N-3 Polyunsaturated Fatty Acids Improve Liver Lipid Oxidation-Related Enzyme Levels and Increased the Peroxisome Proliferator-Activated Receptor α Expression Level in Mice Subjected to Hemorrhagic Shock/Resuscitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Animal Treatments and Groups
2.3. HS Model
2.4. Blood and Tissue Collection
2.5. Western Blot Analysis
2.6. Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.7. Statistical Analyzes
3. Results
3.1. TG Levels in Mice Subjected to HS/R
3.2. Changes in Serum Pro-Inflammation Cytokines Level in Mice Subjected to HS/R
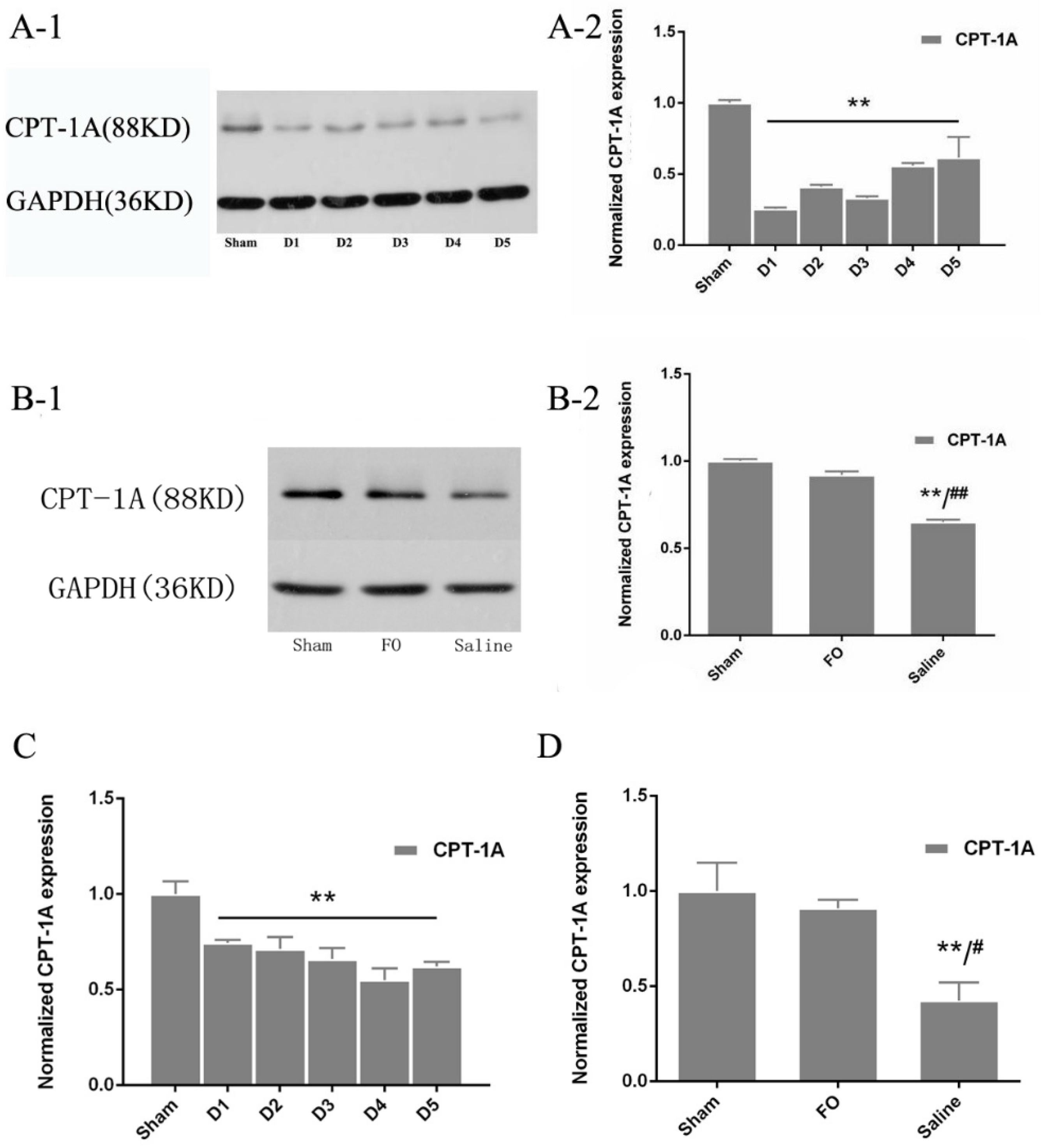
3.3. Changes in CPT-1A Expression in the Liver
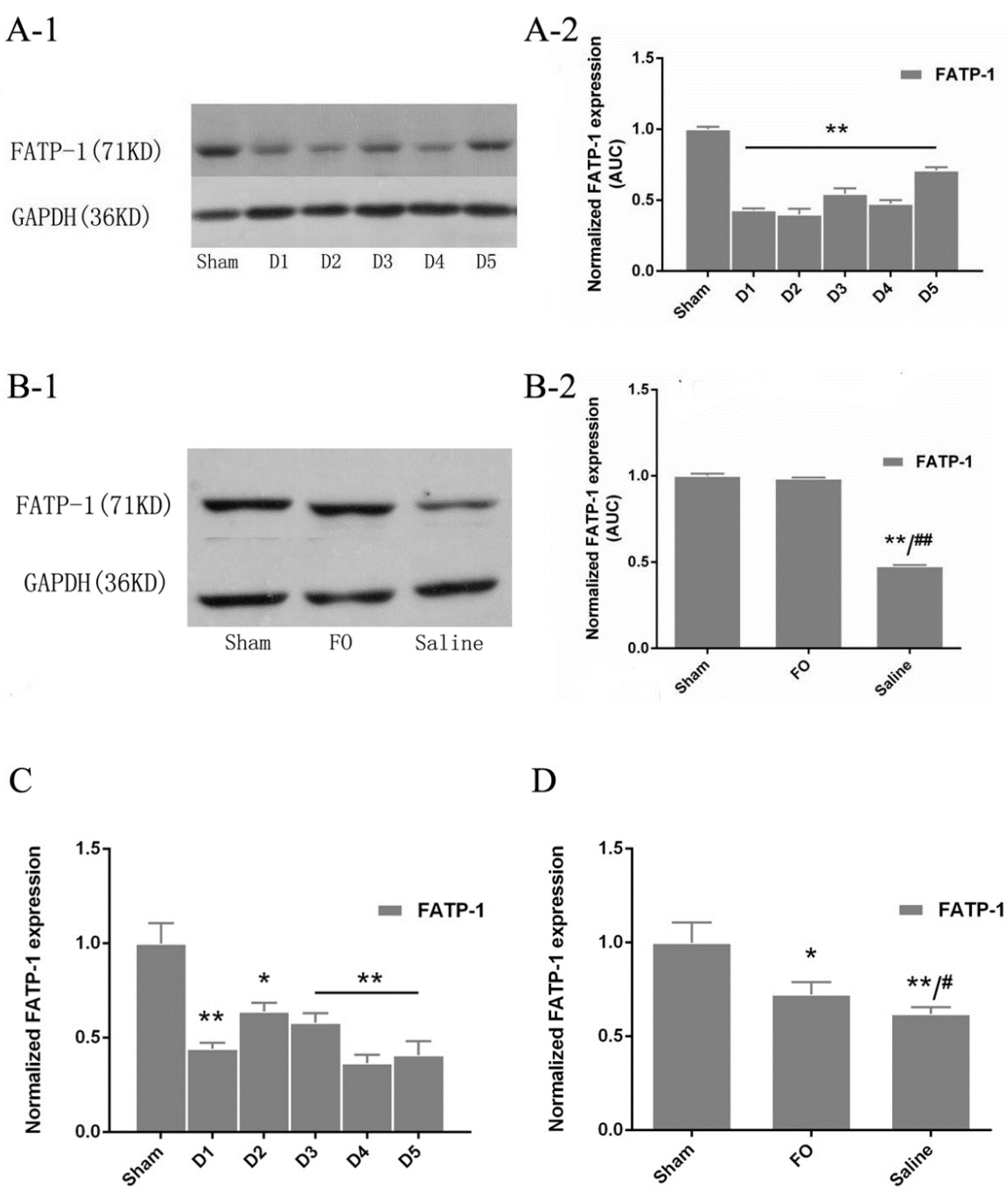
3.4. Changes in FATP-1 Expression in the Liver
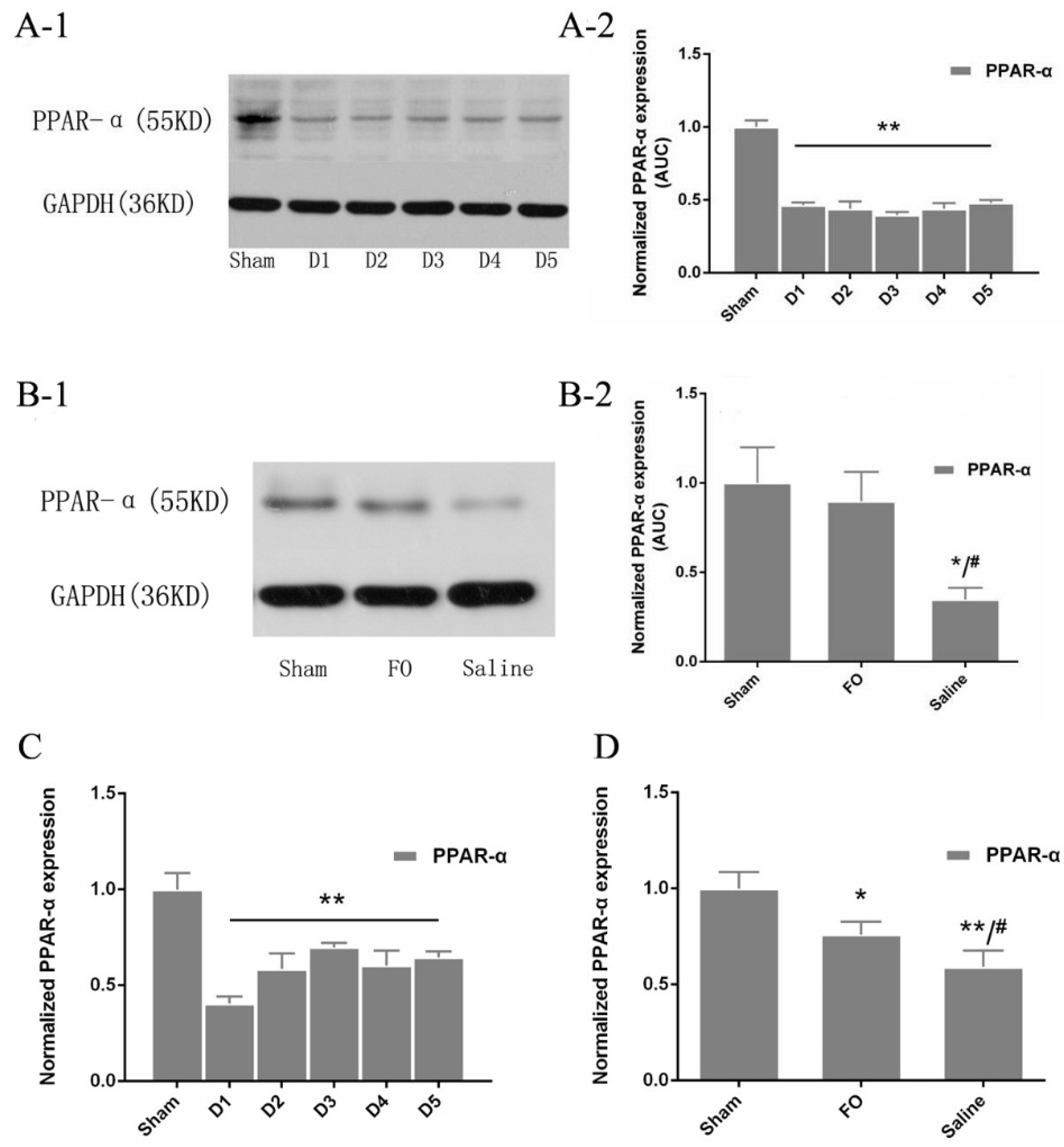
3.5. Changes in PPAR-α Expression in the Liver
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pfeifer, R.; Tarkin, I.S.; Rocos, B.; Pape, H.C. Patterns of mortality and causes of death in polytrauma patients—Has anything changed? Injury 2009, 40, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Cairns, C.B. Rude unhinging of the machinery of life: Metabolic approaches to hemorrhagic shock. Curr. Opin. Crit. Care 2001, 7, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Khardori, R.; Castillo, D. Endocrine and metabolic changes during sepsis: An update. Med. Clin. North Am. 2012, 96, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R. Substrate utilization/insulin resistance in sepsis/trauma. Baillieres Clin. Endocrinol. Metab. 1997, 11, 645–657. [Google Scholar] [CrossRef]
