Protein Content and Methyl Donors in Maternal Diet Interact to Influence the Proliferation Rate and Cell Fate of Neural Stem Cells in Rat Hippocampus
Abstract
:1. Introduction
2. Experimental Section
2.1. Animals and Diets
| Control (C) | Control MD-Supplemented (Csup) | Restricted (R) | Restricted MD-Supplemented (Rsup) | |
|---|---|---|---|---|
| Dextrose (%) | 10 | 10 | 10 | 10 |
| Sucrose (%) | 10 | 10 | 10 | 10 |
| Soybean oil (%) | 4.3 | 4.3 | 4.3 | 4.3 |
| Cellulose (%) | 5 | 5 | 5 | 5 |
| Corn starch (%) | 43.6 | 37.8 | 56.6 | 50.3 |
| Casein (%) | 22 | 22 | 9 | 9 |
| Methionine(g/kg) | 7.2 | 12 | 2.9 | 12 |
| Choline (g/kg) | 1 | 15 | 1 | 15 |
| Betaine (g/kg) | 0 | 15 | 0 | 15 |
| Vitamin B12 (mg/kg) | 25 | 1000 | 25 | 1000 |
| Folic acid (mg/kg) | 2 | 15 | 2 | 15 |
| Zinc (mg/kg) | 30 | 180 | 30 | 180 |
| Energy (kcal/kg) | 3260.8 | 3064.6 | 3261.6 | 3051.4 |
2.2. Preparation of Rat Hippocampal NPC Cultures
2.3. Immunocytochemistry
2.4. Treatment of Rat Hippocampal NPCs
2.5. RT-PCR
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Actb | CTATCGGCAATGAGCGGTTCC | GCACTGTGTTGGCATAGAGGTC |
| Dcx | CTTGGATGAGAATGAATGCAGAG | GCTTGTGGGTGTAGAGATAGG |
| Dnmt1 | AAACGCAAATGAATCTGCTG | TGTCATCTTCCTGTTCACCT |
| Dnmt3b | CTCATGGAAGATGTGACACCT | AACTCCTTGTCATCCTGATAC |
| Gapdh | CGCCAAGTTCAACGGCACAG | TCCACGACATACTCAGCACCA |
| Gfap | GATCTGGAGAGGAAGGTTGAG | GGGAGTTCTCGAACTTCCTCC |
| Igf1 | AAGCCTACAAAGTCAGCTCG | GGTCTTGTTTCCTGCACTTC |
| Igf1r | ATGACACGAGACATCTACGA | TAAGTTCAAACAGCATATCGGG |
| Igf2 | AAGTCGATGTTGGTGCTTCTC | GAAGGCCTGCTGAAGTAGAA |
| Igf2r | TGTATCCGTGAACCTGTGTC | AGTTGTCCTCTTCCTGATATTCTG |
| Insr | CATTGTCAGAAAGTTTGCCCA | GGAAGTGATAGTAGGGTGGTG |
