Vitamin C Status Correlates with Markers of Metabolic and Cognitive Health in 50-Year-Olds: Findings of the CHALICE Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Sample Collection
2.3. Sample Preparation for Vitamin C Analysis
2.4. Vitamin C HPLC Analysis
2.5. Metabolic and Heart Health Assessments
2.6. Dietary Intake Assessment
2.7. Wellbeing, Depression and Cognition
2.7.1. Mental Wellbeing
2.7.2. Depression
2.7.3. Cognition
2.8. Socio-Economic Status
2.9. Statistical Analyses
3. Results
3.1. Characteristics of the Study Population
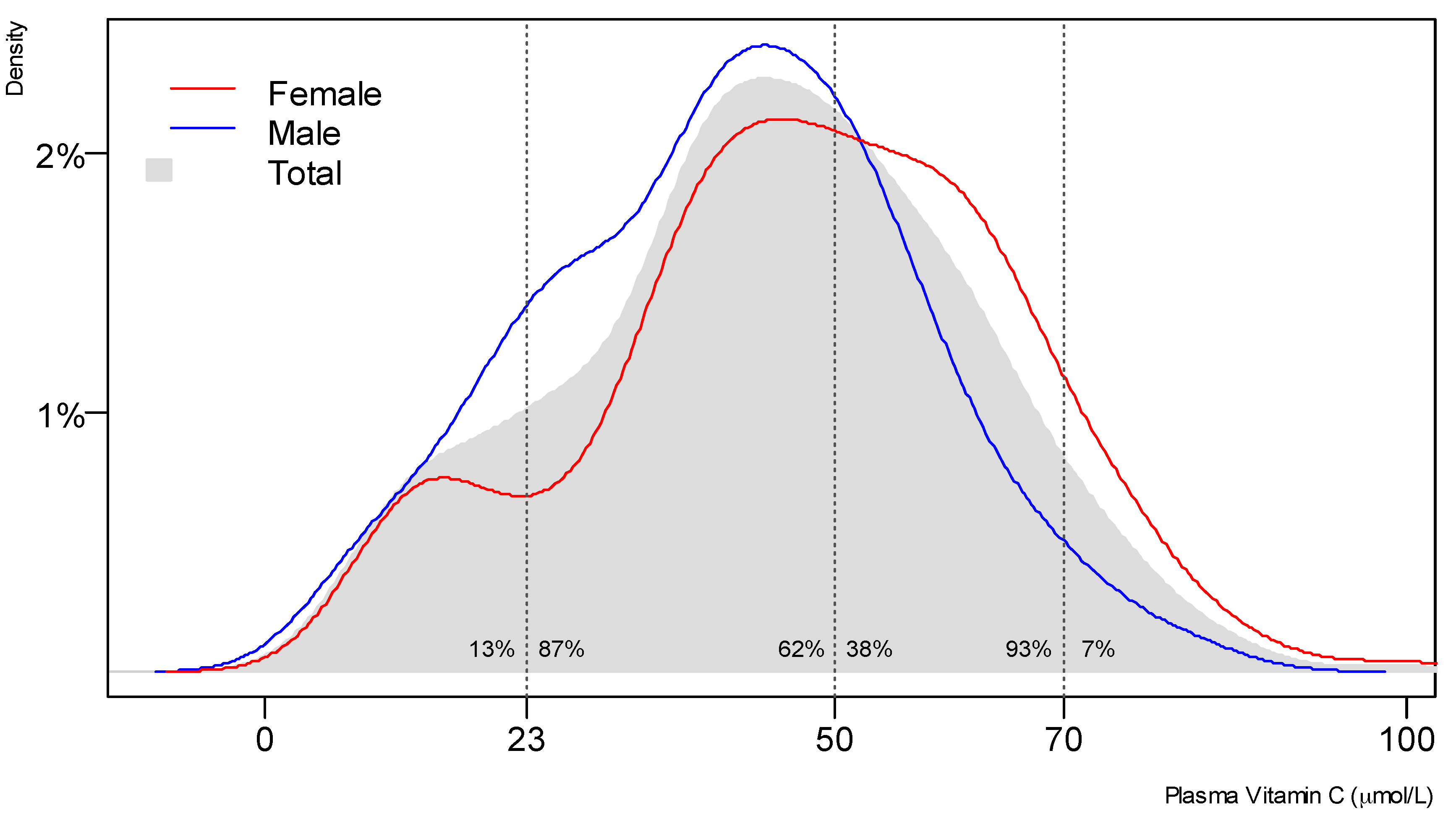
3.2. Vitamin C Status of the Study Population
3.3. Associations of Vitamin C Status with Markers of Metabolic and Mental Health
3.4. Dietary Vitamin C Intake
3.5. Associations of Dietary Vitamin C Intake with Markers of Metabolic and Mental Health
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Svirbely, J.L.; Szent-Gyorgyi, A. The chemical nature of vitamin C. Biochem. J. 1933, 27, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Englard, S.; Seifter, S. The biochemical functions of ascorbic acid. Annu. Rev. Nutr. 1986, 6, 365–406. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.C.; Kuiper, C.; Dachs, G.U. Regulation of the 2-oxoglutarate-dependent dioxygenases and implications for cancer. Biochem. Soc. Trans. 2014, 42, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.; Frei, B. Does vitamin C act as a pro-oxidant under physiological conditions? Faseb J. 1999, 13, 1007–1024. [Google Scholar] [PubMed]
- Bruno, R.S.; Leonard, S.W.; Atkinson, J.; Montine, T.J.; Ramakrishnan, R.; Bray, T.M.; Traber, M.G. Faster plasma vitamin E disappearance in smokers is normalized by vitamin C supplementation. Free. Radic. Biol. Med. 2006, 40, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Selim, M.A.; Shea, C.R.; Grichnik, J.M.; Omar, M.M.; Monteiro-Riviere, N.A.; Pinnell, S.R. UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J. Am. Acad. Dermatol. 2003, 48, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Krebs, H.A. The Sheffield Experiment on the vitamin C requirement of human adults. Proc. Nutr. Soc. 1953, 12, 237–246. [Google Scholar] [CrossRef]
- Schleicher, R.L.; Carroll, M.D.; Ford, E.S.; Lacher, D.A. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am. J. Clin. Nutr. 2009, 90, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- CDC’s Second Nutrition Report. Available online: https://www.cdc.gov/nutritionreport/report.html (accessed on 28 June 2017).