- Wolfe, R.R.; Herndon, D.N.; Jahoor, F.; Miyoshi, H.; Wolfe, M. Effect of severe burn injury on substrate cycling by glucose and fatty acids. N. Engl. J. Med. 1987, 317, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R.; Herndon, D.N.; Peters, E.J.; Jahoor, F.; Desai, M.H.; Holland, O.B. Regulation of lipolysis in severely burned children. Ann. Surg. 1987, 206, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, G.; Commerford, S.R.; Richard, A.M.; Adams, S.H.; Corkey, B.E.; O’Doherty, R.M.; Brown, N.F. Increased beta-oxidation in muscle cells enhances insulin-stimulated glucose metabolism and protects against fatty acid-induced insulin resistance despite intramyocellular lipid accumulation. J. Biol. Chem. 2004, 279, 27177–27186. [Google Scholar] [CrossRef] [PubMed]
- Cheatham, W.W. Peroxisome proliferator-activated receptor translational research and clinical experience. Am. J. Clin. Nutr. 2010, 91, 262S–266S. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Xie, W.; Lei, T.; Hamilton, J.A. Eicosapentaenoic acid, but not oleic acid, stimulates beta-oxidation in adipocytes. Lipids 2005, 40, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pan, L.; Lu, J.; Li, N.; Li, J. N-3 PUFAs attenuate ischemia/reperfusion induced intestinal barrier injury by activating I-FABP-PPARgamma pathway. Clin. Nutr. 2012, 31, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.C.; Ding, S.T. N-3 polyunsaturated fatty acids regulate lipid metabolism through several inflammation mediators: Mechanisms and implications for obesity prevention. J. Nutr. Biochem. 2010, 21, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Cree, M.G.; Wolfe, R.R. Postburn trauma insulin resistance and fat metabolism. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E1–E9. [Google Scholar] [CrossRef] [PubMed]