| Kcnj10 | CTGTGCCAAGATGACATCAG | AACGCTTGTCAGCAATATGC |
| Mecp2 | GGACCTATGTATGATGACCC | TCTACTTTAGAGCGAAAGGCT |
| Nestin | ACATACAGGACTCTGCTGGAG | GAAATTCGGCTTCAGCTTGG |
| Neun (Rbfox3) | CTTCCAGGGTCGTGTATCAG | CTCTACCATAACTGTCACTGTAGG |
2.6. Pyrosequencing
2.7. Statistical Analysis
3. Results
3.1. Methyl Donor Supplementation Associated with Normal Protein Content Increased NPC Proliferation Rate in Vitro and Promoted Neuronal Differentiation
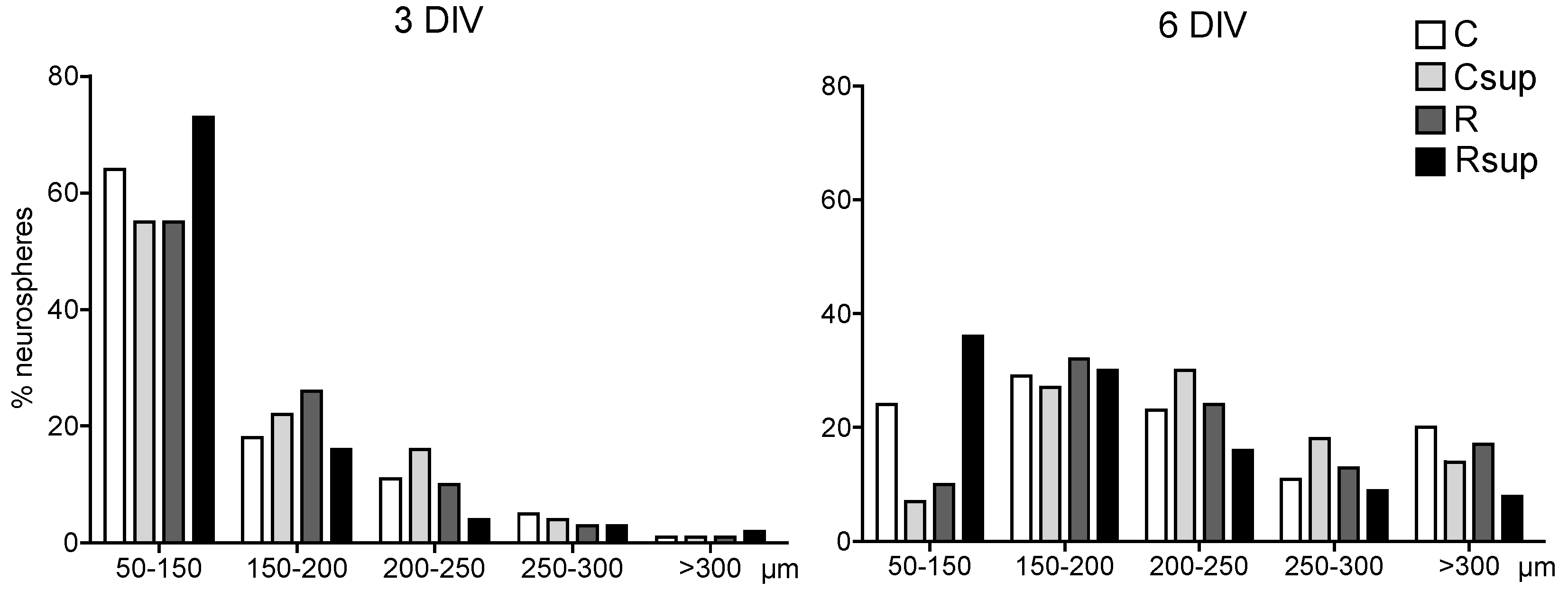
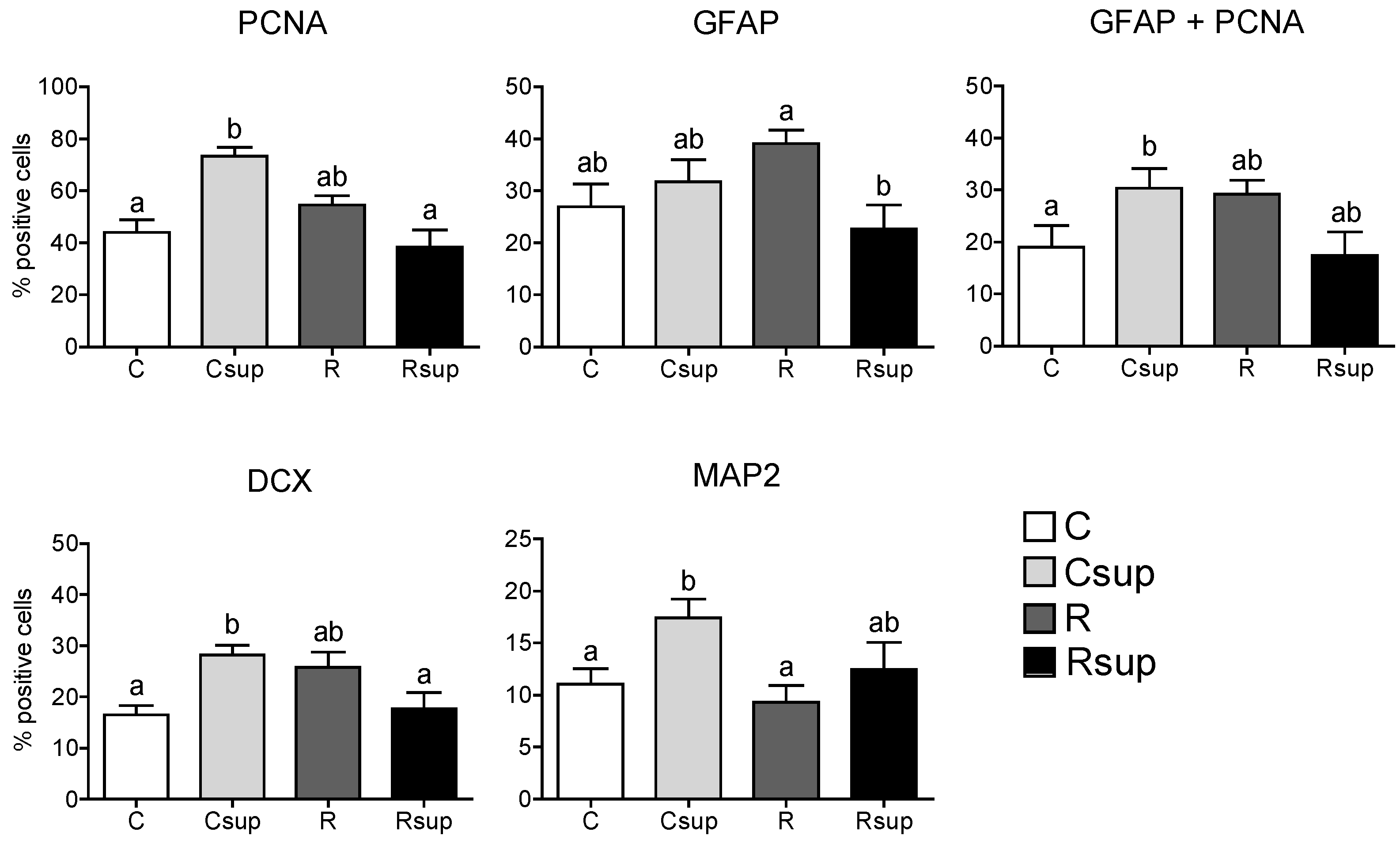
3.2. Protein Content and Methyl Donors in Maternal Diet Interacted to Influence Gene Expression in Whole Hippocampus at Weaning
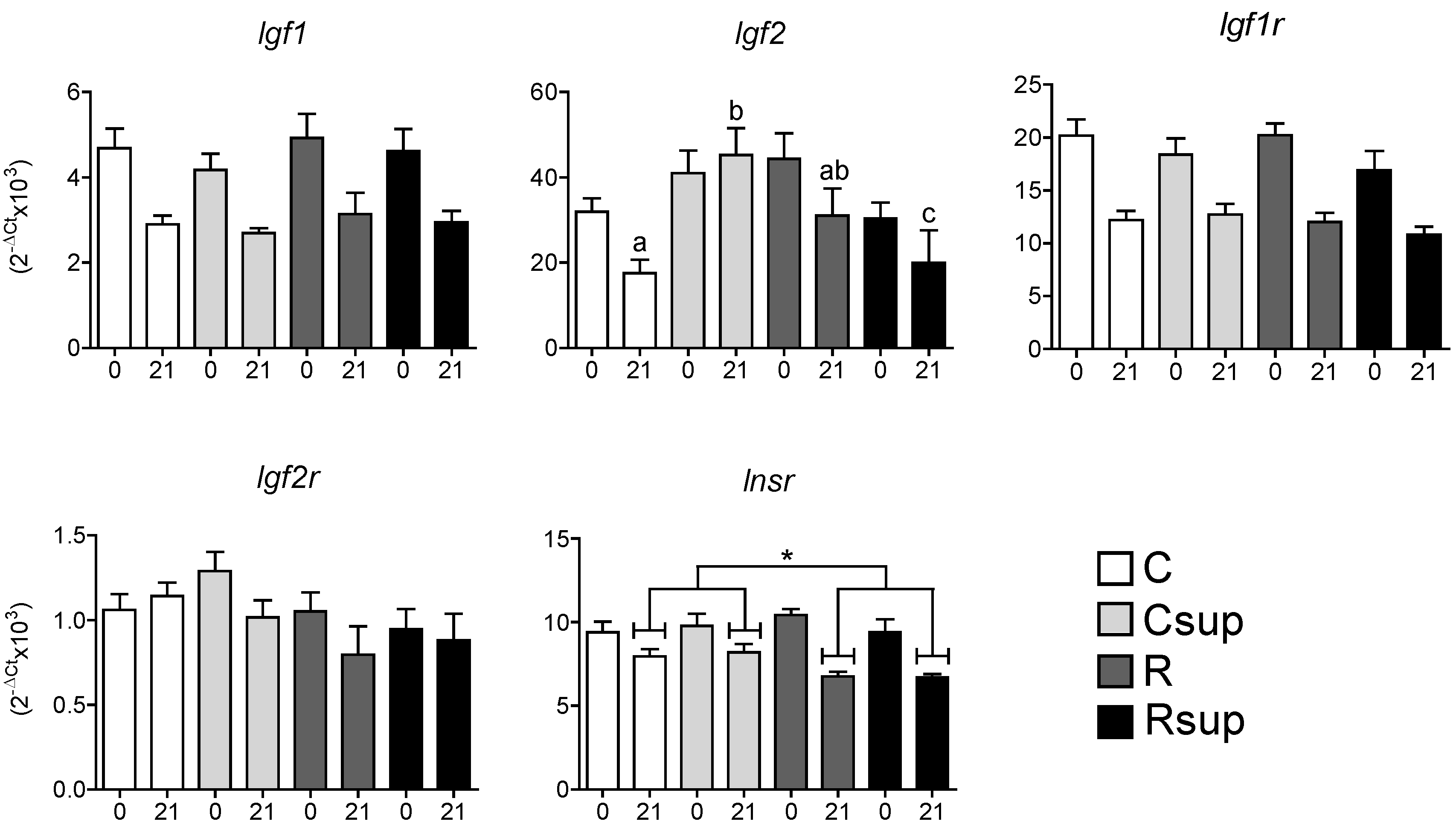
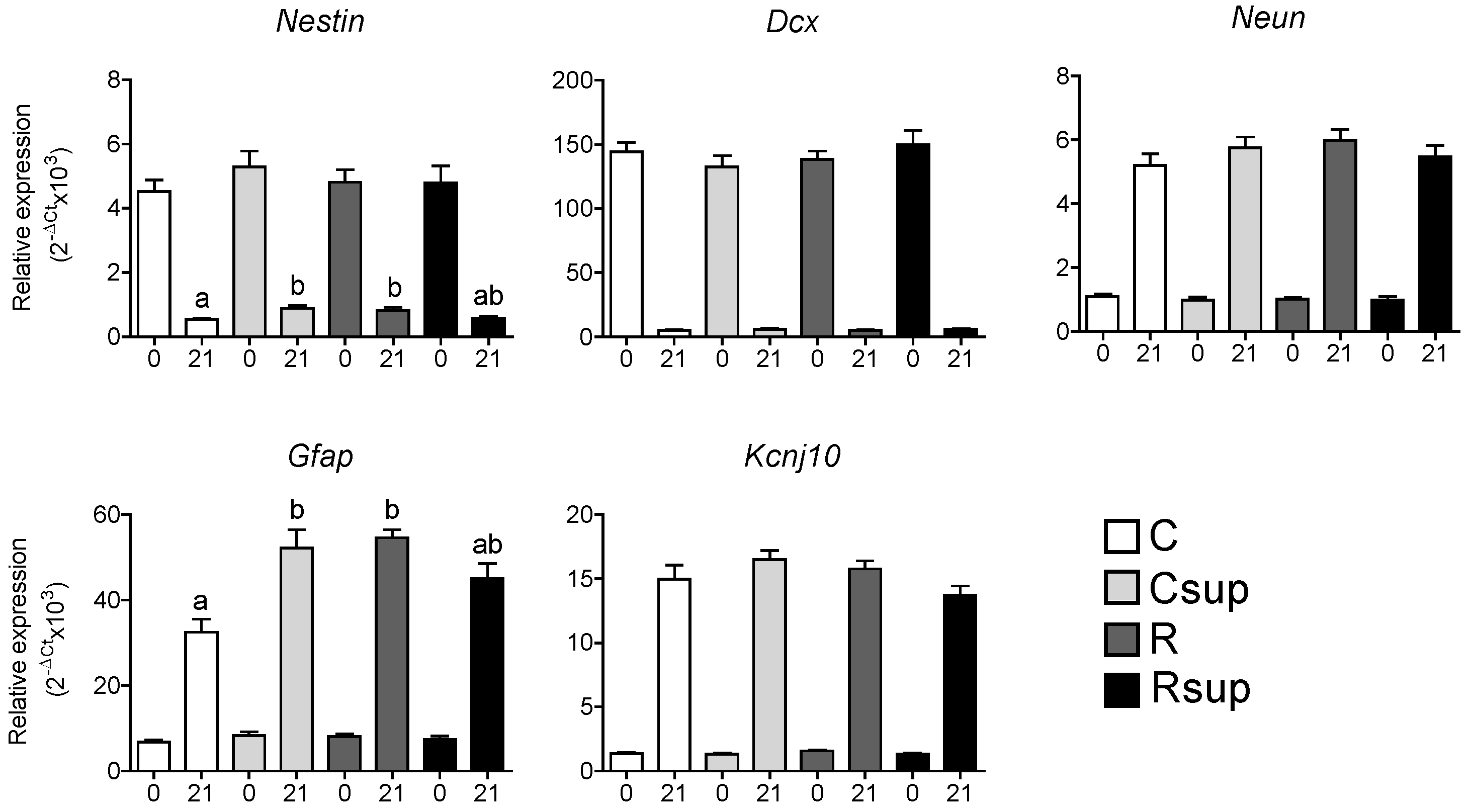

3.3. DNA Methylation of the Gfap Gene in Whole Hippocampus Changed between Birth and Weaning, but Was Barely Affected by Maternal Diet
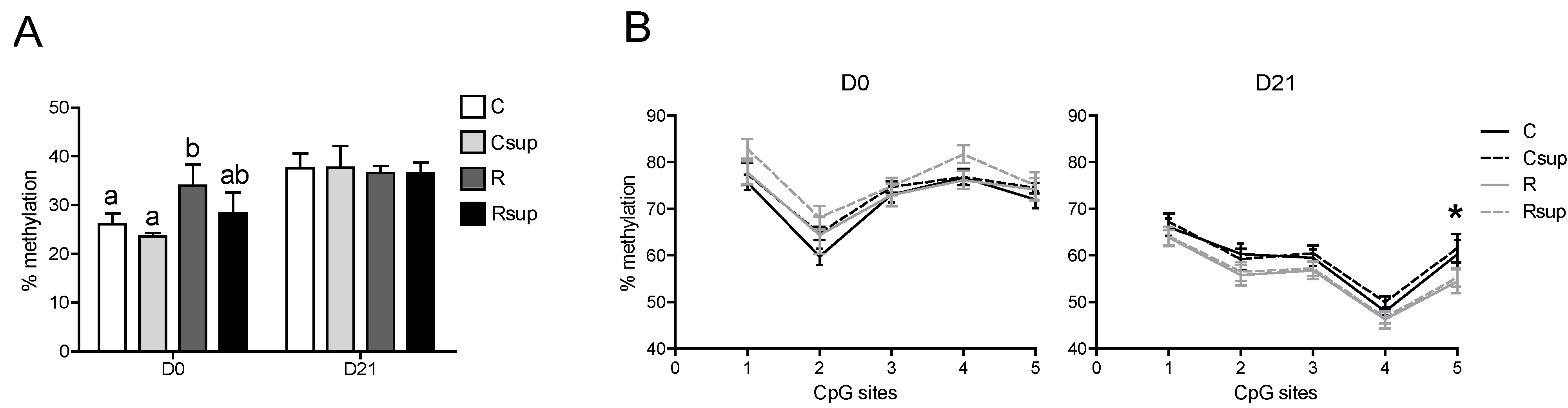
3.4. Influencing DNA Methylation of NPCs in Vitro Affects the Expression of Lineage-Specific Genes
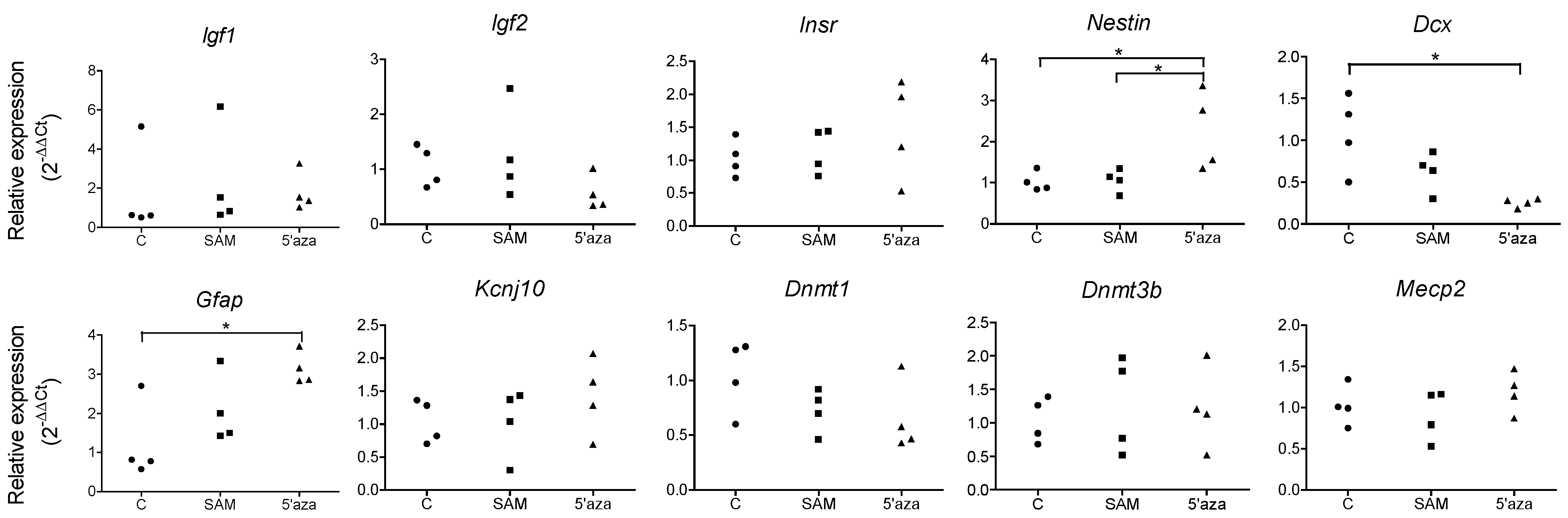
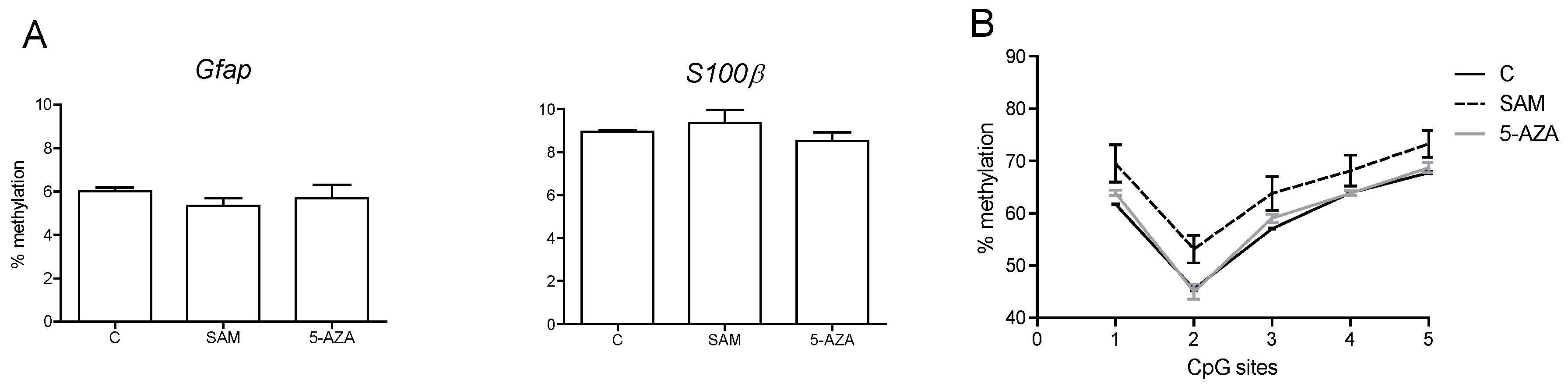
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ranade, S.C.; Rose, A.; Rao, M.; Gallego, J.; Gressens, P.; Mani, S. Different types of nutritional deficiencies affect different domains of spatial memory function checked in a radial arm maze. Neuroscience 2008, 152, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Bouret, S.G.; Draper, S.J.; Simerly, R.B. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 2004, 304, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Casey, P.H.; Whiteside-Mansell, L.; Barrett, K.; Bradley, R.H.; Gargus, R. Impact of prenatal and/or postnatal growth problems in low birth weight preterm