- A Focus on Nutrition: Key findings from the 2008/09 NZ Adult Nutrition Survey. Available online: http://www.health.govt.nz/publication/focus-nutrition-key-findings-2008-09-nz-adult-nutrition-survey (accessed on 8 June 2017).
- Carr, A.C.; Frei, B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am. J. Clin. Nutr. 1999, 69, 1086–1107. [Google Scholar] [PubMed]
- Lykkesfeldt, J.; Poulsen, H.E. Is vitamin C supplementation beneficial? Lessons learned from randomised controlled trials. Br. J. Nutr. 2010, 103, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.A. Assessment of human vitamin C status. J. Nutr. 1990, 120, 1480–1485. [Google Scholar] [PubMed]
- Tetens, I. Scientific opinion on dietary reference values for vitamin C. EFSA. J. 2013, 11, 3418–3486. [Google Scholar]
- Nutrient Reference Values for Australia and New Zealand Executive Summary. Available online: https://www.nhmrc.gov.au/guidelines-publications/n35-n36-n37 (accessed on 7 June 2017).
- Schluter, P.J.; Spittlehouse, J.K.; Cameron, V.A.; Chambers, S.; Gearry, R.; Jamieson, H.A.; Kennedy, M.; Lacey, C.J.; Murdoch, D.R.; Pearson, J.; et al. Canterbury Health, Ageing and Life Course (CHALICE) study: Rationale, design and methodology. N. Z. Med. J. 2013, 126, 71–85. [Google Scholar] [PubMed]
- Enrolment Statistics: Comparison of Estimated Eligible Voting Population to Enrolled Electors for Christchurch City. Available online: http://www.elections.org.nz/councils/ages/district_60_christchurch_city.html (accessed on 12 March 2013).
- Population of Canterbury DHB. Available online: http://www.health.govt.nz/new-zealand-health-system/my-dhb/canterbury-dhb/population-canterbury-dhb (accessed on 12 March 2013).
- Sato, Y.; Uchiki, T.; Iwama, M.; Kishimoto, Y.; Takahashi, R.; Ishigami, A. Determination of dehydroascorbic acid in mouse tissues and plasma by using tris(2-carboxyethyl)phosphine hydrochloride as reductant in metaphosphoric acid/ethylenediaminetetraacetic acid solution. Biol. Pharm. Bull. 2010, 33, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Pullar, J.M.; Moran, S.; Vissers, M.C. Bioavailability of vitamin C from kiwifruit in non-smoking males: Determination of ‘healthy’ and ‘optimal’ intakes. J. Nutr. Sci. 2012, 1, e14. [Google Scholar] [CrossRef] [PubMed]
- New Zealand Primary Care Handbook 2012. Available online: http://www.health.govt.nz/publication/new-zealand-primary-care-handbook-2012 (accessed on 9 July 2017).
