Actin Crosslinking Toxins of Gram-Negative Bacteria
Abstract
:1. Bacterial Protein Toxins with an Actin Crosslinking Domain
1.1. Overview of actin targeting toxins
1.2. Discovery of the actin crosslinking domain of MARTXVc
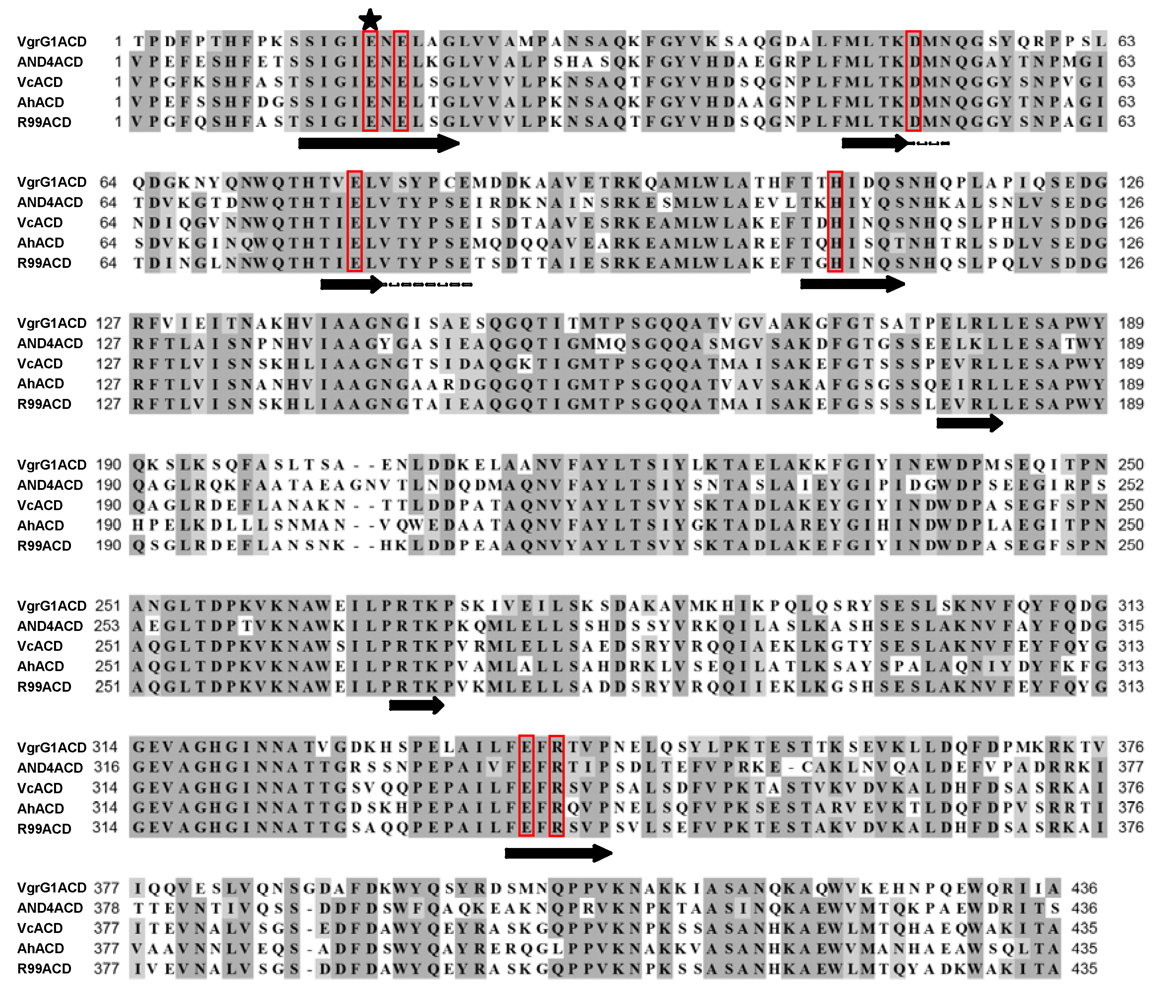
1.3. Other proteins with actin crosslinking domains

2. Characterization of the Actin Crosslinking Activity
2.1. ACD directly crosslinks actin
2.2. Requirement of G-actin, ATP, and Mg2+ for actin crosslinking
2.3. Most G-actin binding proteins do not inhibit the crosslinking reaction
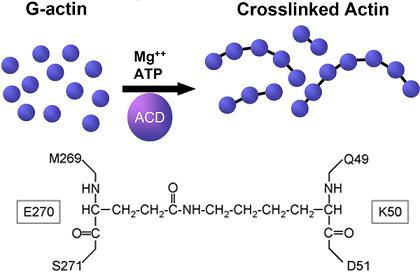
3. The Chemical Nature of the Crosslink

4. The Catalytic Mechanism Is Similar to Glutamate Synthetases

5. Phagocytic Cells as the Primary Target of ACD
Acknowledgements
References
- Espina, B.; Rubiolo, J.A. Marine toxins and the cytoskeleton: pectenotoxins, unusual macrolides that disrupt actin. FEBS J. 2008, 275, 6082–6088. [Google Scholar] [CrossRef] [PubMed]
- Natori, S. Cytochalasins-actin filament modifiers as a group of mycotoxins. Dev. Toxicol. Environ. Sci. 1986, 12, 291–299. [Google Scholar] [PubMed]
- Aktories, K.; Barbieri, J.T. Bacterial cytotoxins: Targeting eukaryotic switches. Nature Rev. 2005, 3, 397–410. [Google Scholar]
- Richard, J.F.; Petit, L.; Gibert, M.; Marvaud, J.C.; Bouchaud, C.; Popoff, M.R. Bacterial toxins modifying the actin cytoskeleton. Int. Microbiol. 1999, 2, 185–194. [Google Scholar] [PubMed]
- Suarez, G.; Sierra, J.C.; Erova, T.E.; Sha, J.; Horneman, A.J.; Chopra, A.K. A type VI secretion system effector protein VgrG1 from Aeromonas hydrophila that induces host cell toxicity by ADP-ribosylation of actin. J. Bacteriol. 2010, 192, 155–168. [Google Scholar] [PubMed]
- Fullner, K.J.; Mekalanos, J.J. In vivo covalent crosslinking of actin by the RTX toxin of Vibrio cholerae. EMBO J. 2000, 19, 5315–5323. [Google Scholar] [PubMed]
- Lin, W.; Fullner, K.J.; Clayton, R.; Sexton, J.A.; Rogers, M.B.; Calia, K.E.; Calderwood, S.B.; Fraser, C.; Mekalanos, J.J. Identification of a Vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc. Natl. Acad. Sci. USA 1999, 96, 1071–1076. [Google Scholar]
- Chow, K.H.; Ng, T.K.; Yuen, K.Y.; Yam, W.C. Detection of RTX toxin gene in Vibrio cholerae by PCR. J. Clin. Microbiol. 2001, 39, 2594–2597. [Google Scholar] [PubMed]
- Cordero, C.L.; Sozhamannan, S.; Satchell, K.J. RTX toxin actin cross-linking activity in clinical and environmental isolates of Vibrio cholerae. J. Clin. Microbiol. 2007, 45, 2289–2292. [Google Scholar] [PubMed]
