Vitamin D and Infectious Diseases: Simple Bystander or Contributing Factor?
Abstract
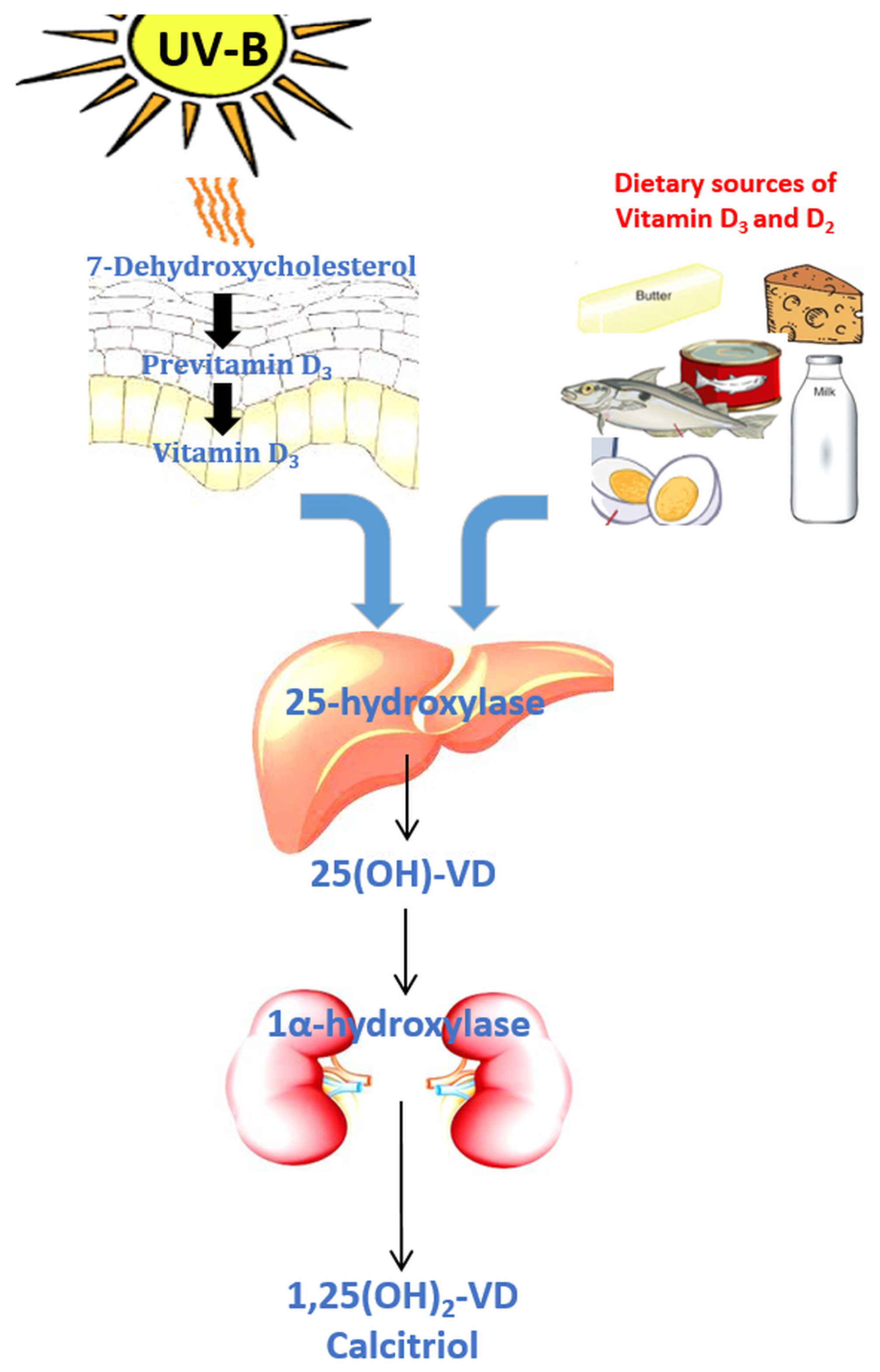
:1. Introduction
2. Vitamin D and the Immune System
3. Vitamin D and Tuberculosis
4. Vitamin D and RTI
5. Vitamin D and Human Immunodeficiency Virus Infection
6. Vitamin D and Fungal Infections
7. Vitamin D and Sepsis
8. Conclusions
Author Contributions
Conflicts of Interest
References
- Vitamin, D. The British Dietetic Association (BDA) Food Fact Sheet. Available online: https://www.bda.uk.com/foodfacts/VitaminD.pdf (accessed on 31 March 2017).