- Tennant, R.; Hiller, L.; Fishwick, R.; Platt, S.; Joseph, S.; Weich, S.; Parkinson, J.; Secker, J.; Stewart-Brown, S. The Warwick-Edinburgh mental well-being scale (WEMWBS): Development and UK validation. Health Qual. Life Outcomes 2007, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Janavs, J.; Weiller, E.; Keskiner, A.; Schinka, J.; Knapp, E.; Sheehan, M.F.; Dunbar, G.C. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. Eur. Psychiatry 1997, 12, 232–241. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- ELSI Short Form: User Manual for a Direct Measure of Living Standards. Available online: https://www.msd.govt.nz/about-msd-and-our-work/publications-resources/monitoring/living-standards/elsi-short-form.html (accessed on 7 June 2017).
- A Portrait of Health: Key results of the 2006/07 New Zealand Health Survey. Available online: http://www.health.govt.nz/publication/portrait-health-key-results-2006-07-new-zealand-health-survey (accessed on 15 May 2017).
- The Royal College of Pathologists of Australasia: RCPA. Available online: https://www.rcpa.edu.au/ (accessed on 9 July 2017).
- Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia. Available online: http://www.who.int/diabetes/publications/diagnosis_diabetes2006/en/ (accessed on 15 May 2017).
- Kallner, A.B.; Hartmann, D.; Hornig, D.H. On the requirements of ascorbic acid in man: Steady-state turnover and body pool in smokers. Am. J. Clin. Nutr. 1981, 34, 1347–1355. [Google Scholar] [PubMed]
- McClean, H.E.; Stewart, A.W.; Riley, C.G.; Beaven, D.W. Vitamin C status of elderly men in a residential home. N. Z. Med. J. 1977, 86, 379–382. [Google Scholar] [PubMed]
- McClean, H.E.; Dodds, P.M.; Abernethy, M.H.; Stewart, A.W.; Beaven, D.W. Vitamin C concentration in plasma and leucocytes of men related to age and smoking habit. N. Z. Med. J. 1976, 83, 226–229. [Google Scholar] [PubMed]
- Mosdol, A.; Erens, B.; Brunner, E.J. Estimated prevalence and predictors of vitamin C deficiency within UK’s low-income population. J. Public Health (Oxf.) 2008, 30, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Galan, P.; Viteri, F.E.; Bertrais, S.; Czernichow, S.; Faure, H.; Arnaud, J.; Ruffieux, D.; Chenal, S.; Arnault, N.; Favier, A.; et al. Serum concentrations of beta-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur. J. Clin. Nutr. 2005, 59, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Jungert, A.; Neuhauser-Berthold, M. The lower vitamin C plasma concentrations in elderly men compared with elderly women can partly be attributed to a volumetric dilution effect due to differences in fat-free mass. Br. J. Nutr. 2015, 113, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Langlois, K.; Cooper, M.; Colapinto, C.K. Vitamin C status of Canadian adults: Findings from the 2012/2013 Canadian Health Measures Survey. Health Rep. 2016, 27, 3–10. [Google Scholar] [PubMed]
- Faure, H.; Preziosi, P.; Roussel, A.M.; Bertrais, S.; Galan, P.; Hercberg, S.; Favier, A. Factors influencing blood concentration of retinol, alpha-tocopherol, vitamin C, and beta-carotene in the French participants of the SU.VI.MAX trial. Eur. J. Clin. Nutr. 2006, 60, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Eating and Activity Guidelines for New Zealand Adults. Available online: http://www.health.govt.nz/publication/eating-and-activity-guidelines-new-zealand-adults (accessed on 7 July 2017).