- Cree, M.G.; Newcomer, B.R.; Herndon, D.N.; Qian, T.; Sun, D.; Morio, B.; Zwetsloot, J.J.; Dohm, G.L.; Fram, R.Y.; Mlcak, R.P.; et al. PPAR-alpha agonism improves whole body and muscle mitochondrial fat oxidation, but does not alter intracellular fat concentrations in burn trauma children in a randomized controlled trial. Nutr. Metab. (Lond.) 2007, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Beylot, M.; Guiraud, M.; Grau, G.; Bouletreau, P. Regulation of ketone body flux in septic patients. Am. J. Physiol. 1989, 257 5 Pt 1, E665–E674. [Google Scholar] [PubMed]
- Cree, M.G.; Fram, R.Y.; Herndon, D.N.; Qian, T.; Angel, C.; Green, J.M.; Mlcak, R.; Aarsland, A.; Wolfe, R.R. Human mitochondrial oxidative capacity is acutely impaired after burn trauma. Am. J. Surg. 2008, 196, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Takeyama, N.; Itoh, Y.; Kitazawa, Y.; Tanaka, T. Altered hepatic mitochondrial fatty acid oxidation and ketogenesis in endotoxic rats. Am. J. Physiol. 1990, 259, E498–E505. [Google Scholar] [PubMed]
- Brooks, B.J.; Arch, J.R.; Newsholme, E.A. Effect of some hormones on the rate of the triacylglycerol/fatty-acid substrate cycle in adipose tissue of the mouse in vivo. Biosci. Rep. 1983, 3, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Heath, D.F.; Stoner, H.B. Studies on the mechanism of shock. Non-esterified fatty acid metabolism in normal and injured rats. Br. J. Exp. Pathol. 1968, 49, 160–169. [Google Scholar] [PubMed]
- Liu, Y.; Ma, Z.; Zhao, C.; Wang, Y.; Wu, G.; Xiao, J.; McClain, C.J.; Li, X.; Feng, W. HIF-1alpha and HIF-2alpha are critically involved in hypoxia-induced lipid accumulation in hepatocytes through reducing PGC-1alpha-mediated fatty acid beta-oxidation. Toxicol. Lett. 2014, 226, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Ilias, I.; Vassiliadi, D.A.; Theodorakopoulou, M.; Boutati, E.; Maratou, E.; Mitrou, P.; Nikitas, N.; Apollonatou, S.; Dimitriadis, G.; Armaganidis, A.; et al. Adipose tissue lipolysis and circulating lipids in acute and subacute critical illness: effects of shock and treatment. J. Crit. Care 2014, 29, e5–e9. [Google Scholar] [CrossRef] [PubMed]