- Deluca, H. History of the discovery of vitamin D and its active metabolites. Bonekey Rep. 2014, 3, 479. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Frommer, J.E.; McNeill, S.C.; Richtand, N.M.; Henley, J.W.; Potts, J.T. Photometabolism of 7-dehydrocholesterol to previtamin D3 in skin. Biochem. Biophys. Res. Commun. 1977, 76, 107–114. [Google Scholar] [CrossRef]
- Dusso, A.S.; Brown, A.J.; Slatopolsky, E. Vitamin D. Am. J. Physiol. Ren. Physiol. 2005, 289, F8–F28. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.R.; Kodicek, E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature 1970, 228, 764–766. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D status: Measurement, interpretation, and clinical application. Ann. Epidemiol. 2009, 19, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Nowson, C.A.; McGrath, J.J.; Ebeling, P.R.; Haikerwal, A.; Daly, R.M.; Sanders, K.M.; Seibel, M.J.; Mason, R.S. Vitamin D and health in adults in Australia and New Zealand: A position statement. Med. J. Aust. 2012, 196, 686–687. [Google Scholar] [CrossRef] [PubMed]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.D.; Cuppari, L.; Titan, S.M.; Magalhães, M.C.; Sassaki, A.L.; dos Reis, L.M.; Jorgetti, V.; Moysés, R.M. Vitamin D status in a sunny country: Where has the sun gone? Clin. Nutr. 2010, 29, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Mithal, A.; Wahl, D.A.; Bonjour, J.P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj Fuleihan, G.; Josse, R.G.; Lips, P.; Morales-Torres, J. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin. Proc. 2006, 81, 353–373. [Google Scholar] [CrossRef] [PubMed]
- Van der Wielen, R.; Lowik, M.; van den Berg, H.; de Groot, L.; Haller, J.; Moreiras, O.; van Staveren, W. Serum vitamin D concentrations among elderly people in Europe. Lancet 1995, 346, 207–210. [Google Scholar] [CrossRef]
- Harris, S.S. Vitamin D and African Americans. J. Nutr. 2006, 136, 1126–1129. [Google Scholar] [PubMed]
- Harris, S.S.; Dawson-Hughes, B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. Am. J. Clin. Nutr. 1998, 67, 1232–1326. [Google Scholar] [PubMed]
- Hewison, M. Antibacterial effects of vitamin D. Nat. Rev. Endocrinol. 2011, 7, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, M.E.; Coleman, L.A. Vitamin D and influenza. Adv. Nutr. 2012, 3, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D and the immune system: Role in protection against bacterial infection. Curr. Opin. Nephrol. Hypertens. 2008, 17, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.A.; Bearden, A.; Striker, R. Vitamin D and the anti-viral state. J. Clin. Virol. 2011, 50, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Veldman, C.M.; Cantorna, M.T.; DeLuca, H.F. Expression of 1,25-dihydroxyvitamin D3 receptor in the immune system. Arch. Biochem. Biophys. 2000, 374, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Mahon, B.D.; Wittke, A.; Weaver, V.; Cantorna, M.T. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J. Cell. Biochem. 2003, 89, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Lyakh, L.A.; Sanford, M.; Chekol, S.; Young, H.A.; Roberts, A.B. TGF-beta and vitamin D3 utilize distinct pathways to suppress IL-12 production and modulate rapid differentiation of human monocytes into CD83+ dendritic cells. J. Immunol. 2005, 174, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Adorini, L.; Penna, G. Dendritic cell tolerogenicity: A key mechanism in immunomodulation by vitamin D receptor agonists. Hum. Immunol. 2009, 70, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and the immune system: New perspectives on an old theme. Endocrinol. Metab. Clin. N. Am. 2010, 39, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Koeffler, H.P.; Reichel, H.; Bishop, J.E.; Norman, A.W. gamma-Interferon stimulates production of 1,25-dihydroxyvitamin D3 by normal human macrophages. Biochem. Biophys. Res. Commun. 1985, 127, 596–603. [Google Scholar] [CrossRef]
- Rook, G.A.; Steele, J.; Fraher, L.; Barker, S.; Karmali, R.; O’Riordan, J.; Stanford, J. Vitamin D3, gamma interferon, and control of proliferation of mycobacterium tuberculosis by human monocytes. Immunology 1986, 57, 159–163. [Google Scholar] [PubMed]