- Block, G.; Jensen, C.D.; Dalvi, T.B.; Norkus, E.P.; Hudes, M.; Crawford, P.B.; Holland, N.; Fung, E.B.; Schumacher, L.; Harmatz, P. Vitamin C treatment reduces elevated C-reactive protein. Free Radic. Biol. Med. 2009, 46, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Canoy, D.; Wareham, N.; Welch, A.; Bingham, S.; Luben, R.; Day, N.; Khaw, K.T. Plasma ascorbic acid concentrations and fat distribution in 19,068 British men and women in the European Prospective Investigation into Cancer and Nutrition Norfolk cohort study. Am. J. Clin. Nutr. 2005, 82, 1203–1209. [Google Scholar] [PubMed]
- Garcia, O.P.; Ronquillo, D.; Caamano Mdel, C.; Camacho, M.; Long, K.Z.; Rosado, J.L. Zinc, vitamin A, and vitamin C status are associated with leptin concentrations and obesity in Mexican women: Results from a cross-sectional study. Nutr. Metab. (Lond.) 2012, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.S.; Beezhold, B.L.; Mostow, B.; Swan, P.D. Plasma vitamin C is inversely related to body mass index and waist circumference but not to plasma adiponectin in nonsmoking adults. J. Nutr. 2007, 137, 1757–1762. [Google Scholar] [PubMed]
- Moor de Burgos, A.; Wartanowicz, M.; Ziemlanski, S. Blood vitamin and lipid levels in overweight and obese women. Eur. J. Clin. Nutr. 1992, 46, 803–808. [Google Scholar] [PubMed]
- Block, G.; Mangels, A.R.; Patterson, B.H.; Levander, O.A.; Norkus, E.P.; Taylor, P.R. Body weight and prior depletion affect plasma ascorbate levels attained on identical vitamin C intake: A controlled-diet study. J. Am. Coll. Nutr. 1999, 18, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Pullar, J.M.; Bozonet, S.M.; Vissers, M.C. Marginal Ascorbate Status (Hypovitaminosis C) Results in an Attenuated Response to Vitamin C Supplementation. Nutrients 2016, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Rahmat, A.; Patimah, I.; Khaza’ai, H.; Abed, Y. Effect of vitamin C on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: A randomized controlled trial. Drug Des. Dev. Ther. 2015, 9, 3405–3412. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, H.V.; Dakhale, G.N.; Chaudhari, S.; Mahatme, M. The beneficial effect of vitamin C supplementation on serum lipids in type 2 diabetic patients: A randomised double blind study. Int. J. Diabetes Metab. 2012, 20, 53–58. [Google Scholar]
- Paolisso, G.; Balbi, V.; Volpe, C.; Varricchio, G.; Gambardella, A.; Saccomanno, F.; Ammendola, S.; Varricchio, M.; D’Onofrio, F. Metabolic benefits deriving from chronic vitamin C supplementation in aged non-insulin dependent diabetics. J. Am. Coll. Nutr. 1995, 14, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Karne, R.J.; Hall, G.; Campia, U.; Panza, J.A.; Cannon, R.O.; Wang, Y.; Katz, A.; Levine, M.; Quon, M.J. High-dose oral vitamin C partially replenishes vitamin C levels in patients with Type 2 diabetes and low vitamin C levels but does not improve endothelial dysfunction or insulin resistance. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H137–H145. [Google Scholar] [CrossRef] [PubMed]
- Dakhale, G.N.; Chaudhari, H.V.; Shrivastava, M. Supplementation of vitamin C reduces blood glucose and improves glycosylated hemoglobin in type 2 diabetes mellitus: A randomized, double-blind study. Adv. Pharmacol. Sci. 2011, 2011, 195271. [Google Scholar] [CrossRef] [PubMed]
- Godala, M.M.; Materek-Kusmierkiewicz, I.; Moczulski, D.; Rutkowski, M.; Szatko, F.; Gaszynska, E.; Tokarski, S.; Kowalski, J. Lower Plasma Levels of Antioxidant Vitamins in Patients with Metabolic Syndrome: A Case Control Study. Adv. Clin. Exp. Med. 2016, 25, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, Y.H. Physical activity, dietary vitamin C, and metabolic syndrome in the Korean adults: The Korea National Health and Nutrition Examination Survey 2008 to 2012. Public Health 2016, 135, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zeng, C.; Gong, Q.Y.; Li, X.X.; Lei, G.H.; Yang, T.B. Associations between Dietary Antioxidant Intake and Metabolic Syndrome. PLoS ONE 2015, 10, e0130876. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Kengne, A.P.; Mikhailidis, D. P.; Cicero, A.F.; Banach, M. Effects of selected dietary constituents on high-sensitivity C-reactive protein levels in U.S. adults. Ann. Med. 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mikirova, N.; Casciari, J.; Rogers, A.; Taylor, P. Effect of high-dose intravenous vitamin C on inflammation in cancer patients. J. Transl. Med. 2012, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health: Virtual Diabetes Register. Available online: http://www.health.govt.nz/our-work/diseases-and-conditions/diabetes/about-diabetes/virtual-diabetes-register-vdr (accessed on 10 July 2017).
- Understanding Excess Body Weight: New Zealand Health Survey. Available online: http://www.health.govt.nz/publication/understanding-excess-body-weight-new-zealand-health-survey (accessed on 12 June 2017).
