Peculiarities of Enhancing Resistant Starch in Ruminants Using Chemical Methods: Opportunities and Challenges
Abstract
:1. Introduction
1.1. Grain Histological Features
1.2. Is Starch Always Starch?
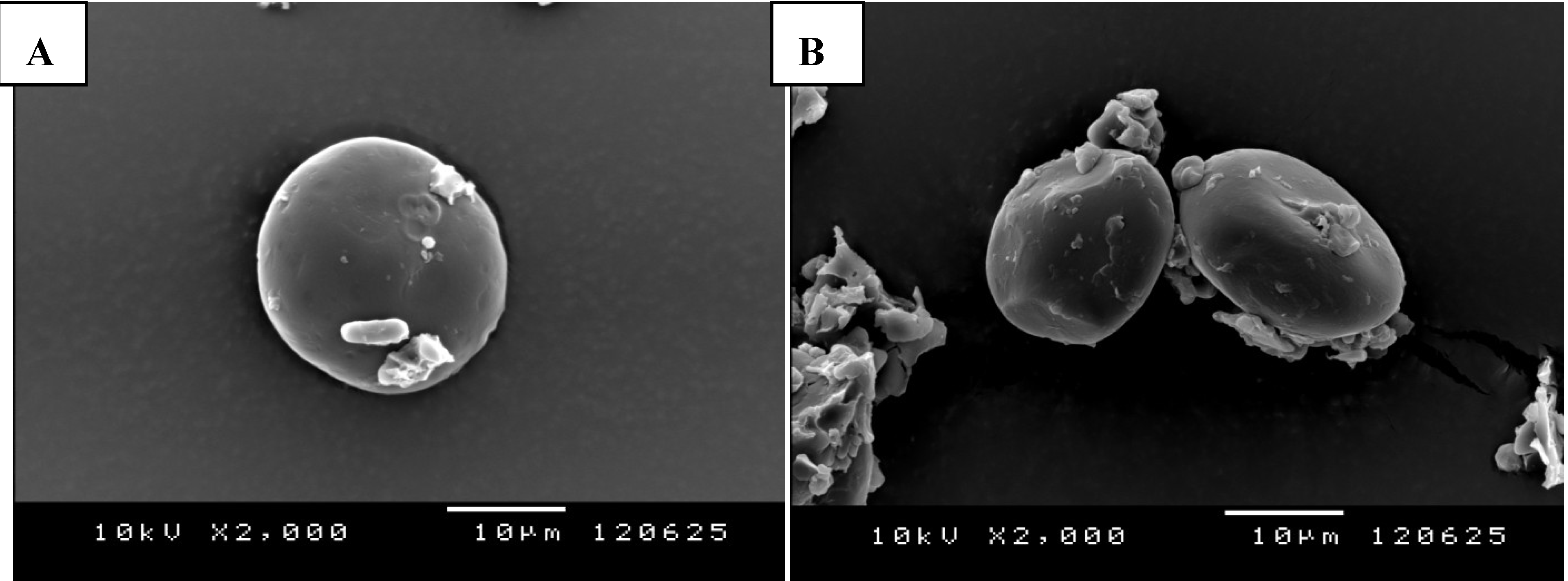
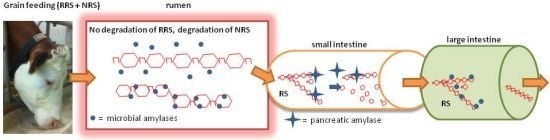
2. Enhancing RRS Starch Content in Concentrates for Ruminants
2.2. New Chemical Grain Processing Methods and Potential Metabolic Effects in Cattle
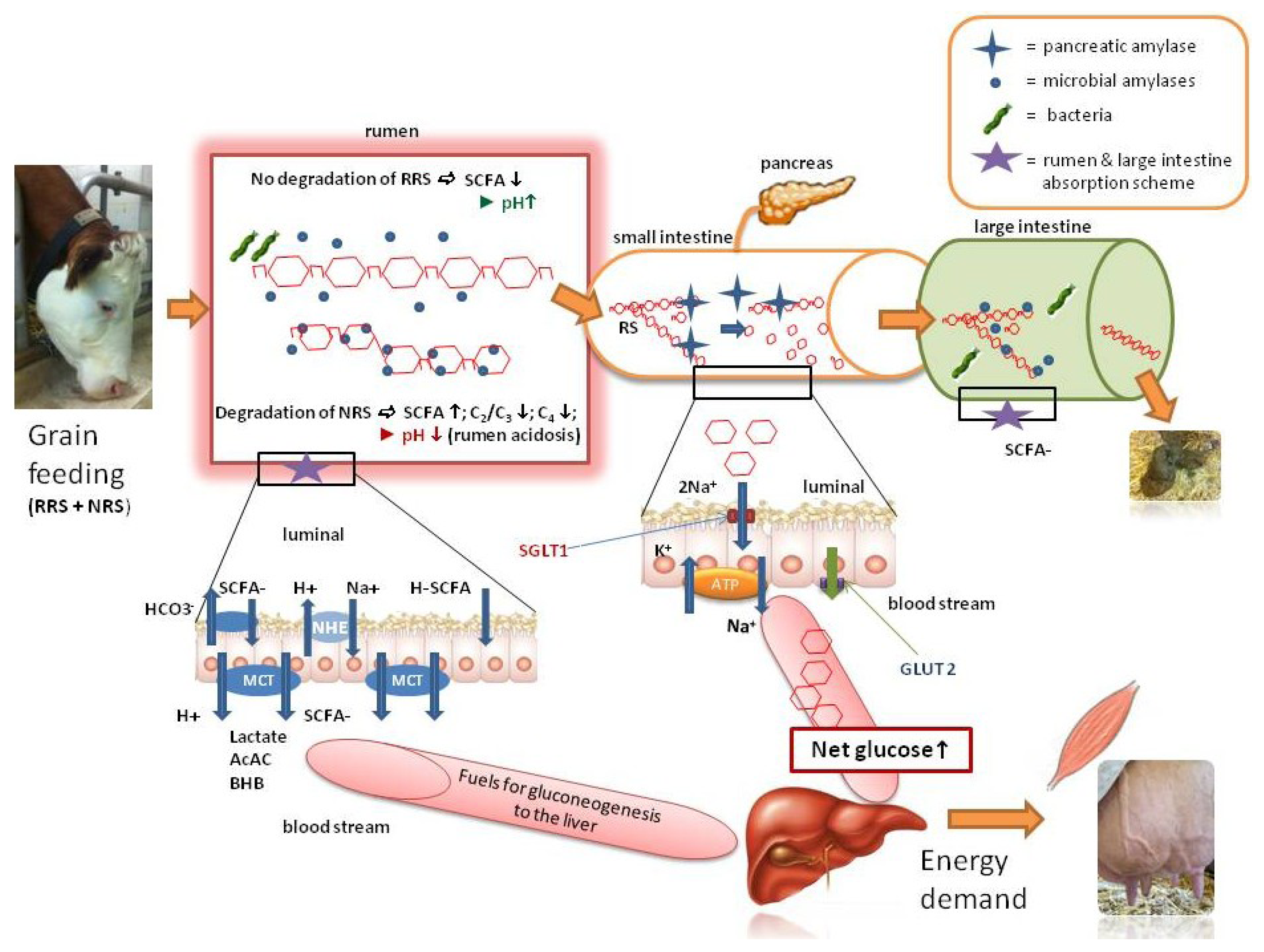
2.3. The RRS in Ruminants Lowers the Risk of Acidosis and may also Provide Energy—A Model
3. Conclusions
Acknowledgments
Conflict of Interest
References
- Khiaosa-Ard, R.; Zebeli, Q. Dietary modulation of rumen metabolism: A key factor to enhancing ruminant production. Albanian J. Agric. Sci. 2012, 3, 131–140. [Google Scholar]
- Nocek, J.E. Bovine acidosis implications on laminitis. J. Dairy Sci. 1997, 80, 1005–1028. [Google Scholar] [CrossRef]
- Nocek, J.E.; Tamminga, S. Site of digestion of starch in the gastrointestinal tract of dairy cows and its effect on milk yield and composition. J. Dairy Sci. 1991, 74, 3598–3629. [Google Scholar] [CrossRef]
- Zebeli, Q.; Mansmann, D.; Steingass, H.; Ametaj, B.N. Balancing diets for physically effective fibre and ruminally degradable starch: A key to lower the risk of sub-acute rumen acidosis and improve productivity of dairy cattle. Livest. Sci. 2010, 127, 1–10. [Google Scholar] [CrossRef]
- Aschenbach, J.R.; Penner, G.B.; Stumpff, F.; Gabel, G. Ruminant Nutrition Symposium: Role of fermentation acid absorption in the regulation of ruminal pH. J. Anim. Sci. 2011, 89, 1092–1107. [Google Scholar] [CrossRef]
- Stone, W.C. Nutritional approaches to minimize subacute ruminal acidosis and laminitis in dairy cattle. J. Dairy Sci. 2004, 87, E12–E26. [Google Scholar] [CrossRef]
- Owens, F.N.; Secrist, D.S.; Hill, W.J.; Gill, D.R. Acidosis in cattle: A review. J.Anim. Sci. 1998, 76, 275–286. [Google Scholar]
- Nagaraja, T.G.; Titgemeyer, E.C. Ruminal acidosis in beef cattle: The current microbiological and nutritional outlook. J. Dairy Sci. 2007, 90, E17–E38. [Google Scholar] [CrossRef]
- Plaizier, J.C.; Krause, D.O.; Gozho, G.N.; McBride, B.W. Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. Vet. J. 2008, 176, 21–31. [Google Scholar] [CrossRef]