- Astrakas, L.G.; Goljer, I.; Yasuhara, S.; Padfield, K.E.; Zhang, Q.; Gopalan, S.; Mindrinos, M.N.; Dai, G.; Yu, Y.M.; Martyn, J.A.; et al. Proton NMR spectroscopy shows lipids accumulate in skeletal muscle in response to burn trauma-induced apoptosis. FASEB J. 2005, 19, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Moser, A.; Patzek, S.M.; Shigenaga, J.K.; Grunfeld, C. Infection decreases fatty acid oxidation and nuclear hormone receptors in the diaphragm. J. Lipid Res. 2009, 50, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Memon, R.A.; Feingold, K.R.; Moser, A.H.; Fuller, J.; Grunfeld, C. Regulation of fatty acid transport protein and fatty acid translocase mRNA levels by endotoxin and cytokines. Am. J. Physiol. 1998, 274, E210–E217. [Google Scholar] [PubMed]
- Memon, R.A.; Grunfeld, C.; Moser, A.H.; Feingold, K.R. Tumor necrosis factor mediates the effects of endotoxin on cholesterol and triglyceride metabolism in mice. Endocrinology 1993, 132, 2246–2253. [Google Scholar] [PubMed]
- Nonogaki, K.; Fuller, G.M.; Fuentes, N.L.; Moser, A.H.; Staprans, I.; Grunfeld, C.; Feingold, K.R. Interleukin-6 stimulates hepatic triglyceride secretion in rats. Endocrinology 1995, 136, 2143–2149. [Google Scholar] [PubMed]
- Feingold, K.R.; Staprans, I.; Memon, R.A.; Moser, A.H.; Shigenaga, J.K.; Doerrler, W.; Dinarello, C.A.; Grunfeld, C. Endotoxin rapidly induces changes in lipid metabolism that produce hypertriglyceridemia: low doses stimulate hepatic triglyceride production while high doses inhibit clearance. J. Lipid Res. 1992, 33, 1765–1776. [Google Scholar] [PubMed]