infants on school-age outcomes: an 8-year longitudinal evaluation. Pediatrics 2006, 118, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.J. Neuropsychological outcomes of children born very preterm. Semin. Fetal Neonatal Med. 2014, 19, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Li, T.; Ross, M.G. Hypothalamic neurosphere progenitor cells in low birth-weight rat newborns: Neurotrophic effects of leptin and insulin. Brain Res. 2011, 1378, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Schechter, R.; Abboud, M.; Johnson, G. Brain endogenous insulin effects on neurite growth within fetal rat neuron cell cultures. Brain Res. Dev. Brain Res. 1999, 116, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Giudicelli, F.; Brabant, A.L.; Grit, I.; Parnet, P.; Amarger, V. Excess of methyl donor in the perinatal period reduces postnatal leptin secretion in rat and interacts with the effect of protein content in diet. PLoS One 2013, 8, e68268. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, M.D.; Craciunescu, C.N.; Zeisel, S.H. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 2006, 20, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jin, L.; Zhang, X. Effects of maternal mild zinc deficiency and zinc supplementation in offspring on spatial memory and hippocampal neuronal ultrastructural changes. Nutrition 2013, 29, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Craciunescu, C.N.; Brown, E.C.; Mar, M.H.; Albright, C.D.; Nadeau, M.R.; Zeisel, S.H. Folic acid deficiency during late gestation decreases progenitor cell proliferation and increases apoptosis in fetal mouse brain. J. Nutr. 2004, 134, 162–166. [Google Scholar] [PubMed]
