Cocoa Phenolic Extract Protects Pancreatic Beta Cells against Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. CPE Preparation
2.3. Cell Culture
2.4. CPE and t-BOOH Treatment
2.5. Evaluation of Cell Viability and ROS Production
2.6. Determination of GSH Concentration and GPx and GR Activity
2.7. Determination of Carbonyl Groups
2.8. Statistics
3. Results
3.1. Effect of CPE on Redox Status of Cultured Ins-1E Cells
| Condition | Concentration | % Cell Viability | ROS (% of Fluorescence Units) |
|---|---|---|---|
| C | 100.5 ± 9.6 a | 100.6 ± 8.9 a | |
| CPE | 5 μg/mL | 108.3 ± 6.6 a | 102.3 ± 8.1 a |
| 10 μg/mL | 109.7 ± 9.4 a | 110.3 ± 9.2 a | |
| 20 μg/mL | 111.4 ± 7.1 a | 113.5 ± 9.8 a |
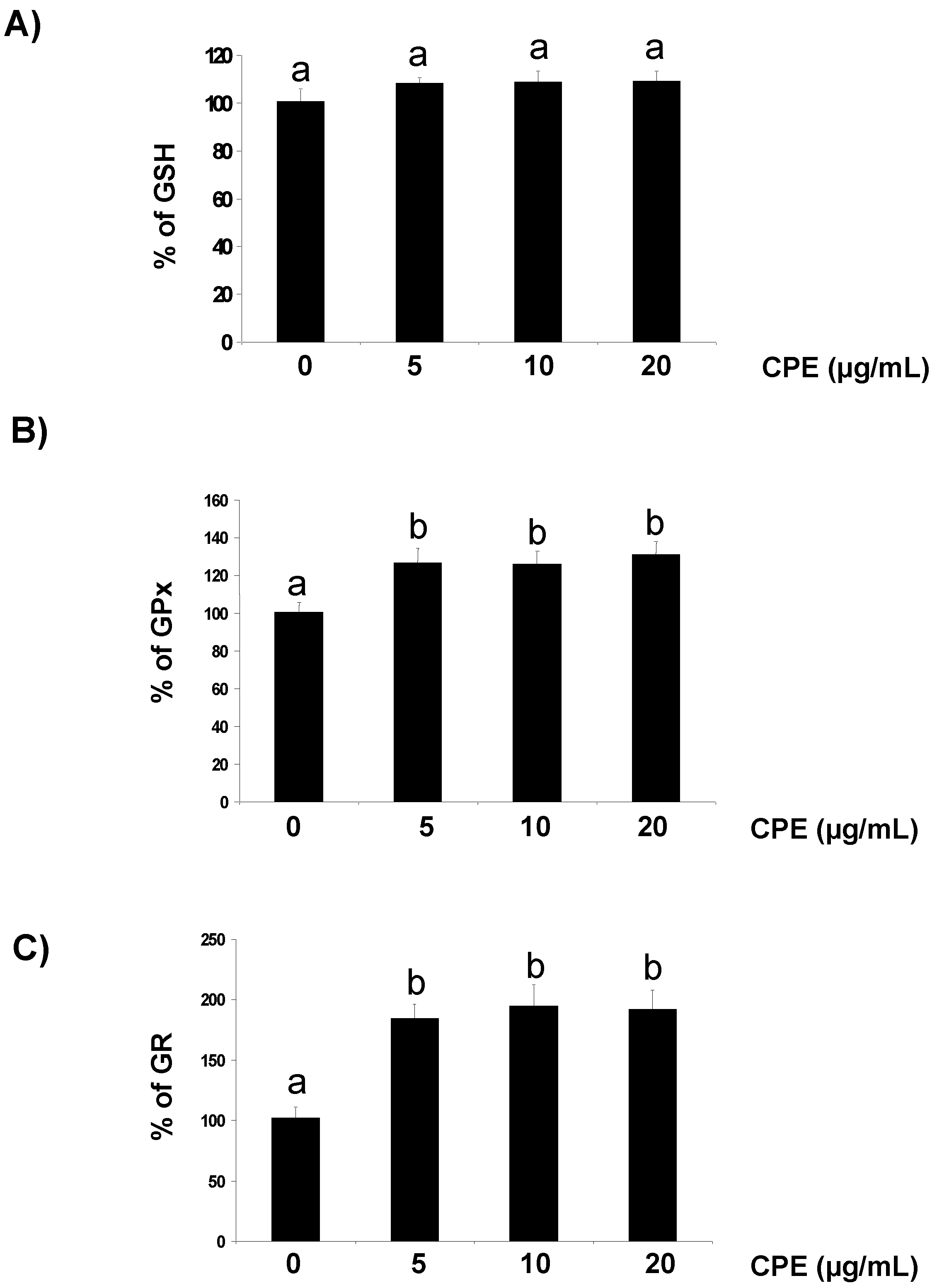
3.2. Response of Cultured Ins-1E Cells to a Chemically-Induced Oxidative Stress
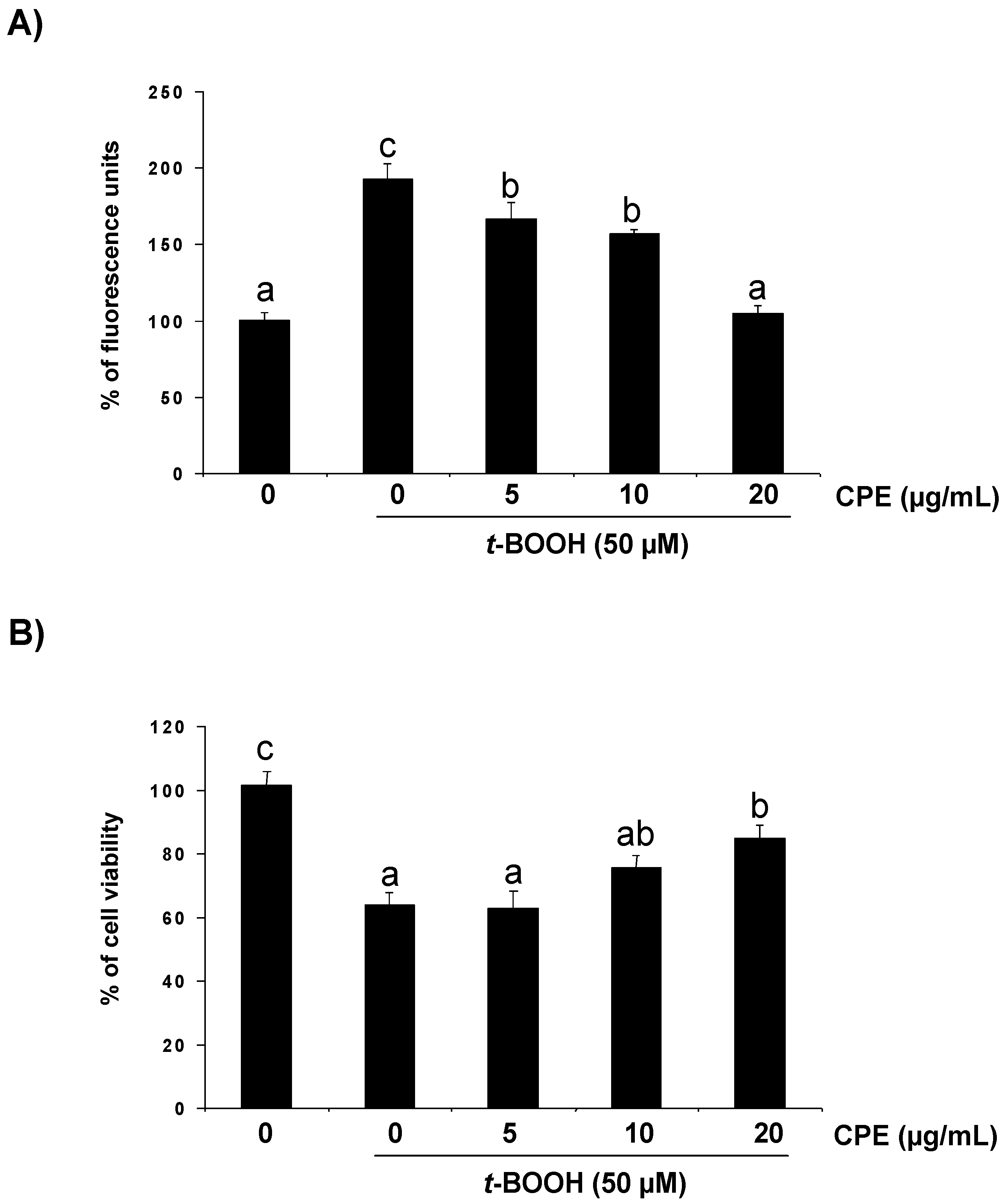
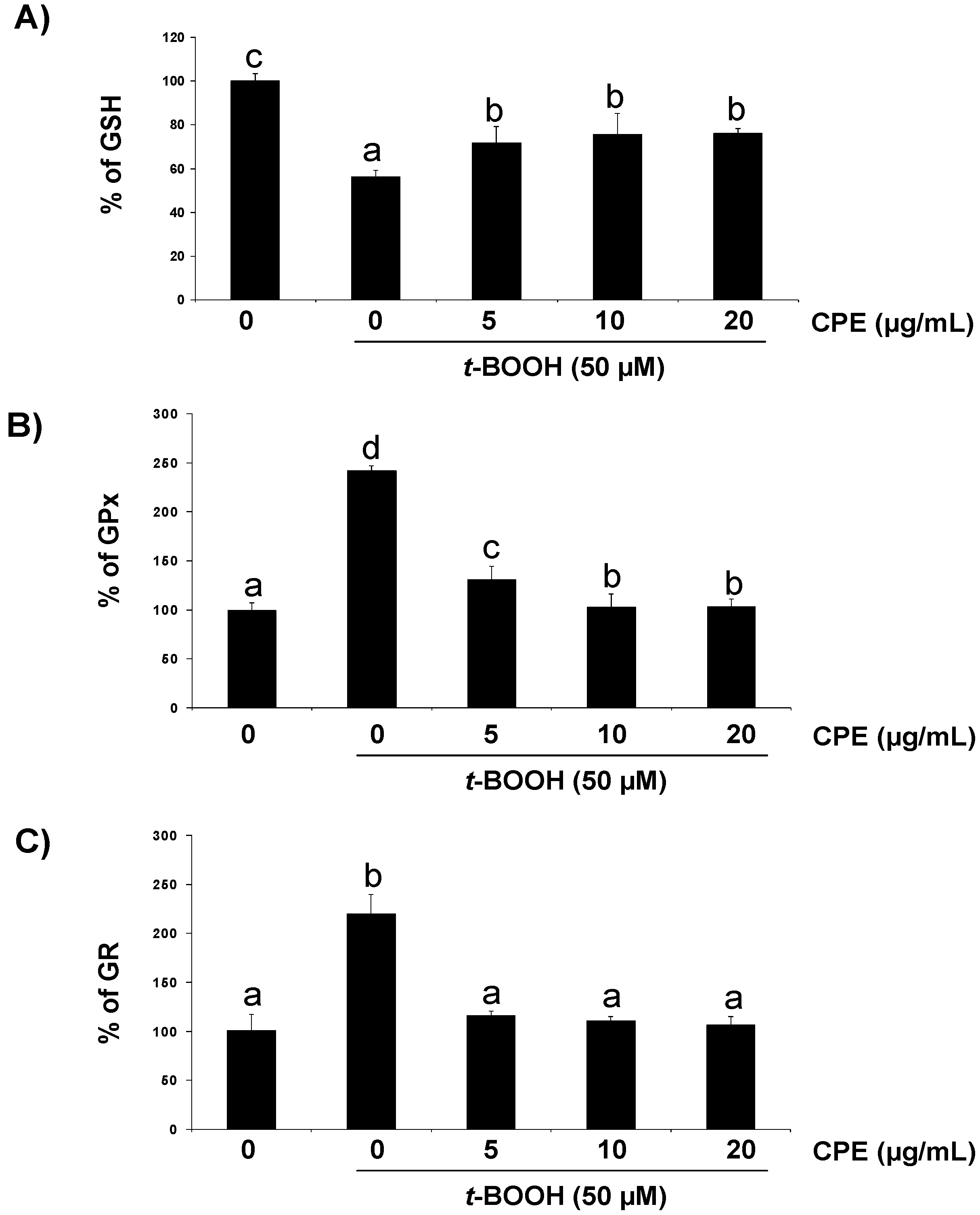
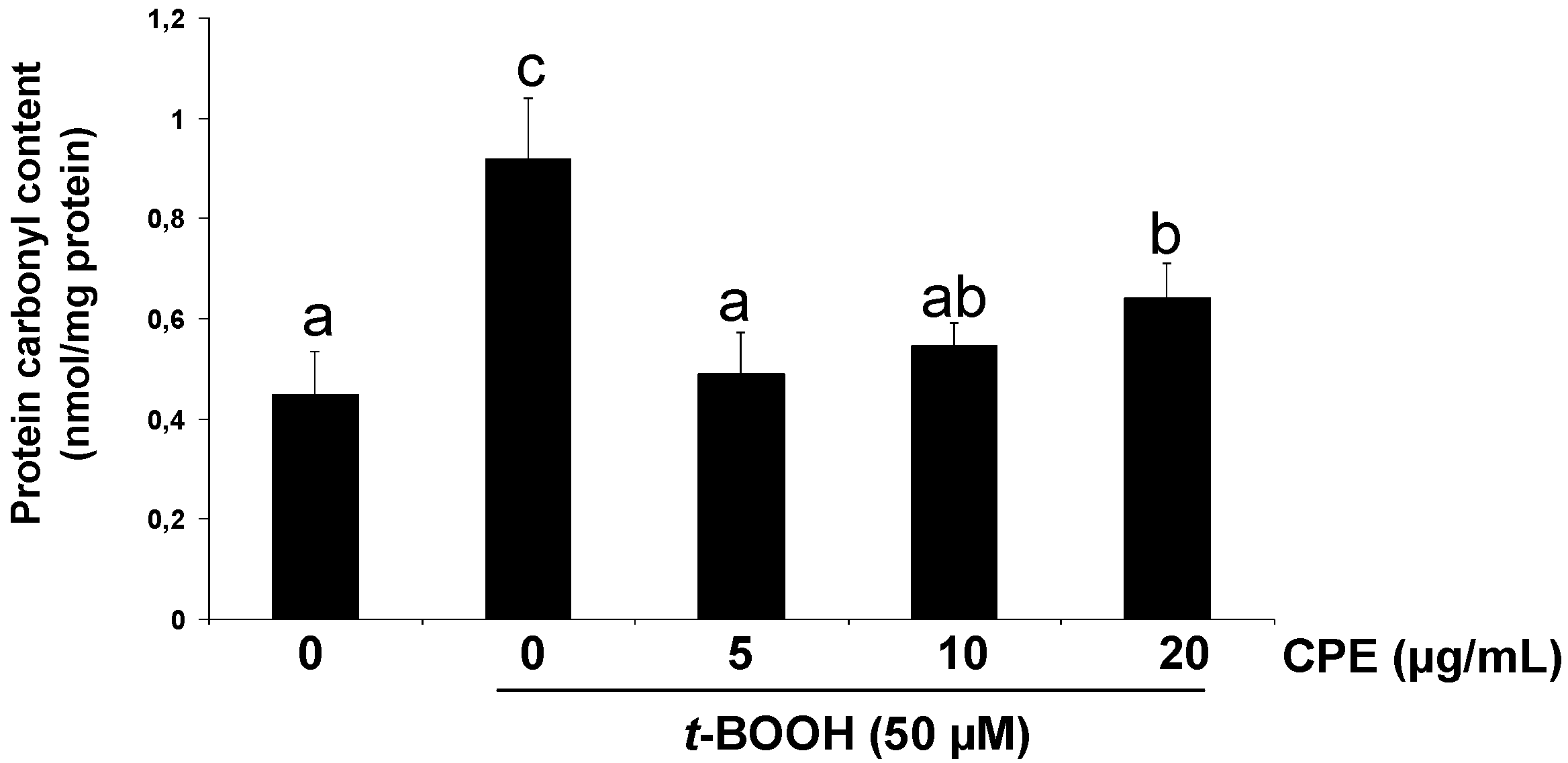
3.3. Protective Effect of CPE on Cultured Ins-1E Cells Submitted to Oxidative Stress
4. Discussion
5. Conclusions
Acknowledgements
Conflict of Interest
References
- Robertson, R.P.; Harmon, J.S. Pancreatic islet β-cell and oxidative stress: The importance of glutathione peroxidase. FEBS Lett. 2007, 581, 3743–3748. [Google Scholar] [CrossRef]
- Forester, S.C.; Lambert, J.D. The role of antioxidant versus pro-oxidant of green tea polyphenols in cancer prevention. Mol. Nutr. Food Res. 2011, 55, 844–854. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Vinson, J.A.; Proch, J.; Bose, P.; Muchler, S.; Taffera, P.; Shutta, D.; Samman, N.; Agbor, G.A. Chocolate is a powerful ex vivo and in vitro antioxidant, antiatherosclerotic agent in an animal model, and a significant contributor to antioxidants in the European and American diets. J. Agric. Food Chem. 2006, 54, 8071–8076. [Google Scholar] [CrossRef]