- Dalsgaard, A.; Serichantalergs, O.; Forslund, A.; Lin, W.; Mekalanos, J.; Mintz, E.; Shimada, T.; Wells, J.G. Clinical and environmental isolates of Vibrio cholerae serogroup O141 carry the CTX phage and the genes encoding the toxin-coregulated pili. J. Clin. Microbiol. 2001, 39, 4086–4092. [Google Scholar] [PubMed]
- Rahman, M.H.; Biswas, K.; Hossain, M.A.; Sack, R.B.; Mekalanos, J.J.; Faruque, S.M. Distribution of genes for virulence and ecological fitness among diverse Vibrio cholerae population in a cholera endemic area: tracking the evolution of pathogenic strains. DNA Cell Biol. 2008, 27, 347–355. [Google Scholar] [PubMed]
- Satchell, K.J. MARTX: Multifunctional-autoprocessing RTX toxins. Infect. Immun. 2007, 75, 5079–5084. [Google Scholar] [PubMed]
- Sheahan, K.L.; Cordero, C.L.; Satchell, K.J. Identification of a domain within the multifunctional Vibrio cholerae RTX toxin that covalently cross-links actin. Proc. Natl. Acad. Sci. USA 2004, 101, 9798–9803. [Google Scholar]
- Prochazkova, K.; Shuvalova, L.A.; Minasov, G.; Voburka, Z.; Anderson, W.F.; Satchell, K.J. Structural and molecular mechanism for autoprocessing of MARTX Toxin of Vibrio cholerae at multiple sites. J. Biol. Chem. 2009, 284, 26557–26568. [Google Scholar] [PubMed]
- Seshadri, R.; Joseph, S.W.; Chopra, A.K.; Sha, J.; Shaw, J.; Graf, J.; Haft, D.; Wu, M.; Ren, Q.; Rosovitz, M.J.; Madupu, R.; Tallon, L.; Kim, M.; Jin, S.; Vuong, H.; Stine, O.C.; Ali, A.; Horneman, A.J.; Heidelberg, J.F. Genome sequence of Aeromonas hydrophila ATCC 7966T: jack of all trades. J. Bacteriol. 2006, 188, 8272–8282. [Google Scholar] [PubMed]
- Chen, C.Y.; Wu, K.M.; Chang, Y.C.; Chang, C.H.; Tsai, H.C.; Liao, T.L.; Liu, Y.M.; Chen, H.J.; Shen, A.B.; Li, J.C.; Su, T.L.; Shao, C.P.; Lee, C.T.; Hor, L.I.; Tsai, S.F. Comparative genome analysis of Vibrio vulnificus, a marine pathogen. Genome Res. 2003, 13, 2577–2587. [Google Scholar] [PubMed]
- Kim, Y.R.; Lee, S.E.; Kim, C.M.; Kim, S.Y.; Shin, E.K.; Shin, D.H.; Chung, S.S.; Choy, H.E.; Progulske-Fox, A.; Hillman, J.D.; Handfield, M.; Rhee, J.H. Characterization and pathogenic significance of Vibrio vulnificus antigens preferentially expressed in septicemic patients. Infect. Immun. 2003, 71, 5461–5471. [Google Scholar] [PubMed]
- Kim, Y.R.; Lee, S.E.; Kook, H.; Yeom, J.A.; Na, H.S.; Kim, S.Y.; Chung, S.S.; Choy, H.E.; Rhee, J.H. Vibrio vulnificus RTX toxin kills host cells only after contact of the bacteria with host cells. Cell Microbiol. 2008, 10, 848–862. [Google Scholar] [PubMed]