- Craciunescu, C.N.; Albright, C.D.; Mar, M.H.; Song, J.; Zeisel, S.H. Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J. Nutr. 2003, 133, 3614–3618. [Google Scholar] [PubMed]
- Jin, Y.; Amaral, A.; McCann, A.; Brennan, L. Homocysteine levels impact directly on epigenetic reprogramming in astrocytes. Neurochem. Int. 2011, 58, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Salas, P.; Moore, S.E.; Cole, D.; da Costa, K.A.; Cox, S.E.; Dyer, R.A.; Fulford, A.J.; Innis, S.M.; Waterland, R.A.; Zeisel, S.H.; et al. DNA methylation potential: dietary intake and blood concentrations of one-carbon metabolites and cofactors in rural African women. Am. J. Clin. Nutr. 2013, 97, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Gueant, J.L.; Namour, F.; Gueant-Rodriguez, R.M.; Daval, J.L. Folate and fetal programming: A play in epigenomics? Trends Endocrinol. Metab. 2013, 24, 279–289. [Google Scholar]
- Hirabayashi, Y.; Gotoh, Y. Epigenetic control of neural precursor cell fate during development. Nat. Rev. Neurosci. 2010, 11, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Juliandi, B.; Abematsu, M.; Nakashima, K. Epigenetic regulation in neural stem cell differentiation. Dev. Growth Differ. 2010, 52, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Kohyama, J.; Kojima, T.; Takatsuka, E.; Yamashita, T.; Namiki, J.; Hsieh, J.; Gage, F.H.; Namihira, M.; Okano, H.; Sawamoto, K.; et al. Epigenetic regulation of neural cell differentiation plasticity in the adult mammalian brain. Proc. Natl. Acad. Sci. USA 2008, 105, 18012–18017. [Google Scholar] [CrossRef] [PubMed]