- Sánchez Rabaneda, F.; Jáuregui, O.; Casals, I.; Andrés Lacueva, C.; Izquierdo Pulido, M.; Lamuela Raventós, R.M. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). J. Mass Spectrom. 2003, 38, 35–42. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, Y.J.; Lee, H.J.; Lee, C.Y. Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. J. Agric. Food Chem. 2003, 51, 7292–7295. [Google Scholar] [CrossRef]
- Lamuela-Raventós, R.M.; Romero-Pérez, A.I.; Andrés-Lacueva, C.; Tornero, A. Review: Health effects of cocoa flavonoids. Food Sci. Technol. Int. 2005, 11, 159–176. [Google Scholar] [CrossRef]
- Pérez-Berezo, T.; Franch, A.; Ramos-Romero, S.; Castellote, C.; Pérez-Cano, F.J.; Castell, M. Cocoa-Enriched diets modulate intestinal and systemic humoral immune response in young adult rats. Mol. Nutr. Food Res. 2011, 55, S56–S66. [Google Scholar] [CrossRef]
- Martín, M.A.; Goya, L.; Ramos, R. Potential for preventive effects of cocoa and cocoa polyphenols in cancer. Food Chem. Toxicol. 2013, 56, 336–351. [Google Scholar] [CrossRef]
- Martín, M.A.; Ramos, S.; Mateos, R.; Granado-Serrano, A.B.; Izquierdo-Pulido, M.; Bravo, L.; Goya, L. Protection of human HepG2 cells against oxidative stress by cocoa phenolic extract. J. Agric. Food Chem. 2008, 56, 7765–7772. [Google Scholar] [CrossRef]
- Martín, M.A.; Granado-Serrano, A.B.; Ramos, S.; Izquierdo-Pulido, M.; Bravo, L.; Goya, L. Cocoa flavonoids up-regulate antioxidant enzymes activity via ERK1/2 pathway to protect against oxidative stress-induced apoptosis in HepG2 cells. J. Nutr. Biochem. 2010, 21, 196–205. [Google Scholar] [CrossRef]
