands from the ectoparasitoid Nasonia vitripennis (Walker) (Hymenoptera: Pteromalidae) produce a calreticulin-like protein that functions in developmental arrest and cell death in the flesh fly host, Sarcophaga bullata Parker (Diptera: Sarcophagidae). In Insect Physiology: New Research; Maes, R.P., Ed.; Nova Science Publishers: New York, NY, USA, 2008; pp. 259–278. [Google Scholar]
- The Nasonia Genome Working Group. Functional and evolutionary insights from the genomes of three parasitoid Nasonia species. Science 2010, 327, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Nakamatsu, Y.; Tanaka, T. Development of a gregarious ectoparasitoid, Euplectrus separatae (Hymenoptera: Eulophidae), that parasitizes Pseudaletia separate (Lepidoptera: Noctuidae). Arthr. Struct. Dev. 2003, 32, 329–336. [Google Scholar] [CrossRef]
- Strand, M.R.; Pech, L.L. Immunological basis for compatability in parasitoid host relationships. Ann. Rev. Entomol. 1995, 40, 31–56. [Google Scholar]
- de Buron, I.; Beckage, N.E. Characterization of a polydnavirus (PDV) and virus-like filamentous particles (VLFP) in the braconid wasp Cotesia congregate (Hymenoptera: Braconidae). J. Invertebr. Pathol. 1992, 59, 315–327. [Google Scholar]
- Rivers, D.B. Evasion of host defense by ectoparasitic wasps. Tr. Entom. 2003, 3, 131–141. [Google Scholar]
- Beckage, N.; Metcalf, J.; Nesbit, K.; Schleifer, K.; Zetlan, S.R.; de Buron, I. Host hemolymph monophenoloxidase activity in parasite Manduca sexta larvae and evidence for inhibition by wasp polydnavirus. Insect Biochem. 1990, 20, 285–294. [Google Scholar]
- Asgari, S.; Zhan, G.; Zareie, R.; Schmidt, O. A serine proteinase homolog venom protein from an endoparasitoid wasp inhibits melanization of the host hemolymph. Insect Biochem. Mol. Biol. 2003, 33, 1017–1024. [Google Scholar] [PubMed]
- de Graaf, D.C.; Aerts, M.; Brunain, M.; Desjardins, C.A.; Jacobs, F.J.; Werren, J.H.; Devreese, B. Insights into the venom composition of the ectoparasitoid wasp Nasonia vitripennis from bioinformatics and proteomic studies. Insect Mol. Biol. 2010, 19 (S1), 11–26. [Google Scholar]
- Kanost, M.R. Serine Protease Inhibitors from the Serpin Gene Family in Manduca sexta and Drosophila Melanogaster; Hagedorn, H.H., Hildebrand, J.G., Kidwell, M.G., Law, J.H., Eds.; Plenum Press: New York, NY, USA, 1990; pp. 139–146. [Google Scholar]
- Jiang, H.; Kanost, M.R. Characterization and functional analysis of 12 naturally occurring reactive site variants of serpin-1 from Manduca sexta. J. Biol. Chem. 1997, 272, 1082–1087. [Google Scholar] [PubMed]
- Beck, M.; Theopold, U.; Schmidt, O. Evidence for serine protease inhibitor activity in the ovarian calyx fluid of the endoparasitoid Venturia canescens. J. Insect Physiol. 2000, 46, 1275–1283. [Google Scholar] [PubMed]
- Colinet, D.; Dubuffet, A.; Cazes, D.; Moreau, S.; Drezen, J.M.; Poirie, M. A serpin from the parasitoid wasp Leptopilina boulardi targets the Drosophila phenoloxidase cascade. Dev. Comp. Immunol. 2009, 33, 681–689. [Google Scholar] [PubMed]