- Medzhitov, R.; Janeway, C., Jr. Innate immune recognition: Mechanisms and pathways. Immunol. Rev. 2000, 173, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T. Toll-Like Receptor Triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.H.; Mader, S.; et al. Cutting edge: 1,25-Dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Borregaard, N.; Koeffler, H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005, 19, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009, 4, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Saito, T.; Koeffler, H.P. Exaptation of an ancient Alu short interspersed element provides a highly conserved vitamin D-mediated innate immune response in humans and primates. BMC Genom. 2009, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Ren, S.; Liu, P.T.; Chun, R.F.; Lagishetty, V.; Gombart, A.F.; Borregaard, N.; Modlin, R.L.; Hewison, M. Vitamin D-directed rheostatic regulation of monocyte antibacterial responses. J. Immunol. 2009, 182, 4289–4295. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Lindh, Å.U.; Björkhem-Bergman, L.; Lindh, J.D. Vitamin D and respiratory tract infections: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2013, 8, e65835. [Google Scholar] [CrossRef] [PubMed]
- Hansdottir, S.; Monick, M.M.; Hinde, S.L.; Lovan, N.; Look, D.C.; Hunninghake, G.W. Respiratory epithelial cells convert inactive vitamin D to its active form: Potential effects on host defense. J. Immunol. 2008, 181, 7090–7099. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.; Garcia-Sastre, A.; Ruchala, P.; Lehrer, R.I.; Chang, T.; Klotman, M.E. alpha-Defensin inhibits influenza virus replication by cell-mediated mechanism(s). J. Infect. Dis. 2007, 196, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.L.; Vargas, J.; DelPortillo, A.; Klotman, M.E. Dual role of alpha-defensin-1 in anti-HIV-1 innate immunity. J. Clin. Investig. 2005, 115, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.P.; Bellido, T.; Manolagas, S.C. Down-regulation of NF-kappa B protein levels in activated human lymphocytes by 1,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. USA 1995, 92, 10990–10994. [Google Scholar] [CrossRef] [PubMed]
- Giarratana, N.; Penna, G.; Amuchastegui, S.; Mariani, R.; Daniel, K.C.; Adorini, L. A vitamin D analog down-regulates proinflammatory chemokine production by pancreatic islets inhibiting T cell recruitment and type 1 diabetes development. J. Immunol. 2004, 173, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Greiller, C.L.; Martineau, A.R. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients 2015, 7, 4240–4270. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Yu, S.; Bruce, D. The paradoxical effects of vitamin D on type 1 mediated immunity. Mol. Aspects Med. 2008, 29, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef] [PubMed]
- Provvedini, D.M.; Tsoukas, C.D.; Deftos, L.J.; Manolagas, S.C. 1 alpha,25-Dihydroxyvitamin D3-binding macromolecules in human B lymphocytes: Effects on immunoglobulin production. J. Immunol. 1986, 136, 2734–2740. [Google Scholar] [PubMed]
- Smith, I. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin. Microbiol. Rev. 2003, 16, 463–496. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Tuberculosis Report. Available online: http://www.who.int/tb/publications/global_report/en/ (accessed on 15 February 2017).
- Barter, D.M.; Agboola, S.O.; Murray, M.B.; Bärnighausen, T. Tuberculosis and poverty: The contribution of patient costs in sub-Saharan Africa—A systematic review. BMC Public Health 2012, 12, 980. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Nutritional Care and Support for Patient with Tuberculosis, GUIDELINE, 2013. Available online: http://www.who.int/nutrition/publications/guidelines/nutcare_support_patients_with_tb/en/ (accessed on 25 March 2017).
- Cegielski, J.P.; McMurray, D.N. The relationship between malnutrition and tuberculosis: Evidence from studies in humans and experimental animals. Int. J. Tuberc. Lung Dis. 2004, 8, 286–298. [Google Scholar] [PubMed]