- Rodríguez-Ramiro, I.; Ramos, S.; López-Oliva, E.; Agis-Torres, A.; Bravo, L.; Goya, L.; Martín, M.A. Cocoa polyphenols prevent inflammation in the colon of azoxymethane-treated rats and in TNF-α-stimulated Caco-2 cells. Br. J. Nutr. 2013. [Google Scholar] [CrossRef]
- Chakravarthy, B.K.; Gupta, S.; Gode, K.D. Functional beta cell regeneration in the islets of pancreas in alloxan induced diabetic rats by (−)-epicatechin. Life Sci. 1982, 31, 2693–2697. [Google Scholar] [CrossRef]
- Si, H.; Fu, Z.; Babu, P.V.A.; Zhen, W.; LeRoith, T.; Meaney, M.P.; Voelker, K.A.; Jia, Z.; Grange, R.W.; Liu, D. Dietary epicatechin promotes survival of obese diabetic mice and Drosophila melanogaster. J. Nutr. 2011, 141, 1095–1100. [Google Scholar] [CrossRef]
- Stote, K.S.; Clevidence, B.A.; Novotny, J.A.; Henderson, T.; Radecki, S.V.; Baer, D.J. Effect of cocoa and green tea on biomarkers of glucose regulation, oxidative stress, inflammation and hemostasis in obese adults at risk for insulin resistance. Eur. J. Clin. Nutr. 2012, 66, 1153–1159. [Google Scholar] [CrossRef]
- Cai, E.P.; Lin, J.K. Epigallocatechin gallate (EGCG) and rutin suppresss the glucotoxicity through activating IRS2 and AMPK signalling in rat pancreatic beta cells. J. Agric. Food Chem. 2009, 57, 9817–9827. [Google Scholar] [CrossRef]
- Lin, N.; Zhang, H.; Su, Q. Advanced glycation end-products induce injury to pancreatic beta cells through oxidative stress. Diabetes Metab. 2012, 38, 250–257. [Google Scholar] [CrossRef]