- Zhu, Y.; Gorman, M.; Jiang, H.; Kanost, M.R. Manduca sexta serpin-3 regulates prophenoloxidase activation in response to infection by inhibiting prophenoloxidase-activating proteinases. J. Biol. Chem. 2003, 278, 46556–46564. [Google Scholar] [PubMed]
- Wang, Y.; Jiang, H. Purification and characterization of Manduca sexta serpin-6: A serine proteinase inhibitor that selectively inhibits prophenoloxidase-activating proteinase-3. Insect Biochem. Mol. Biol. 2004, 34, 387–395. [Google Scholar] [PubMed]
- Kellenberger, C.; Roussel, A. Structure-activity relationship within the serine protease inhibitors of the Pacifastin family. Prot. Pept. Lett. 2005, 12, 409–414. [Google Scholar]
- Parkinson, N.M.; Conyers, C.; Keen, J.; MacNicoll, A.; Smith, I.; Audsley, N.; Weaver, R. Towards a comprehensive view of the primary structure of venom proteins from the parasitoid wasp Pimpla hypochondriaca. Insect Biochem. Mol. Biol. 2004, 34, 565–571. [Google Scholar] [PubMed]
- Beck, M.H.; Strand, M.R. A novel polydnavirus protein inhibits the insect phenoloxidase activation pathway. Proc. Nat. Acad. Sci. USA 2007, 104, 19267–19272. [Google Scholar]
- Yu, X.Q.; Jiang, H.; Wang, Y.; Kanost, M.R. Nonproteolytic serine proteinase homologs are involved in prophenoloxidase activation in the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 2003, 33, 197–208. [Google Scholar] [PubMed]
- Zhang, G.; Lu, Z.; Jiang, H.; Asgari, S. Negative regulation of prophenoloxidase (proPO) activation by a clip-domain serine proteinase homolog (SPH) from endoparasitoid venom. Insect Biochem. Mol. Biol. 2004, 34, 477–483. [Google Scholar] [PubMed]
- Parkinson, N.; Smith, I.; Weaver, R.; Edwards, J.P. A new form of arthropod phenoloxidase is abundant in the parasitoid wasp Pimpla hypochondriaca. Insect Biochem. Mol. Biol. 2001, 31, 57–63. [Google Scholar] [PubMed]
- Rivers, D.B. Changes in oviposition behavior of the ectoparasitoids Nasonia vitripennis and Muscidifurax zaraptor (Hymenoptera: Pteromalidae) when using different species of fly hosts, prior oviposition experience, and allospecific competition. Ann. Entomol. Soc. A 1996, 89, 466–474. [Google Scholar]
- Whiting, A.R. The biology of the parasitic wasp Mormoniella vitripennis [=Nasonia brevicornis] (Walker). Q. Rev. Biol. 1967, 42, 333–406. [Google Scholar]
- Richards, E.H.; Edwards, J.P. Parasitism of Lacanobia oleracea (Lepidoptera) by the ectoparasitoid, Eulophus pennicornis, is associated with a reduction in host haemolymph phenoloxidase activity. Comp. Biochem. Physiol. B-Biochem. Mol. Biol. 2000, 127, 289–298. [Google Scholar] [PubMed]
- Coudron, T.A.; Kelly, T.J.; Puttler, B. Developmental responses of Trichoplusia ni (Lepidoptera, Noctuidae) to parasitism by the ectoparasite Euplectrus Plathypenae (Hymenoptera, Eulophidae). Arch. Insect Biochem. Phys. 1990, 13, 83–94. [Google Scholar]
- Theopold, U.; Schmidt, O.; Söderhäll, K.; Dushay, M.S. Coagulation in arthropods: Defence, wound closure and healing. Trends Immunol. 2004, 25, 289–294. [Google Scholar] [PubMed]