- Lee, C.T.; Amaro, C.; Wu, K.M.; Valiente, E.; Chang, Y.F.; Tsai, S.F.; Chang, C.H.; Hor, L.I. A common virulence plasmid in biotype 2 Vibrio vulnificus and its dissemination aided by a conjugal plasmid. J. Bacteriol. 2008, 190, 1638–1648. [Google Scholar] [PubMed]
- Pukatzki, S.; Ma, A.T.; Sturtevant, D.; Krastins, B.; Sarracino, D.; Nelson, W.C.; Heidelberg, J.F.; Mekalanos, J.J. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc. Natl. Acad. Sci. USA 2006, 103, 1528–1533. [Google Scholar]
- Ma, A.T.; McAuley, S.; Pukatzki, S.; Mekalanos, J.J. Translocation of a Vibrio cholerae type VI secretion effector requires bacterial endocytosis by host cells. Cell Host Microbe 2009, 5, 234–243. [Google Scholar] [PubMed]
- Pukatzki, S.; Ma, A.T.; Revel, A.T.; Sturtevant, D.; Mekalanos, J.J. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc. Natl. Acad. Sci. USA 2007, 104, 15508–15513. [Google Scholar]
- Hagstrom, A.; Ferriera, S.; Johnson, J.; Kravitz, S.; Beeson, K.; Sutton, G.; Rogers, Y.-H.; Friedman, R.; Frazier, M.; Venter, J.C. Vibrio sp. AND4 1103602000423, whole genome shotgun sequence, direct submission. NCBI reference sequence NZ_ABGR01000041.1, 2007. [Google Scholar]
- Sheahan, K.L.; Satchell, K.J. Inactivation of small Rho GTPases by the multifunctional RTX toxin from Vibrio cholerae. Cell Microbiol. 2007, 9, 1324–1335. [Google Scholar] [PubMed]
- Milne, J.C.; Blanke, S.R.; Hanna, P.C.; Collier, R.J. Protective antigen-binding domain of anthrax lethal factor mediates translocation of a heterologous protein fused to its amino- or carboxy-terminus. Mol. Microbiol. 1995, 15, 661–666. [Google Scholar] [PubMed]
- Spyres, L.M.; Qa'Dan, M.; Meader, A.; Tomasek, J.J.; Howard, E.W.; Ballard, J.D. Cytosolic delivery and characterization of the TcdB glucosylating domain by using a heterologous protein fusion. Infect. Immun. 2001, 69, 599–601. [Google Scholar] [PubMed]
- Cordero, C.L.; Kudryashov, D.S.; Reisler, E.; Satchell, K.J. The actin cross-linking domain of the Vibrio cholerae RTX toxin directly catalyzes the covalent cross-linking of actin. J. Biol. Chem. 2006, 281, 32366–32374. [Google Scholar] [PubMed]
- Korn, E.D.; Carlier, M.F.; Pantaloni, D. Actin polymerization and ATP hydrolysis. Science 1987, 238, 638–644. [Google Scholar] [PubMed]
- Kudryashov, D.S.; Cordero, C.L.; Reisler, E.; Satchell, K.J. Characterization of the enzymatic activity of the actin cross-linking domain from the Vibrio cholerae MARTXVc toxin. J. Biol. Chem. 2008, 283, 445–452. [Google Scholar] [PubMed]