- Grobler, L.; Nagpal, S.; Sudarsanam, T.D.; Sinclair, D. Nutritional supplements for people being treated for active tuberculosis. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.-S.; Cho, Y.-J.; Yoon, H.-I.; Song, J.H.; Lee, C.-T.; Lee, J.H. Low serum 25-hydroxyvitamin D level: An independent risk factor for tuberculosis? Clin. Nutr. 2013, 33, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ho-Pham, L.T.; Nguyen, N.D.; Nguyen, T.T.; Nguyen, D.H.; Bui, P.K.; Nguyen, V.N.; Nguyen, T.V. Association between vitamin D insufficiency and tuberculosis in a Vietnamese population. BMC Infect. Dis. 2010, 10, 306. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Kim, S.Y.; Chung, K.S.; Kim, E.Y.; Jung, J.Y.; Park, M.S.; Kim, Y.S.; Kim, S.K.; Chang, J.; Kang, Y.A. Association between vitamin D deficiency and tuberculosis in a Korean population. Int. J. Tuberc. Lung Dis. 2014, 18, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Nnoaham, K.E.; Clarke, A. Low serum vitamin D levels and tuberculosis: A systematic review and meta-analysis. Int. J. Epidemiol. 2008, 37, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Wu, G.; Yang, W.; Gu, X.; Liang, W.; Yao, Y.; Song, Y. A serum vitamin D level <25 nmol/L pose high tuberculosis risk:A meta-analysis. PLoS ONE 2015, 10, e0126014. [Google Scholar]
- Martineau, A.R.; Honecker, F.U.; Wilkinson, R.J.; Griffiths, C.J. Vitamin D in the treatment of pulmonary tuberculosis. J. Steroid Biochem. Mol. Biol. 2007, 103, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.J.B. On the use and administration of cod-liver oil in pulmonary consumption. Am. J. Med. Sci. 1849, 11, 467–468. [Google Scholar] [CrossRef]
- Daley, P.; Jagannathan, V.; John, K.R.; Sarojini, J.; Latha, A.; Vieth, R.; Suzana, S.; Jeyaseelan, L.; Christopher, D.J.; Smieja, M.; et al. Adjunctive vitamin D for treatment of active tuberculosis in India: A randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2015, 15, 528–534. [Google Scholar] [CrossRef]
- Martineau, A.R.; Timms, P.M.; Bothamley, G.H.; Hanifa, Y.; Islam, K.; Claxton, A.P.; Packe, G.E.; Moore-Gillon, J.C.; Darmalingam, M.; Davidson, R.N.; et al. High-dose vitamin D3 during intensive-phase antimicrobial treatment of pulmonary tuberculosis: A double-blind randomised controlled trial. Lancet 2011, 377, 242–250. [Google Scholar] [CrossRef]
- Wejse, C.; Gomes, V.F.; Rabna, P.; Gustafson, P.; Aaby, P.; Lisse, I.M.; Andersen, P.L.; Glerup, H.; Sodemann, M. Vitamin D as supplementary treatment for tuberculosis: A double-blind, randomized, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 2009, 179, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Ralph, A.P.; Waramori, G.; Pontororing, G.J.; Kenangalem, E.; Wiguna, A.; Tjitra, E.; Sandjaja; Lolong, D.B.; Yeo, T.W.; Chatfield, M.D.; et al. l-arginine and vitamin D adjunctive therapies in pulmonary tuberculosis: A randomised, double-blind, placebo-controlled trial. PLoS ONE 2013, 8, e70032. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, N.; Ali, F.; Hasan, Z.; Rao, N.; Aqeel, M.; Mahmood, F. Vitamin D accelerates clinical recovery from tuberculosis: Results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonar. BMC Infect. Dis. 2013, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Tukvadze, N.; Sanikidze, E.; Kipiani, M.; Hebbar, G.; Easley, K.A.; Shenvi, N.; Kempker, R.R.; Frediani, J.K.; Mirtskhulava, V.; Alvarez, J.A.; et al. High-dose vitamin D 3 in adults with pulmonary tuberculosis: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Mily, A.; Rekha, R.S.; Kamal, S.M.M.; Arifuzzaman, A.S.M.; Rahim, Z.; Khan, L.; Haq, M.A.; Zaman, K.; Bergman, P.; Brighenti, S.; et al. Significant effects of oral phenylbutyrate and vitamin D3 adjunctive therapy in pulmonary tuberculosis: A randomized controlled trial. PLoS ONE 2015, 10, e0138340. [Google Scholar] [CrossRef] [PubMed]
- Nursyam, E.W.; Amin, Z.; Rumende, C.M. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med. Indones. 2006, 38, 3–5. [Google Scholar] [PubMed]
- Coussens, A.K.; Wilkinson, R.J.; Hanifa, Y.; Nikolayevskyy, V.; Elkington, P.T.; Islam, K.; Timms, P.M.; Venton, T.R.; Bothamley, G.H.; Packe, G.E.; et al. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proc. Natl. Acad. Sci. USA 2012, 109, 15449–15454. [Google Scholar] [CrossRef] [PubMed]
- Hope-Simpson, R.E. The role of season in the epidemiology of influenza. J. Hyg. (Lond.) 1981, 86, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.J.; Hesketh, K.; Power, C.; Hyppönen, E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br. J. Nutr. 2011, 106, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Ginde, A.A.; Mansbach, J.M.; Camargo, C.A. Association Between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third Naional Health and Nutrition Examination Survey. Arch. Intern. Med. 2009, 169, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Laaksi, I.; Ruohola, J.; Tuohimaa, P.; Auvinen, A.; Haataja, R.; Pihlajama, H. An association of serum vitamin D concentrations <40 nmol/L with acute respiratory tract infection in young Finnish men. Am. J. Clin. Nutr. 2007, 25, 714–717. [Google Scholar]