- Francischetti, I.M.B.; Mather, T.N.; Ribeiro, J.M.C. Cloning of a salivary gland metalloprotease and characterization of gelatinase and fibrin(ogen)lytic activities in the saliva of the Lyme disease tick vector Ixodes scapularis. Biochem. Biophys. Res. Comm. 2003, 305, 869–875. [Google Scholar]
- Jia, L.; Shimokawa, K.; Bjarnason, J.B.; Fox, J.W. Snake venom metalloproteinases: Structure, function and relationship to the adams family of proteins. Toxicon 1996, 34, 1269–1276. [Google Scholar] [PubMed]
- Parkinson, N.; Conyers, C.; Smith, I. A venom protein from the endoparasitoid wasp Pimpla hypochondriaca is similar to snake venom reprolysin-type metalloproteases. J. Invert. Pathol. 2002, 79, 129–131. [Google Scholar]
- Iwanaga, S.; Kawabata, S.; Muta, T. New types of clotting factors and defense molecules found in horseshoe crab hemolymph: Their structures and functions. J. Biochem. 1998, 123, 1–15. [Google Scholar] [PubMed]
- Miura, Y.; Kawabata, S.; Wakamiya, Y.; Nakamura, T.; Iwanaga, S. A limulus intracellular coagulation inhibitor type 2. J. Biol. Chem. 1995, 270, 558–565. [Google Scholar] [PubMed]
- Prevot, P.; Adam, B.; Boudjeltia, K.Z.; Brossard, M.; Lins, L.; Cauchie, P.; Brasseur, R.; Vanhaeverbeek, M.; Vanhamme, L.; Godfroid, E. Anti-hemostatic effects of a serpin from the saliva of the tick Ixodes ricinus. J. Biol. Chem. 2006, 281, 26361–26369. [Google Scholar] [PubMed]
- Friedrich, T.; Dröger, B.; Bialojan, S.; Lemaire, H.G.; Höffken, H.W.; Reuschenbach, P.; Otte, M.; Dodt, J. A Kazal-type inhibitor with thrombin specificity from Rhodnius prolixus. J. Biol. Chem. 1993, 268, 16216–16222. [Google Scholar] [PubMed]
- Waxman, L.; Smith, D.E.; Arcuri, K.E.; Vlasuk, G.P. Tick anticoagulant peptide (TAP) is a novel inhibitor of blood coagulation factor Xa. Science 1990, 248, 593–596. [Google Scholar] [PubMed]
- Francischetti, I.M.; Valenzuela, J.G.; Andersen, J.F.; Mather, T.N.; Ribeiro, J.M. Ixolaris, a novel recombinant tissue factor pathway inhibitor (TFPI) from the salivary gland of the tick, Ixodes scapularis: Identification of factor X and factor Xa as scaffolds for the inhibition of factor VIIa/tissue factor complex. Blood 2002, 99, 3602–3612. [Google Scholar] [PubMed]
- Francischetti, I.M.; Mather, T.N.; Ribeiro, J.M. Penthalaris, a novel recombinant five-Kunitz tissue factor pathway inhibitor (TFPI) from the salivary gland of the tick vector of Lyme disease, Ixodes scapularis. Thromb. Haemost. 2004, 91, 886–898. [Google Scholar] [PubMed]
- Yao, L.; Pike, S.E.; Tosato, G. Laminin binding to the calreticulin fragment vasostatin regulates endothelial cell function. J. Leukocyte Biol. 2002, 71, 47–53. [Google Scholar]
- Kuwabara, K.; Pinsky, D.J.; Schmidt, A.M.; Benedict, C.; Brett, J.; Ogawa, S.; Broekman, M.J.; Marcus, A.J.; Sciacca, R.R.; Michalak, M.; Wang, F.; Pan, Y.C.; Grunfeld, S.; Patton, S.; Malinski, T.; Stern, D.M.; Ryan, J. Calreticulin, an antithrombotic agent which binds to vitamin K-dependent coagulation factors, stimulates endothelial nitric oxide production, and limits thrombosis in canine coronary arteries. J. Biol. Chem. 1995, 270, 8179–8187. [Google Scholar] [PubMed]