- Shors, T.J.; Miesegaes, G.; Beylin, A.; Zhao, M.; Rydel, T.; Gould, E. Neurogenesis in the adult is involved in the formation of trace memories. Nature 2001, 410, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.; Bayer, S.A. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J. Comp. Neurol. 1990, 301, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, T.; Nakashima, K.; Namihira, M.; Ochiai, W.; Uemura, A.; Yanagisawa, M.; Fujita, N.; Nakao, M.; Taga, T. DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Dev. Cell 2001, 1, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Choi, E.Y.; Park, M.J.; Lee, M.A. Expression of tyrosine hydroxylase is epigenetically regulated in neural stem cells. Biochem. Biophys. Res. Commun. 2011, 414, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Barve, S.; Chen, T.; Nelson, W.; Uriarte, S.; Hill, D.; McClain, C. S-adenosylmethionine (AdoMet) modulates endotoxin stimulated interleukin-10 production in monocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G949–G955. [Google Scholar] [PubMed]
- Zhao, Y.; Li, J.S.; Guo, M.Z.; Feng, B.S.; Zhang, J.P. Inhibitory effect of S-adenosylmethionine on the growth of human gastric cancer cells in vivo and in vitro. Chin. J. Cancer 2010, 29, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Christman, J.K.; Mendelsohn, N.; Herzog, D.; Schneiderman, N. Effect of 5-azacytidine on differentiation and DNA methylation in human promyelocytic leukemia cells (HL-60). Cancer Res. 1983, 43, 763–769. [Google Scholar] [PubMed]
- Setoguchi, H.; Namihira, M.; Kohyama, J.; Asano, H.; Sanosaka, T.; Nakashima, K. Methyl-CpG binding proteins are involved in restricting differentiation plasticity in neurons. J. Neurosci. Res. 2006, 84, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Namihira, M.; Nakashima, K.; Taga, T. Developmental stage dependent regulation