- Juraschek, S.P.; Guallar, E.; Appel, L.J.; Miller, E.R. Effects of vitamin C supplementation on blood pressure: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 95, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Hornig, D. Distribution of ascorbic acid, metabolites and analogues in man and animals. Ann. N. Y. Acad. Sci. 1975, 258, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.C.; Bozonet, S.M.; Pearson, J.F.; Braithwaite, L.J. Dietary ascorbate intake affects steady state tissue concentrations in vitamin C-deficient mice: Tissue deficiency after suboptimal intake and superior bioavailability from a food source (kiwifruit). Am. J. Clin. Nutr. 2011, 93, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Bernardo, A.; Walker, J.M.; Kennard, J.A.; Kim, G.Y.; Kessler, E.S.; Harrison, F.E. Vitamin C deficiency in the brain impairs cognition, increases amyloid accumulation and deposition, and oxidative stress in APP/PSEN1 and normally aging mice. ACS Chem. Neurosci. 2015, 6, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Masaki, K.H.; Losonczy, K.G.; Izmirlian, G.; Foley, D.J.; Ross, G.W.; Petrovitch, H.; Havlik, R.; White, L.R. Association of vitamin E and C supplement use with cognitive function and dementia in elderly men. Neurology 2000, 54, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; An, Y.; Guo, J.; Zhang, X.; Wang, H.; Rong, H.; Xiao, R. Dietary Intake of Nutrients and Lifestyle Affect the Risk of Mild Cognitive Impairment in the Chinese Elderly Population: A Cross-Sectional Study. Front. Behav. Neurosci. 2016, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Aggarwal, N.; Wilson, R.S.; Scherr, P.A. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA 2002, 287, 3230–3237. [Google Scholar] [CrossRef] [PubMed]
- Zandi, P.P.; Anthony, J.C.; Khachaturian, A.S.; Stone, S.V.; Gustafson, D.; Tschanz, J.T.; Norton, M.C.; Welsh-Bohmer, K.A.; Breitner, J.C. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: The Cache County Study. Arch. Neurol. 2004, 61, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Kean, R.J.; Lamport, D.J.; Dodd, G.F.; Freeman, J.E.; Williams, C.M.; Ellis, J.A.; Butler, L.T.; Spencer, J.P. Chronic consumption of flavanone-rich orange juice is associated with cognitive benefits: An 8-wk, randomized, double-blind, placebo-controlled trial in healthy older adults. Am. J. Clin. Nutr. 2015, 101, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Letenneur, L.; Proust-Lima, C.; Le Gouge, A.; Dartigues, J.F.; Barberger-Gateau, P. Flavonoid intake and cognitive decline over a 10-year period. Am. J. Epidemiol. 2007, 165, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Touvier, M.; Druesne-Pecollo, N.; Kesse-Guyot, E.; Andreeva, V.A.; Fezeu, L.; Galan, P.; Hercberg, S.; Latino-Martel, P. Dual association between polyphenol intake and breast cancer risk according to alcohol consumption level: A prospective cohort study. Breast Cancer Res. Treat 2013, 137, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.P.; Clark, R.E.; Nutt, D.J.; Haller, J.; Hayward, S.G.; Perry, K. Vitamin C, Mood and Cognitive Functioning in the Elderly. Nutr. Neurosci. 1999, 2, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Albert, C.M.; Grodstein, F. Vitamin E, vitamin C, beta carotene, and cognitive function among women with or at risk of cardiovascular disease: The Women’s Antioxidant and Cardiovascular Study. Circulation 2009, 119, 2772–2780. [Google Scholar] [CrossRef] [PubMed]