- Calsamiglia, S.; Blanch, M.; Ferret, A.; Moya, D. Is subacute ruminal acidosis a pH related problem? Causes and tools for its control. Anim. Feed Sci. Technol. 2012, 172, 42–50. [Google Scholar] [CrossRef]
- Wang, Y.; Majak, W.; McAllister, T.A. Frothy bloat in ruminants: Cause, occurrence, and mitigation strategies. Anim. Feed Sci. Technol. 2012, 172, 103–114. [Google Scholar] [CrossRef]
- Kleen, J.L.; Hooijer, G.A.; Rehage, J.; Noordhuizen, J.P.T.M. Subacute ruminal acidosis (SARA): A review. J. Vet. Med. A 2003, 50, 406–414. [Google Scholar] [CrossRef]
- Nagaraja, T.G.; Lechtenberg, K.F. Liver Abscesses in feedlot cattle. Vet. Clin. Food Anim. 2007, 23, 351–369. [Google Scholar] [CrossRef]
- Karapinar, T.; Dabak, M.; Kizil, O. Thiamine status of feedlot cattle fed a high-concentrate diet. Can. Vet. J. 2010, 51, 1251–1253. [Google Scholar]
- Zebeli, Q.; Dijkstra, J.; Tafaj, M.; Steingass, H.; Ametaj, B.N.; Drochner, W. Modeling the adequacy dietary fiber in dairy cows based on responses of ruminal pH and milk fat production to composition of the diet. J. Dairy Sci. 2008, 91, 2046–2066. [Google Scholar] [CrossRef]
- Matthe, A.; Lebzien, P.; Flachowsky, G. On the relevance of bypass-starch for the glucose supply of high-yielding dairy cows. Übers. Tierernähr. 2000, 28, 1–64. [Google Scholar]
- Theurer, C.B. Grain processing effects on starch utilization by ruminants. J. Anim. Sci. 1986, 63, 1649–1662. [Google Scholar]
- Matthe, A.; Lebzien, P.; Hric, I.; Flachowsky, G.; Sommer, A. Effect of starch application into proximal duodenum of ruminants on starch digestibility in the small and total intestine. Arch. Anim. Nutr. 2001, 55, 351–369. [Google Scholar]
- Nozière, P.; Rémond, D.; Lemosquet, S.; Chauvau, B.; Durand, D.; Poncet, C. Effect of site of starch digestion on portal nutrients net fluxes in steers. Br. J. Nutr. 2005, 94, 182–191. [Google Scholar] [CrossRef]
- Svihus, B.; Uhlen, A.K.; Harstad, O.M. Effect of starch granule structure, associated components and processing on nutritive value of cereal starch: A review. Anim. Feed Sci. Technol. 2005, 122, 303–320. [Google Scholar] [CrossRef]
- Dehghan-Banadaky, M.; Corbett, R.; Oba, M. Effects of barley grain processing on productivity of cattle. Anim. Feed Sci. Technol. 2007, 137, 1–24. [Google Scholar] [CrossRef]