of DNA methylation and chromatin modification in an immature astrocyte specific gene promoter. FEBS Lett. 2004, 572, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Werner, H.; Leroith, D. Insulin and insulin-like growth factor receptors in the brain: Physiological and pathological aspects. Eur. Neuropsychopharmacol. 2014. [Google Scholar] [CrossRef]
- Tsujimura, K.; Abematsu, M.; Kohyama, J.; Namihira, M.; Nakashima, K. Neuronal differentiation of neural precursor cells is promoted by the methyl-CpG-binding protein MeCP2. Exp. Neurol. 2009, 219, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Coupe, B.; Amarger, V.; Grit, I.; Benani, A.; Parnet, P. Nutritional programming affects hypothalamic organization and early response to leptin. Endocrinology 2010, 151, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Gressens, P.; Muaku, S.M.; Besse, L.; Nsegbe, E.; Gallego, J.; Delpech, B.; Gaultier, C.; Evrard, P.; Ketelslegers, J.M.; Maiter, D. Maternal protein restriction early in rat pregnancy alters brain development in the progeny. Brain Res. Dev. Brain Res. 1997, 103, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Matos, R.J.; Orozco-Solis, R.; Lopes de Souza, S.; Manhaes-de-Castro, R.; Bolanos-Jimenez, F. Nutrient restriction during early life reduces cell proliferation in the hippocampus at adulthood but does not impair the neuronal differentiation process of the new generated cells. Neuroscience 2011, 196, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Danglot, L.; Triller, A.; Marty, S. The development of hippocampal interneurons in rodents. Hippocampus 2006, 16, 1032–1060. [Google Scholar] [CrossRef] [PubMed]