- Fullner, K.J.; Mekalanos, J.J. Genetic characterization of a new type IV pilus gene cluster found in both classical and El Tor biotypes of Vibrio cholerae. Infect Immun. 1999, 67, 1393–1404. [Google Scholar] [PubMed]
- Pollard, T.D.; Borisy, G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003, 112, 453–465. [Google Scholar] [PubMed]
- Kudryashov, D.S.; Durer, Z.A.; Ytterberg, A.J.; Sawaya, M.R.; Pashkov, I.; Prochazkova, K.; Yeates, T.O.; Loo, R.R.; Loo, J.A.; Satchell, K.J.; Reisler, E. Connecting actin monomers by iso-peptide bond is a toxicity mechanism of the Vibrio cholerae MARTX toxin. Proc. Natl. Acad. Sci. USA 2008, 105, 18537–18542. [Google Scholar]
- Geissler, B.; Bonebrake, A.; Sheahan, K.L.; Walker, M.E.; Satchell, K.J. Genetic determination of essential residues of the Vibrio cholerae actin cross-linking domain reveals functional similarity with glutamine synthetases. Mol. Microbiol. 2009, 73, 858–868. [Google Scholar] [PubMed]
- Hibi, T.; Nii, H.; Nakatsu, T.; Kimura, A.; Kato, H.; Hiratake, J.; Oda, J. Crystal structure of gamma-glutamylcysteine synthetase: insights into the mechanism of catalysis by a key enzyme for glutathione homeostasis. Proc. Natl. Acad. Sci. USA 2004, 101, 15052–15057. [Google Scholar]
- Olivier, V.; Queen, J.; Satchell, K.J. Successful small intestine colonization of adult mice by Vibrio cholerae requires ketamine anesthesia and accessory toxins. PLoS One 2009, 4, e7352. [Google Scholar]
- Olivier, V.; Salzman, N.H.; Satchell, K.J. Prolonged colonization of mice by Vibrio cholerae El Tor O1 depends on accessory toxins. Infect. Immun. 2007, 75, 5043–5051. [Google Scholar] [PubMed]
- Suarez, G.; Sierra, J.C.; Sha, J.; Wang, S.; Erova, T.E.; Fadl, A.A.; Foltz, S.M.; Horneman, A.J.; Chopra, A.K. Molecular characterization of a functional type VI secretion system from a clinical isolate of Aeromonas hydrophila. Microbial Pathog. 2008, 44, 344–361. [Google Scholar]
- Satchell, K.J. Bacterial martyrdom: Phagocytes disabled by type VI secretion after engulfing bacteria. Cell Host Microbe 2009, 5, 213–214. [Google Scholar] [PubMed]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Satchell, K.J.F. Actin Crosslinking Toxins of Gram-Negative Bacteria. Toxins 2009, 1, 123-133. https://doi.org/10.3390/toxins1020123
Satchell KJF. Actin Crosslinking Toxins of Gram-Negative Bacteria. Toxins. 2009; 1(2):123-133. https://doi.org/10.3390/toxins1020123
Chicago/Turabian StyleSatchell, Karla J. F. 2009. "Actin Crosslinking Toxins of Gram-Negative Bacteria" Toxins 1, no. 2: 123-133. https://doi.org/10.3390/toxins1020123