- Owens, F.N.; Secrist, D.S.; Hill, W.J.; Gill, D.R. The effect of grain source and grain processing on performance of feedlot cattle. J. Anim. Sci. 1997, 75, 868–879. [Google Scholar]
- Östman, E.M.; Nilsson, M.; Liljeberg-Elmstahl, H.G.M.; Molin, G.; Björck, I.M.E. On the effect of lactic acid on blood glucose and insulin responses to cereal products: Mechanistic studies in healthy subjects and in vitro. J. Cereal Sci. 2002, 36, 339–346. [Google Scholar] [CrossRef]
- Iqbal, S.; Zebeli, Q.; Mazzolari, A.; Bertoni, G.; Dunn, S.M.; Yang, W.Z.; Ametaj, B.N. Feeding barley grain steeped in lactic acid modulates rumen fermentation patterns and increases milk fat content in dairy cows. J. Dairy Sci. 2009, 92, 6023–6032. [Google Scholar] [CrossRef]
- Iqbal, S.; Zebeli, Q.; Mazzolari, A.; Dunn, S.M.; Ametaj, B.N. Feeding rolled barley grain steeped in lactic acid modulated energy status and innate immunity in dairy cows. J. Dairy Sci. 2010, 93, 5147–5156. [Google Scholar] [CrossRef]
- Martínez, T.F.; Moyano, F.J.; Díaz, M.; Barroso, F.G.; Alarcón, F.J. Use of tannic acid to protect barley meal against ruminal degradation. J. Sci. Food Agric. 2005, 85, 1371–1378. [Google Scholar] [CrossRef]
- Seal, C.J.; Jones, A.R.; Whitney, A.D. Whole grains uncovered. Nutr. Bull. 2006, 31, 129–137. [Google Scholar] [CrossRef]
- Cromey, M.; Wright, D.; Boddington, H. Effects of frost during grain filling on wheat yield and grain structure. N. Z. J. Crop Hortic. Sci. 1998, 26, 279–290. [Google Scholar] [CrossRef]
- Amrein, T.M.; Gränicher, P.; Arrigoni, E.; Amadò, R. In vitro digestibility and colonic fermentability of aleurone isolated from wheat bran. LWT-Food Sci. Technol. 2003, 26, 451–460. [Google Scholar]
- Huntington, G.B. Starch utilization by ruminants: From basics to the bunk. J. Anim. Sci. 1997, 75, 852–867. [Google Scholar]
- Kotarski, S.F.; Waniska, R.D.; Thurn, K.K. Starch hydrolysis by the ruminal microflora. J. Nutr. 1992, 122, 178–190. [Google Scholar]
- Andersson, L.; Fredriksson, H.; Oscarsson Bergh, M.; Andersson, R.; Åman, P. Characterisation of starch from inner and peripheral parts of normal and waxy barley kernels. J. Cereal Sci. 1999, 30, 165–171. [Google Scholar] [CrossRef]