- Kesse-Guyot, E.; Fezeu, L.; Jeandel, C.; Ferry, M.; Andreeva, V.; Amieva, H.; Hercberg, S.; Galan, P. French adults’ cognitive performance after daily supplementation with antioxidant vitamins and minerals at nutritional doses: A post hoc analysis of the Supplementation in Vitamins and Mineral Antioxidants (SU.VI.MAX) trial. Am. J. Clin. Nutr. 2011, 94, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Naeini, A.M.; Elmadfa, I.; Djazayery, A.; Barekatain, M.; Ghazvini, M.R.; Djalali, M.; Feizi, A. The effect of antioxidant vitamins E and C on cognitive performance of the elderly with mild cognitive impairment in Isfahan, Iran: A double-blind, randomized, placebo-controlled trial. Eur. J. Nutr. 2014, 53, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
| Chalice (n, %) | Census 2006 (%) | p | |||
|---|---|---|---|---|---|
| Gender | Female | 215 | 53.2 | 50.9 | NS |
| Male | 189 | 46.8 | 49.1 | ||
| Ethnicity | Māori | 60 | 14.9 | 4.5 | <0.0001 |
| NZ European | 338 | 83.7 | 74.2 | ||
| Socio-Economic Status | Low (ELSISF score 0–16) | 30 | 7.4 | 8.2 | NS |
| Medium (ELSISF score 17–24) | 122 | 30.2 | 29.4 | ||
| High (ELSISF score 25–31) | 252 | 62.4 | 62.5 | ||
| Education | No Qualification | 53 | 13.1 | 23.9 | <0.0001 |
| Secondary School Qualification | 110 | 27.2 | 35.2 | ||
| Post-secondary | 168 | 41.6 | 25.6 | ||
| University Degree | 73 | 18.1 | 15.3 | ||
| Current Smoker | 60 | 14.9 | 16.6 | NS | |
| Female | Male | |||||||
|---|---|---|---|---|---|---|---|---|
| Body Measurements | Mean | Min | Max | NZ Female Mean | Mean | Min | Max | NZ Male Mean |
| Weight kg | 78.6 | 49.1 | 149.9 | 74.8 (73.5–76.1) | 88.4 | 50.8 | 143.8 | 88.0 (86.9–89.1) |
| BMI kg/m2 | 29.1 | 17.4 | 63.4 | 28.1 (27.6–28.6) | 28.1 | 19.2 | 48.6 | 28.6 (28.2–28.9) |
| Waist cm | 92.0 | 63.0 | 144.0 | 86.6 (85.5–87.6) | 98.3 | 72.5 | 148.0 | 98.4 (97.4–99.3) |
| Metabolism | Mean | Min | Max | Healthy Range | Mean | Min | Max | Healthy Range |
| Triglycerides mmol/L | 1.3 | 0.4 | 11.7 | <1.7 | 1.6 | 0.4 | 11.7 | <1.7 |
| HDL mmol/L | 1.4 | 0.8 | 2.7 | 1.0–2.2 | 1.2 | 0.7 | 1.9 | 0.9–2.0 |
| Glucose mmol/L | 5.1 | 3.2 | 10.8 | <6.1 | 5.4 | 3.7 | 17.9 | <6.1 |
| HbA1c mmol/L | 38.2 | 27.0 | 74.0 | <40 | 39.9 | 28.0 | 102.0 | <40 |
| Insulin pmol/L | 60.9 | 10.0 | 277.0 | 10–80 | 61.2 | 4.0 | 480.0 | 10–80 |
| Heart Health | Mean | Min | Max | Healthy Range | Mean | Min | Max | Healthy Range |
| BP (systolic) mmHg | 131.1 | 104.0 | 183.7 | 120 | 134.2 | 97.7 | 185.7 | 120 |
| BP (diastolic) mmHg | 82.5 | 60.3 | 106.0 | 80 | 85.0 | 61.0 | 128.3 | 80 |
| CVD risk score % | 2.5–5 | <2.5 | 20–25 | <2.5 | 5–10 | 2.5–5 | 20–25 | <2.5 |
| Mental Health | Mean | Min | Max | Mean | Min | Max | ||
| Wellbeing | 53.0 | 16 | 70 | 52.7 | 30 | 70 | ||
| Cognition | 27.1 | 19 | 30 | 26.6 | 16 | 30 | ||
| Current Depression n (%) | 17 (7.9) | 12 (6.3) | ||||||
| Plasma Vitamin C | Deficient | Marginal | Inadequate | Adequate | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | n | % | n | % | n | % | n | % | |||