- Napoli, I.; Blusztajn, J.K.; Mellott, T.J. Prenatal choline supplementation in rats increases the expression of IGF2 and its receptor IGF2R and enhances IGF2-induced acetylcholine release in hippocampus and frontal cortex. Brain Res. 2008, 1237, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Li, T.; Ross, M.G. Fetal hypothalamic neuroprogenitor cell culture: preferential differentiation paths induced by leptin and insulin. Endocrinology 2011, 152, 3192–3201. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.N.; Schneider, J.S.; Qin, M.; Tyler, W.A.; Pintar, J.E.; Fraidenraich, D.; Wood, T.L.; Levison, S.W. IGF-II promotes stemness of neural restricted precursors. Stem Cells 2012, 30, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Bracko, O.; Singer, T.; Aigner, S.; Knobloch, M.; Winner, B.; Ray, J.; Clemenson, G.D.; Suh, H.; Couillard-Despres, S.; Aigner, L.; et al. Gene expression profiling of neural stem cells and their neuronal progeny reveals IGF2 as a regulator of adult hippocampal neurogenesis. J. Neurosci. 2012, 32, 3376–3387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reusens, B.; Theys, N.; Dumortier, O.; Goosse, K.; Remacle, C. Maternal malnutrition programs the endocrine pancreas in progeny. Am. J. Clin. Nutr. 2011, 94, 1824S–1829S. [Google Scholar] [CrossRef] [PubMed]
- Dahri, S.; Reusens, B.; Remacle, C.; Hoet, J.J. Nutritional influences on pancreatic development and potential links with non-insulin-dependent diabetes. Proc. Nutr. Soc. 1995, 54, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Hoet, J.J.; Ozanne, S.; Reusens, B. Influences of pre- and postnatal nutritional exposures on vascular/endocrine systems in animals. Environ. Health Perspect. 2000, 108 (Suppl.3), S563–S568. [Google Scholar]