- Faudry, E.; Santana, J.M.; Ebel, C.; Vernet, T.; Teixeira, A.R.L. Salivary apyrases of Triatoma infestans are assembled into homo-oligomers. Biochem. Soc. 2006, 396, 509–515. [Google Scholar]
- Felföldi, G.; Marokhazi, J.; Képiro, M.; Venekei, I. Identification of natural target proteins indicates functions of a serralysin-type metalloprotease, PrtA, in anti-immune mechanisms. Appl. Environ. Microbiol. 2009, 75, 3120–3126. [Google Scholar] [PubMed]
- Cönsoli, F.L.; Brandt, S.L.; Coudron, T.A.; Vinson, S.B. Host regulation and release of parasitism-specific proteins in the system Toxoneuron nigriceps-Helitohis virescens. Comp. Biochem. Physiol. 2005, 142, 181–191. [Google Scholar]
- Huber, M.; Cabib, E.; Miller, L.H. Malaria parasite chitinase and penetration of the mosquito peritrophic membrane. Proc. Natl. Acad. Sci. USA 1991, 88, 2807–2810. [Google Scholar]
- Xia, Y.; Judge, A.J.; Gillespie, J.P.; Clarkson, J.M.; Charnley, A.K. Acid phosphatases in the haemolymph of the desert locust, Schistocerca gregaria, infected with the entomopathogenic fungus Metarhizium anisopliae. J. Insect Physiol. 2000, 46, 1249–1257. [Google Scholar] [PubMed]
- Zhang, G.M.; Schmidt, O.; Asgari, S. A calreticulin-like protein from endoparasitoid venom fluid is involved in host hemocyte inactivation. Dev. Comp. Immunol. 2006, 30, 756–764. [Google Scholar] [PubMed]
- Pagh, R.; Duus, K.; Laursen, I.; Hansen, P.R.; Mangor, J.; Thielens, N.; Arlaud, G.J.; Kongerslev, L.; Hojrup, P.; Houen, G. The chaperone and potential mannan-binding lectin (MBL) co-receptor calreticulin interacts with MBL through the binding site for MBL-associated serine proteases. FEBS Lett. 2008, 275, 515–526. [Google Scholar]
- Kishore, U.; Sontheimer, R.D.; Sastry, K.N.; Zaner, K.S.; Zapp, E.G.; Hughes, G.R.V.; Khamashta, M.A.; Strong, P.; Reid, K.B.M.; Eggleton, P. Release of calreticulin from neutrophils may alter C1q-mediated immune functions. Biochem. J. 1997, 322, 543–550. [Google Scholar] [PubMed]
- de Graaf, D.C.; Brunain, M.; Scharlaken, B.; Peiren, N.; Devreese, B.; Ebo, D.G.; Stevens, W.J.; Desjardins, C.A.; Werren, J.H.; Jacobs, F.J. Novel Apis mellifera and Nasonia vitripennis venom associated protein with ancient C1q-like domain. Insect Mol. Biol. 2010, 19 (S1), 1–10. [Google Scholar]
- Kawabata, S.; Nagayama, R.; Hirata, M.; Shigenaga, T.; Lala Agarwala, K.; Saito, T.; Cho, J.; Nakajima, H.; Takagi, T.; Iwanaga, S. Tachycitin, a small granular component in horseshoe crab hemocytes, is an antimicrobial protein with chitin-binding activity. J. Biochem. 1996, 120, 1253–1260. [Google Scholar] [PubMed]
- Peiren, N.; de Graaf, D.C.; Vanrobaeys, F.; Danneels, E.L.; Devreese, B.; Van Beeumen, J.; Jacobs, F.J. Proteomic analysis of the honey bee worker venom gland focusing on the mechanisms of protection against tissue damage. Toxicon 2008, 52, 72–83. [Google Scholar] [PubMed]
- Ochiai, M.; Ashida, M. A pattern-recognition protein for β-1,3-glucan. J. Biol. Chem. 2000, 275, 4995–5002. [Google Scholar] [PubMed]