- Feingold, K.R.; Grunfeld, C. Tumor necrosis factor-alpha stimulates hepatic lipogenesis in the rat in vivo. J. Clin. Invest. 1987, 80, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, D.P.; Forbes, S.; Walker, B.R. Glucocorticoids and fatty acid metabolism in humans: fuelling fat redistribution in the metabolic syndrome. J. Endocrinol. 2008, 197, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.V.; Menezes-Garcia, Z.; Mario, E.G.; Delpuerto, H.L.; Martins, A.S.; Botion, L.M. Increased expression of oxidative enzymes in adipose tissue following PPARalpha-activation. Metabolism 2014, 63, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Sweeney, T.R.; Shigenaga, J.K.; Chui, L.G.; Moser, A.; Grunfeld, C.; Feingold, K.R. Tumor necrosis factor and interleukin 1 decrease RXRalpha, PPARalpha, PPARgamma, LXRalpha, and the coactivators SRC-1, PGC-1alpha, and PGC-1beta in liver cells. Metabolism 2007, 56, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Youssef, J.; Badr, M. Role of Peroxisome Proliferator-Activated Receptors in Inflammation Control. J. Biomed. Biotechnol. 2004, 2004, 156–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Primer | Primer Sequences (5′to3′) |
|---|---|
| FATP1 Forward | TCTGTTCTGATTCGTGTTCGG |
| FATP1 Reverse | CAGCATATACCACTACTGGCG |
| CPT-1A Forward | CTATGCGCTACTCGCTGAAGG |
| CPT-1A Reverse | GGCTTTCGACCCGAGAAGA |
| PPAR-α Forward | TACTGCCGTTTTCACAAGTGC |
| PPAR-α Reverse | AGGTCGTGTTCACAGGTAAGA |
| 36B4 (internal control) Forward | TGAGATTCGGGATATGCTGTTGG |
| 36B4 (internal control) Reverse | CGGGTCCTAGACCAGTGTTCT |
| Group | TG (mmol/L) | p |
|---|---|---|
| Sham | 0.32 ± 0.09 | |
| D1 | 0.30 ± 0.07 | 0.699 |
| D2 | 0.39 ± 0.08 | 0.210 |
| D3 | 0.42 ± 0.09 | 0.055 |
| D4 | 0.44 ± 0.11 | 0.024 * |
| D5 | 0.51 ± 0.13 | 00.001 ** |
| Group | TG (mmol/L) | p |
|---|---|---|
| Sham | 0.34 ± 0.19 | |