| Total | 44.2 | (42.4, 46.0) | 9 | 2.4 | 39 | 10.6 | 183 | 49.6 | 138 | 37.4 | ||
| Gender | Female | 47.4 | (44.9, 49.9) | 2 | 1.0 | 20 | 10.3 | 85 | 43.6 | 88 | 45.1 | 0.005 |
| Male | 40.6 | (38.2, 43.0) | 7 | 4.0 | 19 | 10.9 | 98 | 56.3 | 50 | 28.7 | ||
| Ethnicity | Non Māori | 44.5 | (42.6, 46.4) | 7 | 2.2 | 31 | 9.8 | 159 | 50.3 | 119 | 37.7 | NS |
| Māori | 42.4 | (37.2, 47.6) | 2 | 3.8 | 8 | 15.1 | 24 | 45.3 | 19 | 35.8 | ||
| Socio- Economic Status | Low | 36.8 | (28.3, 45.3) | 2 | 8.0 | 7 | 28.0 | 9 | 36.0 | 7 | 28.0 | 0.003 |
| Medium | 43.7 | (40.3, 47.1) | 4 | 3.5 | 14 | 12.3 | 53 | 46.5 | 43 | 37.7 | ||
| High | 45.3 | (43.2, 47.4) | 3 | 1.3 | 18 | 7.8 | 121 | 52.6 | 88 | 38.3 | ||
| Education | None | 38.7 | (33.6, 43.9) | 3 | 6.1 | 6 | 12.2 | 26 | 53.1 | 14 | 28.6 | NS |
| Secondary School | 45.9 | (42.1, 49.7) | 1 | 1.0 | 13 | 12.6 | 49 | 47.6 | 40 | 38.8 | ||
| Post-secondary | 43.1 | (40.6, 45.7) | 5 | 3.3 | 16 | 10.6 | 75 | 49.7 | 55 | 36.4 | ||
| University Degree | 48.1 | (44.4, 51.9) | 0 | 0.0 | 4 | 6.1 | 33 | 50.0 | 29 | 43.9 | ||
| Tobacco | Not Current Smoker | 45.9 | (44.1, 47.8) | 6 | 1.9 | 26 | 8.2 | 157 | 49.7 | 127 | 40.2 | <0.001 |
| Current Smoker | 34.1 | (29.2, 38.9) | 3 | 5.7 | 13 | 24.5 | 26 | 49.1 | 11 | 20.8 | ||
| Vitamin C <23 µmol/L (n = 47) | Vitamin C >23 µmol/L (n = 321) | p | p Adjusted | |||
|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |||
| Body measurements | ||||||
| Weight | 90.3 | (83.3, 97.4) | 81.7 | (79.8, 83.6) | 0.024 | 0.004 |
| BMI | 31.4 | (28.7, 34.0) | 28.1 | (27.5, 28.7) | 0.021 | <0.001 |
| Waist | 103.3 | (97.6, 108.9) | 93.3 | (91.8, 94.8) | 0.001 | <0.001 |
| Metabolism | ||||||
| Triglycerides | 1.8 | (1.4, 2.3) | 1.4 | (1.3, 1.5) | 0.061 | 0.029 |
| HDL | 1.3 | (1.2, 1.3) | 1.4 | (1.3, 1.4) | 0.033 | NS |
| Glucose | 5.6 | (5.2, 6.0) | 5.2 | (5.0, 5.3) | 0.072 | 0.073 |
| HbA1c | 42.2 | (39.6, 44.8) | 38.5 | (37.7, 39.3) | 0.009 | 0.015 |
| Insulin | 91.0 | (68.4, 113.6) | 56.3 | (51.9, 60.8) | 0.004 | 0.000 |
| Heart health | ||||||
| BP (systolic) | 132.2 | (128.0, 136.4) | 132.5 | (130.8, 134.2) | NS | NS |
| BP (diastolic) | 83.6 | (81.0, 86.3) | 83.5 | (82.4, 84.6) | NS | NS |
| CVD risk score | 5–10% | (<2.5%, 20–25%) | 2.5–5% | (3.5–5%, 5–10%) | 0.057 | NS |
| Mental Health | ||||||
| Wellbeing | 50.9 | (48.4, 53.4) | 53.0 | (52.0, 53.9) | NS | NS |
| n | % | n | % | |||
| MCI | 17 | 40.5 | 66 | 21.5 | 0.012 | 0.02 |
| Current Depression | 6 | 12.5 | 20 | 6.2 | NS | NS |
| Dietary Vitamin C | Below RDI | RDI-Average | Above Average | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | n | % | n | % | n | % | |||
| Total | 109.8 | (101.5, 118.1) | 30 | 12 | 126 | 50.4 | 94 | 37.6 | ||
| Gender | Female | 107.4 | (96.6, 118.2) | 13 | 9.7 | 73 | 54.5 | 48 | 35.8 | NS |
| Male | 112.6 | (99.7, 125.6) | 17 | 14.7 | 53 | 45.7 | 46 | 39.7 | ||
| Ethnicity | Non Māori | 112.0 | (102.7, 121.2) | 22 | 10.3 | 111 | 51.9 | 81 | 37.9 | NS |
| Māori | 97.2 | (79.6, 114.7) | 8 | 22.2 | 15 | 41.7 | 13 | 36.1 | ||
| Socio-Economic Status | Low | 78.8 | (54.4, 103.1) | 4 | 26.7 | 8 | 53.3 | 3 | 20.0 | NS |