- Kim, Y.; Ryu, J.; Han, S.; Choi, K.; Nam, K.; Jang, I.; Lemaitre, B.; Bry, P.T.; Lee, W. Gram-negative bacteria-binding protein, a pattern recognition receptor for lipopolysaccharide and β-1,3-glucan that mediates the signaling for the induction of innate immune genes in Drosophila melanogaster cells. J. Biol. Chem. 2000, 275, 32721–32727. [Google Scholar] [PubMed]
- Boonacker, E.; Van Noorden, C.J.F. The multifunctional or moonlighting protein CD26/DPPIV. Eur. J. Cell Biol. 2003, 82, 53–73. [Google Scholar] [PubMed]
- de Graaf, D.C.; Aerts, M.; Danneels, E.; Devreese, B. Bee, wasp and ant venomics pave the way for a component-resolved diagnosis of sting allergy. J. Prot. 2009, 72, 145–154. [Google Scholar]
- Isaac, R.E.; Schoofs, L.; Williams, T.A.; Veelaerts, D.; Sajid, M.; Corvol, P.; Coates, D. A novel peptide-processing activity of insect peptidyl-dipeptidase A (angiotensin I-converting enzyme): The hydrolysis of lysyl-arginine and arginyl-arginine from the C-terminus of an insect prohormone peptide. Biochem. J. 1998, 330, 61–65. [Google Scholar] [PubMed]
- Dani, M.P.; Richards, E.H.; Isaac, R.E.; Edwards, J.P. Antibacterial and proteolytic activity in venom from the endoparasitic wasp Pimpla hypochondriaca (Hymenoptera: Ichneumonidae). J. Insect Phys. 2003, 49, 945–954. [Google Scholar]
- Kawabata, S.; Tokunaga, F.; Kugi, Y.; Motoyama, S.; Miura, Y.; Hirata, M.; Iwanaga, S. Limulus factor D, a 43-kDa protein isolated from horseshoe crab hemocytes, is a serine protease homologue with antimicrobial activity. FEBS Lett. 1996, 398, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Buchon, N.; Poidevin, M.; Kwon, H.M.; Guillou, A.; Sottas, V.; Lee, B.L.; Lemaitre, B. A single modular serine protease integrates signals from pattern-recognition receptors upstream of the Drosophila Toll pathway. Proc. Natl. Acad. Sci. USA 2009, 106, 12442–12447. [Google Scholar]
- Nirmala, X.; Kodrik, D.; Zurovec, M.; Sehnal, F. Insect silk contains both a Kunitz-type and a unique Kazal-type proteinase inhibitor. Eur. J. Biochem. 2001, 268, 2064–2073. [Google Scholar] [PubMed]
- Dahlman, D.L.; Rana, R.L.; Schepers, E.J.; Schepers, T.; DiLuna, F.A.; Webb, B.A. A teratocyte gene from a parasitic wasp that is associated with inhibition of insect growth and development inhibits host protein synthesis. Insect Mol. Biol. 2003, 12, 527–534. [Google Scholar] [PubMed]
- Tanaka, K.; Matsumoto, H.; Hayakawa, Y. Analysis in the course of polydnavirus replication in ovarian calyx cells of the parasitoid wasp, Cotesia kariyai (Hymenoptera: Braconidae). Appl. Entomol. Zool. 2002, 37, 323–328. [Google Scholar] [CrossRef]
- Rivers, D.B.; Hink, W.F.; Denlinger, D.L. Toxicity of the venom from Nasonia vitripennis (Hymenoptera: Pteromalidae) toward fly hosts, nontarget insects, different developmental stages and cultured insect cells. Toxicon 1993, 31, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Honegger, B.; Galic, M.; Köhler, K.; Wittwer, F.; Brogiolo, W.; Hafen, E.; Stocker, H. Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. J. Biol. 2008, 7, 1–11. [Google Scholar] [PubMed]