| FO | 0.39 ± 0.07 | 0.240 |
| Saline | 0.47 ± 0.11 | 0.048 * |
| Group | TNF-α (pg/mL) | IL-1 (pg/mL) | IL-6 (pg/mL) |
|---|---|---|---|
| Sham | 22.01 ± 9.14 | 10.10 ± 7.23 | 13.00 ± 10.23 |
| D1 | 23.66 ± 13.44 | 12.14 ± 7.67 | 11.75 ± 8.06 |
| D2 | 26.15 ± 10.67 | 14.33 ± 13.04 | 19.75 ± 12.95 |
| D3 | 22.06 ± 6.63 | 12.37 ± 7.49 | 13.25 ± 7.68 |
| D4 | 22.18 ± 7.48 | 11.43 ± 8.03 | 10.25 ± 7.93 |
| D5 | 24.38 ± 10.19 | 10.95 ± 8.98 | 14.25 ± 9.29 |
| Group | TNF-α (pg/mL) | IL-1 (pg/mL) | IL-6 (pg/mL) |
|---|---|---|---|
| Sham | 23.97 ± 9.35 | 10.18 ± 7.42 | 13.12 ± 9.73 |
| FO | 23.66 ± 13.44 | 12.14 ± 7.67 | 11.75 ± 8.06 |
| Saline | 26.15 ± 10.67 | 14.33 ± 13.04 | 13.25 ± 12.95 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Tian, F.; Gao, X.; Wang, X.; Wu, C.; Li, N.; Li, J. N-3 Polyunsaturated Fatty Acids Improve Liver Lipid Oxidation-Related Enzyme Levels and Increased the Peroxisome Proliferator-Activated Receptor α Expression Level in Mice Subjected to Hemorrhagic Shock/Resuscitation. Nutrients 2016, 8, 237. https://doi.org/10.3390/nu8040237
Zhang L, Tian F, Gao X, Wang X, Wu C, Li N, Li J. N-3 Polyunsaturated Fatty Acids Improve Liver Lipid Oxidation-Related Enzyme Levels and Increased the Peroxisome Proliferator-Activated Receptor α Expression Level in Mice Subjected to Hemorrhagic Shock/Resuscitation. Nutrients. 2016; 8(4):237. https://doi.org/10.3390/nu8040237
Chicago/Turabian StyleZhang, Li, Feng Tian, Xuejin Gao, Xinying Wang, Chao Wu, Ning Li, and Jieshou Li. 2016. "N-3 Polyunsaturated Fatty Acids Improve Liver Lipid Oxidation-Related Enzyme Levels and Increased the Peroxisome Proliferator-Activated Receptor α Expression Level in Mice Subjected to Hemorrhagic Shock/Resuscitation" Nutrients 8, no. 4: 237. https://doi.org/10.3390/nu8040237