- Wayse, V.; Yousafzai, A.; Mogale, K.; Filteau, S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur. J. Clin. Nutr. 2004, 58, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.E.; Shah, R.; Black, R.E.; Baqui, A.H. Vitamin D status and acute lower respiratory infection in early childhood in Sylhet, Bangladesh. Acta Paediatr. 2010, 99, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [PubMed]
- Coelho, L.; Cardoso, S.W.; Luz, P.M.; Hoffman, R.M.; Mendonça, L.; Veloso, V.G.; Currier, J.S.; Grinsztejn, B.; Lake, J.E. Vitamin D3 supplementation in HIV infection: Effectiveness and associations with antiretroviral therapy. Nutr. J. 2015, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, O.M.; Agniel, D.; French, A.L.; Tien, P.C.; Weber, K.; Glesby, M.J.; Villacres, M.C.; Sharma, A.; Merenstein, D.; Golub, E.T.; et al. Vitamin D deficiency in HIV-infected and HIV-uninfected women in the United States. J. Acquir. Immune Defic. Syndr. 2011, 57, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Schtscherbyna, A.; Gouveia, C.; Pinheiro, M.F.; Luiz, R.R.; Farias, M.L.; Machado, E.S. Vitamin D status in a Brazilian cohort of adolescents and young adults with perinatally acquired human immunodeficiency virus infection. Mem. Inst. Oswaldo Cruz 2016, 111, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Ezeamama, A.E.; Guwatudde, D.; Wang, M.; Bagenda, D.; Kyeyune, R.; Sudfeld, C.; Manabe, Y.C.; Fawzi, W.W. Vitamin-D deficiency impairs CD4+ T-cell count recovery rate in HIV-positive adults on highly active antiretroviral therapy: A longitudinal study. Clin. Nutr. 2016, 35, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Haug, C.; Müller, F.; Aukrust, P.; Frøland, S.S. Subnormal serum concentration of 1,25-vitamin D in human immunodeficiency virus infection: Correlation with degree of immune deficiency and survival. J. Infect. Dis. 1994, 169, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Giovannucci, E.; Mugusi, F.M.; Spiegelman, D.; Aboud, S.; Hertzmark, E.; Msamanga, G.I.; Hunter, D.; Fawzi, W.W. Vitamin D status of HIV-infected women and its association with HIV disease progression, anemia, and mortality. PLoS ONE 2010, 5, e8770. [Google Scholar] [CrossRef] [PubMed]
- Sudfeld, C.R.; Wang, M.; Aboud, S.; Giovannucci, E.L.; Mugusi, F.M.; Fawzi, W.W. Vitamin D and HIV progression among Tanzanian adults initiating antiretroviral therapy. PLoS ONE 2012, 7, e40036. [Google Scholar] [CrossRef] [PubMed]
- Viard, J.-P.; Souberbielle, J.-C.; Kirk, O.; Reekie, J.; Knysz, B.; Losso, M.; Gatell, J.; Pedersen, C.; Bogner, J.R.; Lundgren, J.D.; et al. Study Group vitamin D and clinical disease progression in HIV infection: Results from the EuroSIDA study. AIDS 2011, 25, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Escota, G.V.; Mondy, K.; Bush, T.; Conley, L.; Brooks, J.T.; Önen, N.; Patel, P.; Kojic, E.M.; Henry, K.; Hammer, J.; et al. High prevalence of low bone mineral density and substantial bone loss over 4 years among HIV-infected persons in the era of modern antiretroviral therapy. AIDS Res. Hum. Retrovir. 2015, 32, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Kooij, K.W.; Wit, F.W.; Bisschop, P.H.; Schouten, J.; Stolte, I.G.; Prins, M.; van der Valk, M.; Prins, J.M.; van Eck-Smit, B.L.; Lips, P.; et al. Low bone mineral density in patients with well-suppressed HIV infection: association with body weight, smoking, and prior advanced HIV disease. J. Infect. Dis. 2015, 211, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Negredo, E.; Domingo, P.; Ferrer, E.; Estrada, V.; Curran, A.; Navarro, A.; Isernia, V.; Rosales, J.; Pérez-Álvarez, N.; Puig, J.; et al. Peak bone mass in young HIV-infected patients compared with healthy controls. J. Acquir. Immune Defic. Syndr. 2014, 65, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Piso, R.J.; Rothen, M.; Rothen, J.P.; Stahl, M.; Fux, C. Per oral substitution with 300,000 IU vitamin D (Cholecalciferol) reduces bone turnover markers in HIV-infected patients. BMC Infect. Dis. 2013, 13, 577. [Google Scholar] [CrossRef] [PubMed]