- Andersen, A.S.; Hansen, P.H.; Schäffer, L.; Kristensen, C. A new secreted insect protein belonging to the immunoglobulin superfamily binds insulin and related peptides and inhibits their activities. J. Biol. Chem. 2000, 275, 16948–16953. [Google Scholar]
- Price, D.R.G.; Bell, H.A.; Hinchliffe, G.; Fitches, E.; Weaver, R.; Gatehouse, J.A. A venom metalloproteinase from the parasitic wasp Eulophus pennicornis is toxic towards its host, tomato moth (Lacanobia oleracae). Insect Mol. Biol. 2009, 18, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.S.H.; Pelliccia, J.G.; Wilkinson, C.F. Age-dependent arylsulphatase and sulphotransferase activities in the southern armyworm: A possible insect endocrine regulatory mechanism? Biochem. J. 1973, 136, 817–820. [Google Scholar] [PubMed]
- Dantuma, N.P.; Potters, M.; De Winther, M.P.J.; Tensen, C.P.; Kooiman, F.P.; Bogerd, J.; Van der Horst, D.J. An insect homolog of the vertebrate very low density lipoprotein receptor mediates endocytosis of lipophorins. J. Lipid Res. 2009, 40, 973–978. [Google Scholar]
- Rivers, D.B.; Crawley, T.; Bauser, H. Localization of intracellular calcium release in cells injured by venom from the ectoparasitoid Nasonia vitripennis (Walker) (Hymenoptera: Pteromalidae) and dependence of calcium mobilization on G-protein activation. J. Insect Physiol. 2005, 51, 149–160. [Google Scholar] [PubMed]
- Li, L.Y.; Lou, X.; Wang, X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 2001, 412, 95–99. [Google Scholar] [PubMed]
- Shibayama, K.; Kamachi, K.; Nagata, N.; Yagi, T.; Nada, T.; Doi, Y.; Shibata, N.; Yokoyama, K.; Yamane, K.; Kato, H.; Iinuma, Y.; Arakawa, Y. A novel apoptosis-inducing protein from Helicobacter pylori. Mol. Microbiol. 2003, 47, 443–451. [Google Scholar] [PubMed]
- Falabella, P.; Riviello, L.; Caccialupi, P.; Rossodivita, T.; Valente, M.T.; De Stradis, M.L.; Tranfaglia, A.; Varricchio, P.; Gigliotti, S.; Graziani, F.; Malva, C.; Pennacchio, F. A γ-glutamyl transpeptidase of Aphidius ervi venom induces apoptosis in the ovaries of host aphids. Insect Biochem. Mol. Biol. 2007, 37, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Chen, X.; Liu, Y.; Tian, H.; Liu, J.; Hu, J.; Xu, W.; Zhang, W. Charachterization and expression patterns of a membrane-bound trehalase from Spodoptera exigua. BMC Mol. Biol. 2008, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, N.M.; Conyers, C.M.; Keen, J.N.; MacNicoll, A.D.; Smith, I.; Weaver, R.J. cDNAs encoding large venom proteins from the parasitoid wasp Pimpla hypochondriaca identified by random sequence analysis. Comp. Biochem. Physiol. 2003, 134, 513–520. [Google Scholar]
- Miyoshi, T.; Tsuji, N.; Islam, M.K.; Kamio, T.; Fujisaki, K. Enzymatic characterization of a cubilin-related serine proteinase from the hard tick Haemaphysalis longicornis. J. Vet. Med. Sci. 2004, 66, 1195–1198. [Google Scholar] [PubMed]
- Nakamatsu, Y.; Tanaka, T. The function of a trypsin-like enzyme in the saliva of Euplectrus separatae larvae. J. Insect Physiol. 2004, 50, 847–854. [Google Scholar] [PubMed]
- Nakamatsu, Y.; Fujii, S.; Tanaka, T. Larvae of an endoparasitoid, Cotesia kariyai (Hymenoptera: Braconidae), feed on the host fat body directly in the second stadium with the help of teratocytes. J. Insect Physiol. 2002, 48, 1041–1052. [Google Scholar] [PubMed]