- Williamson, G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol. Nutr. Food Res. 2013, 57, 48–57. [Google Scholar] [CrossRef]
- Kim, M.K.; Jung, H.S.; Yoon, C.S.; Ko, J.H.; Chun, H.J.; Kim, T.K.; Kwon, M.J.; Lee, S.H.; Koh, K.S.; Rhee, B.D.; et al. EGCG and quercetin protected INS-1 cells in oxidative stress via different mechanisms. Front. Biosci. 2010, 2, 810–817. [Google Scholar]
- Granado-Serrano, A.B.; Martín, M.A.; Izquierdo-Pulido, M.; Goya, L.; Bravo, L.; Ramos, S. Molecular mechanisms of (−)-epicatechin and chlorogenic acid on the regulation of the apoptotic and survival/proliferation pathways in a human hepatoma cell line (HepG2). J. Agric. Food Chem. 2007, 55, 2020–2027. [Google Scholar]
- Alía, M.; Ramos, S.; Mateos, R.; Bravo, L.; Goya, L. Quercetin protects human hepatoma cell line (HepG2) against oxidative stress induced by tertbutyl hydroperoxide. Toxicol. Appl. Pharmacol. 2006, 212, 110–118. [Google Scholar] [CrossRef]
- Granado-Serrano, A.B.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. A diet rich in cocoa attenuates N-nitrosodiethylamine-induced liver injury in rats. Food Chem. Toxicol. 2009, 47, 2499–2506. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz-Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef]
- Azam, S.; Hadi, N.; Khan, N.U.; Hadi, S.M. Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: Implications for anticancer properties. Toxicol. In Vitro 2004, 18, 555–561. [Google Scholar] [CrossRef]
- Lapidot, T.; Walter, M.; Kanner, J. Antioxidant and prooxidant effects of phenolics on pancreatic beta-cells in vitro. J. Agric. Food Chem. 2002, 50, 7220–7225. [Google Scholar] [CrossRef]