- McComsey, G.A.; Kendall, M.A.; Tebas, P.; Swindells, S.; Hogg, E.; Alston-Smith, B.; Suckow, C.; Gopalakrishnan, G.; Benson, C.; Wohl, D.A. Alendronate with calcium and vitamin D supplementation is safe and effective for the treatment of decreased bone mineral density in HIV. AIDS 2007, 21, 2473–2482. [Google Scholar] [CrossRef] [PubMed]
- Hileman, C.O.; Overton, E.T.; McComsey, G.A. Vitamin D and bone loss in HIV. Curr. Opin. HIV AIDS 2016, 11, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Sudjaritruk, T.; Bunupuradah, T.; Aurpibul, L.; Kosalaraksa, P.; Kurniati, N.; Prasitsuebsai, W.; Sophonphan, J.; Ananworanich, J.; Puthanakit, T. Hypovitaminosis D and hyperparathyroidism: Effects on bone turnover and bone mineral density among perinatally HIV-infected adolescents. AIDS 2016, 30, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Dave, J.A.; Cohen, K.; Micklesfield, L.K.; Maartens, G.; Levitt, N.S. Antiretroviral therapy, especially efavirenz, is associated with low bone mineral density in HIV-infected South Africans. PLoS ONE 2015, 10, e0144286. [Google Scholar] [CrossRef] [PubMed]
- Dao, C.N.; Patel, P.; Overton, E.T.; Rhame, F.; Pals, S.L.; Johnson, C.; Bush, T.; Brooks, J.T. Study to understand the natural history of HIV and AIDS in the era of effective therapy (SUN) investigators low vitamin D among HIV-infected adults: Prevalence of and risk factors for low vitamin D levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clin. Infect. Dis. 2011, 52, 396–405. [Google Scholar] [PubMed]
- Libório, A.B.; Andrade, L.; Pereira, L.V.; Sanches, T.R.; Shimizu, M.H.; Seguro, A.C. Rosiglitazone reverses tenofovir-induced nephrotoxicity. Kidney Int. 2008, 74, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Canale, D.; De Bragança, A.C.; Gonçalves, J.G.; Shimizu, M.H.M.; Sanches, T.R.; Andrade, L.; Volpini, R.A.; Seguro, A.C. Vitamin D deficiency aggravates nephrotoxicity, hypertension and dyslipidemia caused by tenofovir: Role of oxidative stress and renin-angiotensin system. PLoS ONE 2014, 9, e103055. [Google Scholar] [CrossRef] [PubMed]
- Sifuentes-Osornio, J.; Corzo-León, D.E.; Ponce-De-León, L.A. Epidemiology of invasive fungal infections in Latin America. Curr. Fungal Infect. Rep. 2012, 6, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Hobson, R.P. The global epidemiology of invasive Candida infections—Is the tide turning? J. Hosp. Infect. 2003, 55, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G. Invasive Candidiasis. Infect. Dis. Clin. N. Am. 2006, 20, 485–506. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Roussos, N.; Vardakas, K.Z. Relative frequency of albicans and the various non-albicans Candida spp among candidemia isolates from inpatients in various parts of the world: A systematic review. Int. J. Infect. Dis. 2010, 14, e954–e966. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Jones, R.N.; Messer, S.A.; Edmond, M.B.; Wenzel, R.P. National surveillance of nosocomial blood stream infection due to species of Candida other than Candida albicans: Frequency of occurrence and antifungal susceptibility in the SCOPE program. Diagn. Microbiol. Infect. Dis. 1998, 30, 121–129. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases form a prospective mnationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Lass-Flörl, C. The changing face of epidemiology of invasive fungal disease in Europe. Mycoses 2009, 52, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Iqbal, N.; Cleveland, A.A.; Farley, M.M.; Harrison, L.H.; Bolden, C.B.; Baughman, W.; Stein, B.; Hollick, R.; Park, B.J.; et al. Species Identification and antifungal susceptibility testing of Candida bloodstream isolates from population-based surveillance studies in two US cities from 2008 to 2011. J. Clin. Microbiol. 2012, 50, 3435–3442. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, A.A.; Farley, M.M.; Harrison, L.H.; Stein, B.; Hollick, R.; Lockhart, S.R.; Magill, S.S.; Derado, G.; Park, B.J.; Chiller, T.M. Changes in incidence and antifungal drug resistance in candidemia: Results from population-based laboratory surveillance in Atlanta and Baltimore, 2008–2011. Clin. Infect. Dis. 2012, 55, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.E.; Peck, K.R.; Joo, E.-J.; Kim, S.W.; Jung, S.-I.; Chang, H.H.; Park, K.H.; Han, S.H. Impact of first-line antifungal agents on the outcomes and costs of candidemia. Antimicrob. Agents Chemother. 2012, 56, 3950–3956. [Google Scholar] [CrossRef] [PubMed]