- Benton, A.W. Esterases and phosphatases of honeybee venom. J. Apic. Res. 1967, 6, 91–94. [Google Scholar]
- Xia, Y.; Clarkson, J.M.; Charnley, A.K. Acid phosphatase of Metarhizium anisopliae during infection of the tobacco hornworm Manduca sexta. Arch. Microbiol. 2001, 176, 427–434. [Google Scholar] [PubMed]
- Dani, M.P.; Edwards, J.P.; Richards, E.H. Hydrolase activity in the venom of the pupal endoparasitic wasp Pimpla hypochondriaca. Comp. Biochem. Physiol. 2005, 141, 373–381. [Google Scholar]
- Pelosi, P.; Maida, R. Odorant-binding proteins in insects. Comp. Biochem. Physiol. Biochem. Mol. Biol. 1995, 111, 503–514. [Google Scholar]
- Gopaul, D.N.; Meyer, S.L.; Degano, M.; Sacchettini, J.C.; Schramm, V.L. Inosine-uridine nucleoside hydrolase from Crithidia fasciculate. Genetic characterization, crystallization, and identification of hisitine 241 as a catalytic site residue. Biochemistry 1996, 35, 5963–5970. [Google Scholar] [PubMed]
- Fang, J.; Han, Q.; Li, J. Isolation, characterization, and functional expression of kynurenine aminotransferase cDNA from the yellow fever mosquito, Aedes aegypti. Insect Biochem. Mol. Biol. 2002, 32, 943–950. [Google Scholar] [PubMed]
- Hoffman, D.R. Hymenoptera venom allergens. Clin. Rev. Allergy Immunol. 2006, 30, 109–128. [Google Scholar] [PubMed]
- Evans, A.C. Comparative observations on the morphology and biology of some hymenopterous parasites of carrion-infesting Diptera. Bull. Entomol. Res. 1993, 24, 385–405. [Google Scholar]
- Beard, R.L. Pathogenic stinging of house-fly pupae of Nasonia vitripennis (Walker). J. Invert. Path. 1964, 6, 1–4. [Google Scholar]
- Hickle, L.A.; Fitch, W.L. Analytical Chemistry of Bacillus Thuringiensis; Fickle, L.A., Fitch, W.L., Eds.; American Chemical Society: Washington D.C., USA, 1990; pp. 1–8. [Google Scholar]
- Gill, S.S.; Chang, C.; Chow, E. Mechanism of action of Bacillus thuringiensis CytA and CrylVD mosquitocidal toxins. In Molecular Action of Insecticides on Ion Channels; Clark, J.M., Ed.; American Chemical Society: Washington D.C., USA, 1995; p. 308. [Google Scholar]
- Federici, B.A.; Bigot, Y. Origin and evolution of polydnaviruses by symbiogenesis on insect DNA viruses in endoparasitic wasps. J. Insect Phys. 2003, 49, 419–432. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Danneels, E.L.; Rivers, D.B.; De Graaf, D.C. Venom Proteins of the Parasitoid Wasp Nasonia vitripennis: Recent Discovery of an Untapped Pharmacopee. Toxins 2010, 2, 494-516. https://doi.org/10.3390/toxins2040494
Danneels EL, Rivers DB, De Graaf DC. Venom Proteins of the Parasitoid Wasp Nasonia vitripennis: Recent Discovery of an Untapped Pharmacopee. Toxins. 2010; 2(4):494-516. https://doi.org/10.3390/toxins2040494
Chicago/Turabian StyleDanneels, Ellen L., David B. Rivers, and Dirk C. De Graaf. 2010. "Venom Proteins of the Parasitoid Wasp Nasonia vitripennis: Recent Discovery of an Untapped Pharmacopee" Toxins 2, no. 4: 494-516. https://doi.org/10.3390/toxins2040494