- Rein, D.; Lotito, S.; Holt, R.R.; Keen, C.L.; Schmitz, H.H.; Fraga, C.G. Epicatechin in human plasma: In vivo determination and effect of chocolate consumption on plasma oxidation status. J. Nutr. 2000, 130, 2109–2114. [Google Scholar]
- Holt, R.R.; Schramm, D.D.; Keen, C.L.; Lazarus, S.A.; Schmitz, H.H. Chocolate consumption and platelet function. J. Am. Med. Assoc. 2002, 287, 2212–2213. [Google Scholar] [CrossRef]
- Masella, R.; di Benedetto, R.; Varì, R.; Filesi, C.; Giovannini, C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005, 16, 577–586. [Google Scholar] [CrossRef]
- Rodríguez-Ramiro, I.; Ramos, S.; Bravo, L.; Goya, L.; Martín, M.A. Procyanidin B2 induces Nrf2 translocation and glutathion-S-transferase P1 expresión and via ERKs and p38-MAPK pathways and protect human colonic cells against oxidative stress. Eur. J. Nutr. 2012, 51, 881–892. [Google Scholar] [CrossRef]
- Martín, M.A.; Ramos, S.; Mateos, R.; Izquierdo-Pulido, M.; Bravo, L.; Goya, L. Protection of human HepG2 cells against oxidative stress induced by the flavonoid epicatechin. Phytother. Res. 2010, 24, 503–509. [Google Scholar]
- Lima, C.F.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Phenolic compounds protect HepG2 cells from oxidative damage: Relevance of glutathione levels. Life Sci. 2006, 79, 2056–2068. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Ramiro, I.; Martín, M.A.; Ramos, S.; Bravo, L.; Goya, L. Comparative effects of dietary flavanols on antioxidant defences and their response to oxidant-induced stress on Caco2 cells. Eur. J. Nutr. 2011, 50, 313–322. [Google Scholar] [CrossRef]
- Martín, M.A.; Fernández-Millán, E.; Ramos, S.; Bravo, L.; Goya, L. Cocoa flavonoid epicatechin protects pancreatic beta cell viability and function against oxidative stress. Mol. Nutr. Food Res. 2013, in press. [Google Scholar]