- Franz, R.; Kelly, S.L.; Lamb, D.C.; Kelly, D.E.; Ruhnke, M.; Morschhäuser, J. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob. Agents Chemother. 1998, 42, 3065–3072. [Google Scholar] [PubMed]
- Laverdière, M. Systemic antifungal drugs: Are we making any progress? Can. J. Infect. Dis. 1994, 5, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R. Current epidemiology of Candida infection. Clin. Microbiol. Newsl. 2014, 36, 131–136. [Google Scholar] [CrossRef]
- Neumann, A.; Wieczor, M.; Zielinska, J.; Baginski, M.; Czub, J. Membrane sterols modulate the binding mode of amphotericin B without affecting its affinity for a lipid bilayer. Langmuir 2016, 32, 3452–3461. [Google Scholar] [CrossRef] [PubMed]
- Dórea, E.L.; Yu, L.; De Castro, I.; Campos, S.B.; Ori, M.; Vaccari, E.M.; Lacaz, C.d.S.; Seguro, A.C. Nephrotoxicity of amphotericin B is attenuated by solubilizing with lipid emulsion. J. Am. Soc. Nephrol. 1997, 8, 1415–1422. [Google Scholar]
- Ferreira, D.; Canale, D.; Volpini, R.A.; Gois, P.H.F.; Shimizu, M.H.M.; Girardi, A.C.C.; Seguro, A.C. Vitamin D deficiency induces acute kidney injury in rats treated with lipid formulation of amphotericin B. In Proceedings of the ASN 2016—American Society of Nephrology Kidney Week, Chicago, IL, USA, 15–20 November 2016; p. 670A. [Google Scholar]
- Lim, J.H.; Ravikumar, S.; Wang, Y.-M.; Thamboo, T.P.; Ong, L.; Chen, J.; Goh, J.G.; Tay, S.H.; Chengchen, L.; Win, M.S.; et al. Bimodal influence of vitamin D in host response to systemic Candida infection-vitamin D dose matters. J. Infect. Dis. 2015, 212, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Khoo, A.-L.; Chai, L.Y.; Koenen, H.J.; Kullberg, B.-J.; Joosten, I.; van der Ven, A.J.; Netea, M.G. 1,25-Dihydroxyvitamin D3 modulates cytokine production induced by Candida albicans: Impact of seasonal variation of immune responses. J. Infect. Dis. 2011, 203, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, J.N.; Bicanic, T.; Loyse, A.; Meintjes, G.; Hogan, L.; Roberts, C.H.; Shoham, S.; Perfect, J.R.; Govender, N.P.; Harrison, T.S. Very low levels of 25-hydroxyvitamin D are not associated with immunologic changes or clinical outcome in South African patients With HIV-associated cryptococcal meningitis. Clin. Infect. Dis. 2014, 59, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Cryptococcal Meningitis: A Deadly Fungal Disease Among People Living With HIV/AIDS Cryptococcal Infection. Available online: https://www.cdc.gov/fungal/pdf/at-a-glance-508c.pdf (accessed on 14 March 2017).
- Jarvis, J.N.; Harrison, T.S. HIV-associated cryptococcal meningitis. AIDS 2007, 21, 2119–2129. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Wannemuehler, K.A.; Marston, B.J.; Govender, N.; Pappas, P.G.; Chiller, T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009, 23, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801. [Google Scholar] [CrossRef] [PubMed]
- Remick, D.G. Pathophysiology of sepsis. Am. J. Pathol. 2007, 170, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Tiru, B.; DiNino, E.K.; Orenstein, A.; Mailloux, P.T.; Pesaturo, A.; Gupta, A.; McGee, W.T. The economic and humanistic burden of severe sepsis. Pharmacoeconomics 2015, 33, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Danai, P.A.; Sinha, S.; Moss, M.; Haber, M.J.; Martin, G.S. Seasonal variation in the epidemiology of sepsis. Crit. Care Med. 2007, 35, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Mayr, F.B.; Yende, S.; Angus, D.C. Epidemiology of severe sepsis. Virulence 2014, 5, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Klingberg, E.; Oleröd, G.; Konar, J.; Petzold, M.; Hammarsten, O. Seasonal variations in serum 25-hydroxy vitamin D levels in a Swedish cohort. Endocrine 2015, 49, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Moromizato, T.; Litonjua, A.A.; Braun, A.B.; Gibbons, F.K.; Giovannucci, E.; Christopher, K.B. Association of low serum 25-hydroxyvitamin D levels and sepsis in the critically ill. Crit. Care Med. 2014, 42, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.B.; Gibbons, F.K.; Litonjua, A.A.; Giovannucci, E.; Christopher, K.B. Low serum 25-hydroxyvitamin D at critical care initiation is associated with increased mortality. Crit. Care Med. 2012, 40, 63–72. [Google Scholar] [CrossRef] [PubMed]