| Medium | 105.0 | (90.7, 119.2) | 12 | 15.2 | 36 | 45.6 | 31 | 39.2 | ||
| High | 115.3 | (104.4, 126.1) | 14 | 9.0 | 82 | 52.6 | 60 | 38.5 | ||
| Education | None | 83.5 | (64.2, 102.7) | 8 | 28.6 | 13 | 46.4 | 7 | 25.0 | 0.1 |
| Secondary School | 117.1 | (98.4, 135.7) | 6 | 10.0 | 32 | 53.3 | 22 | 36.7 | ||
| Post-secondary | 108.6 | (97.1, 120.1) | 11 | 9.9 | 59 | 53.2 | 41 | 36.9 | ||
| University Degree | 118.4 | (97.6, 139.2) | 5 | 9.8 | 22 | 43.1 | 24 | 47.1 | ||
| Tobacco | Not Current Smoker | 114.1 | (105.3, 122.8) | 20 | 9.0 | 112 | 50.7 | 89 | 40.3 | <0.001 |
| Current Smoker | 77.5 | (54.6, 100.5) | 10 | 34.5 | 14 | 48.3 | 5 | 17.2 | ||
| Intake < Average (n = 147) | Intake > Average (n = 103) | p | p Adjusted | |||
|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |||
| Body measurements | ||||||
| Weight | 82.2 | (79.2, 85.3) | 79.8 | (76.4, 83.3) | NS | NS |
| BMI | 28.5 | (27.4, 29.6) | 27.2 | (26.2, 28.1) | 0.08 | 0.063 |
| Waist | 94.6 | (92.2, 97.0) | 91.2 | (88.5, 93.8) | 0.06 | 0.047 |
| Metabolism | ||||||
| Triglycerides | 1.4 | (1.3, 1.5) | 1.3 | (1.1, 1.6) | NS | NS |
| HDL | 1.4 | (1.3, 1.4) | 1.4 | (1.3, 1.4) | NS | NS |
| Glucose | 5.3 | (5.1, 5.5) | 5.0 | (4.9, 5.2) | 0.03 | 0.078 |
| HbA1c | 39.6 | (38.3, 41.0) | 37.8 | (36.9, 38.7) | 0.03 | NS |
| Insulin | 64.6 | (55.5, 73.6) | 52.3 | (44.3, 60.3) | 0.05 | 0.041 |
| Heart health | ||||||
| BP (systolic) | 135.0 | (132.5, 137.5) | 130.6 | (127.4, 133.8) | 0.03 | 0.016 |
| BP (diastolic) | 85.2 | (83.6, 86.7) | 82.3 | (80.4, 84.1) | 0.02 | 0.007 |
| CVD risk score | 2.8 | (2.6, 3.0) | 2.6 | (2.2, 2.9) | NS | NS |
| Mental Health | ||||||
| Wellbeing | 52.5 | (51.1, 53.8) | 52.9 | (51.3, 54.4) | NS | NS |
| n | % | n | % | |||
| MCI | 36 | 24.5 | 14 | 13.6 | 0.04 | NS |
| Current Depression | 13 | 8.8 | 4 | 3.9 | NS | NS |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pearson, J.F.; Pullar, J.M.; Wilson, R.; Spittlehouse, J.K.; Vissers, M.C.M.; Skidmore, P.M.L.; Willis, J.; Cameron, V.A.; Carr, A.C. Vitamin C Status Correlates with Markers of Metabolic and Cognitive Health in 50-Year-Olds: Findings of the CHALICE Cohort Study. Nutrients 2017, 9, 831. https://doi.org/10.3390/nu9080831
Pearson JF, Pullar JM, Wilson R, Spittlehouse JK, Vissers MCM, Skidmore PML, Willis J, Cameron VA, Carr AC. Vitamin C Status Correlates with Markers of Metabolic and Cognitive Health in 50-Year-Olds: Findings of the CHALICE Cohort Study. Nutrients. 2017; 9(8):831. https://doi.org/10.3390/nu9080831
Chicago/Turabian StylePearson, John F., Juliet M. Pullar, Renee Wilson, Janet K. Spittlehouse, Margreet C. M. Vissers, Paula M. L. Skidmore, Jinny Willis, Vicky A. Cameron, and Anitra C. Carr. 2017. "Vitamin C Status Correlates with Markers of Metabolic and Cognitive Health in 50-Year-Olds: Findings of the CHALICE Cohort Study" Nutrients 9, no. 8: 831. https://doi.org/10.3390/nu9080831
APA StylePearson, J. F., Pullar, J. M., Wilson, R., Spittlehouse, J. K., Vissers, M. C. M., Skidmore, P. M. L., Willis, J., Cameron, V. A., & Carr, A. C. (2017). Vitamin C Status Correlates with Markers of Metabolic and Cognitive Health in 50-Year-Olds: Findings of the CHALICE Cohort Study. Nutrients, 9(8), 831. https://doi.org/10.3390/nu9080831