- Scharf, G.; Prustomersky, S.; Knasmuller, S.; Schulte-Hermann, R.; Huber, W.W. Enhancement of glutathione and g-glutamylcysteine synthetase, the rate limiting enzyme of glutathione synthesis, by chemoprotective plant-derived food and beverage components in the human hepatoma cell line HepG2. Nutr. Cancer 2003, 45, 74–83. [Google Scholar] [CrossRef]
- Tomaru, M.; Takano, H.; Osakabe, N.; Yasuda, A.; Inoue, K.; Yanagisawa, R.; Ohwatari, T.; Uematsu, H. Dietary supplementation with cacao liquor proanthocyanidins prevents elevation of blood glucose levels in diabetic obese mice. Nutrition 2007, 4, 351–355. [Google Scholar]
- Murakami, C.; Hirakawa, Y.; Inui, H.; Nakano, Y.; Yoshida, H. Effect of tea catechins on cellular lipid peroxidation and cytotoxicity in HepG2 cells. Biosci. Biotechnol. Biochem. 2002, 66, 1559–1562. [Google Scholar] [CrossRef]
- Mateos, R.; Bravo, L. Chromatographic and electrophoretic methods for the analysis of biomarkers of oxidative damage to macromolecules (DNA, lipids, and proteins). J. Sep. Sci. 2007, 30, 175–191. [Google Scholar] [CrossRef]
- Chen, L.; Yang, X.; Jiao, H.; Zhao, B. Tea catechins protect against lead-induced cytotoxicity, lipid peroxidation, and membrane fluidity in HepG2 cells. Toxicol. Sci. 2002, 69, 149–156. [Google Scholar] [CrossRef]
- Goya, L.; Mateos, R.; Bravo, L. Effect of the olive oil phenol hydroxytyrosol on human hepatoma HepG2 cells. Protection against oxidative stress induced by tert-butylhydroperoxide. Eur. J. Nutr. 2007, 46, 70–78. [Google Scholar] [CrossRef]