- De Haan, K.; Groeneveld, A.B.; de Geus, H.R.; Egal, M.; Struijs, A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: Systematic review and meta-analysis. Crit. Care 2014, 18, 660. [Google Scholar] [CrossRef] [PubMed]
- Upala, S.; Sanguankeo, A.; Permpalung, N. Significant association between vitamin D deficiency and sepsis: A systematic review and meta-analysis. BMC Anesthesiol. 2015, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- McNally, J.D.; Ginde, A.A.; Amrein, K. Clarification needed for the systematic review of vitamin D trials in the ICU. Intensive Care Med. 2017, 43, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, S.A.; De Pascale, G.; Needleman, J.S.; Nakazawa, H.; Kaneki, M.; Bajwa, E.K.; Camargo, C.A.; Bhan, I.; Bhan, I. Effect of cholecalciferol supplementation on vitamin D status and cathelicidin levels in sepsis: A randomized, placebo-controlled trial. Crit. Care Med. 2015, 43, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Leaf, D.E.; Raed, A.; Donnino, M.W.; Ginde, A.A.; Waikar, S.S. Randomized controlled trial of calcitriol in severe sepsis. Am. J. Respir. Crit. Care Med. 2014, 190, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Vitamin D—Health Professional Fact Sheet. Available online: https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ (accessed on 12 June 2017).
- Institute of Medicine of the National Academices. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]


| Author | Study Design | Number of Patients | Dose of Vitamin D | Adverse Events | Primary Outcomes | Conclusion |
|---|---|---|---|---|---|---|
| Coussens et al. [64] # | Double-blind, randomized, placebo-controlled | 95 | 100,000 IU VD3 PO (4 doses) | Not reported | Sputum smear and culture conversion Circulating immune response | Improved both outcomes |
| Daley et al. [56] | Double-blind, randomized, placebo-controlled | 247 | 100,000 IU VD3 PO (4 doses) | Not correlated with intervention | Sputum culture conversion | No difference between groups |
| Martineau et al. [57] | Double-blind, randomized, placebo-controlled | 146 | 100,000 IU VD3 PO (4 doses) | Paradoxical upgrading reaction (n = 2) | Sputum culture conversion | Improved outcome only for tt genotype (VDR receptor) |
| Mily et al. [62] | Double-blind, randomized, placebo-controlled | 288 | 5000 IU/day VD3 PO (2 months) | Not correlated with intervention | Sputum culture conversion Clinical symptoms ¶ | Improved only culture conversion |
| Nursyam et al. [63] ± | Double-blind, randomized, placebo-controlled | 67 | 1000 IU/day VD * PO (2 months) | Not reported | Sputum smear conversion Radiological changes | Improved both outcomes |
| Ralph et al. [59] | Double-blind, randomized, placebo-controlled | 200 | 50,000 IU VD3 PO (2 doses) | Similar between groups | Sputum culture conversion Clinical symptoms/lung function test | No difference between groups |
| Salahuddin et al. [60] | Double-blind, randomized, placebo-controlled | 259 | 600,000 IU VD3 IM (2 doses) | Paradoxical upgrading reaction (n = 1) | Weight gain Radiological changes | Improved both outcomes & |
| Tukvadze et al. [61] | Double-blind, randomized, placebo-controlled | 199 | 50,000 IU 3×/week VD3 PO (8 weeks) and 50,000 IU q2week (8 weeks) | Similar between groups | Sputum culture conversion | No difference between groups |
| Wejse et al. [58] | Double-blind, randomized, placebo-controlled | 367 | 100,000 IU VD3 PO (3 doses) | Similar between groups | Clinical symptoms | No difference between groups |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gois, P.H.F.; Ferreira, D.; Olenski, S.; Seguro, A.C. Vitamin D and Infectious Diseases: Simple Bystander or Contributing Factor? Nutrients 2017, 9, 651. https://doi.org/10.3390/nu9070651
Gois PHF, Ferreira D, Olenski S, Seguro AC. Vitamin D and Infectious Diseases: Simple Bystander or Contributing Factor? Nutrients. 2017; 9(7):651. https://doi.org/10.3390/nu9070651
Chicago/Turabian StyleGois, Pedro Henrique França, Daniela Ferreira, Simon Olenski, and Antonio Carlos Seguro. 2017. "Vitamin D and Infectious Diseases: Simple Bystander or Contributing Factor?" Nutrients 9, no. 7: 651. https://doi.org/10.3390/nu9070651