- Sanosaka, T.; Namihira, M.; Nakashima, K. Epigenetic mechanisms in sequential differentiation of neural stem cells. Epigenetics 2009, 4, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Hatada, I.; Namihira, M.; Morita, S.; Kimura, M.; Horii, T.; Nakashima, K. Astrocyte-specific genes are generally demethylated in neural precursor cells prior to astrocytic differentiation. PLoS One 2008, 3, e3189. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhang, X.; Yu, M.; Yan, H.; Liu, H.; Wilson, J.X.; Huang, G. Folic acid acts through DNA methyltransferases to induce the differentiation of neural stem cells into neurons. Cell Biochem. Biophys. 2013, 66, 559–566. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amarger, V.; Lecouillard, A.; Ancellet, L.; Grit, I.; Castellano, B.; Hulin, P.; Parnet, P. Protein Content and Methyl Donors in Maternal Diet Interact to Influence the Proliferation Rate and Cell Fate of Neural Stem Cells in Rat Hippocampus. Nutrients 2014, 6, 4200-4217. https://doi.org/10.3390/nu6104200
Amarger V, Lecouillard A, Ancellet L, Grit I, Castellano B, Hulin P, Parnet P. Protein Content and Methyl Donors in Maternal Diet Interact to Influence the Proliferation Rate and Cell Fate of Neural Stem Cells in Rat Hippocampus. Nutrients. 2014; 6(10):4200-4217. https://doi.org/10.3390/nu6104200
Chicago/Turabian StyleAmarger, Valérie, Angèle Lecouillard, Laure Ancellet, Isabelle Grit, Blandine Castellano, Philippe Hulin, and Patricia Parnet. 2014. "Protein Content and Methyl Donors in Maternal Diet Interact to Influence the Proliferation Rate and Cell Fate of Neural Stem Cells in Rat Hippocampus" Nutrients 6, no. 10: 4200-4217. https://doi.org/10.3390/nu6104200