- Granado-Serrano, A.B.; Martín, M.A.; Goya, L.; Bravo, L.; Ramos, S. Time-course regulation of survival pathways by epicatechin on HepG2 cells. J. Nutr. Biochem. 2009, 20, 115–124. [Google Scholar] [CrossRef]
- Geraets, L.; Moonen, H.J.J.; Wouters, E.F.M.; Bast, A.; Hageman, G.J. Caffeine metabolites are inhibitors of the nuclear enzyme poly(ADP-ribose)polymerase-1 at physiological concentrations. Biochem. Pharmacol. 2006, 72, 902–910. [Google Scholar] [CrossRef]
- Sheehan, E.W.; Stiff, D.D.; Duah, F.; Slatkin, D.J.; Schiff, P.L., Jr.; Zemaitis, M.A. The lack of effectiveness of (−)-epicatechin against alloxan induced diabetes in Wistar rats. Life Sci. 1983, 33, 593–597. [Google Scholar] [CrossRef]
- Cordero-Herrera, I.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Cocoa flavonoids improve insulin signalling and repress glucose production via AKT and AMPK in HepG2 cells. Mol. Nutr. Food Res. 2013. [Google Scholar] [CrossRef] [Green Version]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Martín, M.Á.; Ramos, S.; Cordero-Herrero, I.; Bravo, L.; Goya, L. Cocoa Phenolic Extract Protects Pancreatic Beta Cells against Oxidative Stress. Nutrients 2013, 5, 2955-2968. https://doi.org/10.3390/nu5082955
Martín MÁ, Ramos S, Cordero-Herrero I, Bravo L, Goya L. Cocoa Phenolic Extract Protects Pancreatic Beta Cells against Oxidative Stress. Nutrients. 2013; 5(8):2955-2968. https://doi.org/10.3390/nu5082955
Chicago/Turabian StyleMartín, María Ángeles, Sonia Ramos, Isabel Cordero-Herrero, Laura Bravo, and Luis Goya. 2013. "Cocoa Phenolic Extract Protects Pancreatic Beta Cells against Oxidative Stress" Nutrients 5, no. 8: 2955-2968. https://doi.org/10.3390/nu5082955