- Huntington, G.B.; Harmon, D.L.; Richards, C.J. Sites, rates, and limits of starch digestion and glucose metabolism in growing cattle. J. Anim. Sci. 2006, 84, E14–E24. [Google Scholar]
- Pérez, S.; Bertoft, E. The molecular structures of starch components and their contribution to the architecture of starch granules: A comprehensive review. Starch Stärke 2010, 62, 389–420. [Google Scholar] [CrossRef]
- Buléon, A.; Colonna, P.; Planchot, V.; Ball, S. Mini review Starch granules: Structure and biosynthesis. Int. J. Biol. Macromol. 1998, 23, 85–112. [Google Scholar] [CrossRef]
- Lindeboom, N.; Chang, P.R.; Tyler, R.T. Analytical, biochemical and physicochemical aspects of starch granule size, with emphasis on small granule starches: A review. Starch Stärke 2004, 56, 89–99. [Google Scholar] [CrossRef]
- Panozzo, J.F.; Eagles, H.A. Cultivar and environmental effects on quality characters in wheat. I. Starch. Aust. J. Agric. Res. 1998, 49, 757–766. [Google Scholar] [CrossRef]
- Baldwin, P.M. Starch granule-associated proteins and polypeptides: A review. Starch Stärke 2001, 53, 475–503. [Google Scholar] [CrossRef]
- Salomonsson, A.C.; Sundberg, B. Amylose content and chain profile of amylopectin from normal, high amylose and waxy barleys. Starch Stärke 1994, 46, 325–328. [Google Scholar] [CrossRef]
- Chen, Y.; Fringant, C.; Rinaudo, M. Molecular characterization of starch by SEC: Dependance of the performances on the amylopectin content. Carbohydr. Polym. 1997, 33, 73–78. [Google Scholar]
- Hanashiro, I.; Takeda, Y. Examination of number-average degree of polymerization and molar-based distribution of amylose by fluorescent labeling with 2-aminopyridine. Carbohydr. Res. 1998, 42, 421–426. [Google Scholar]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant starch—A review. Comp. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar]
- Hizukuri, S.; Takagi, T. Estimation of the distribution of molecular weight for amylose by the low-angle laser-light-scattering technique combined with high-performance gel chromatography. Carbohydr. Res. 1984, 134, 1–10. [Google Scholar]
- French, D. Chemical and physical properties of starch. J. Anim. Sci. 1973, 37, 1048–1061. [Google Scholar]
- Topping, D.L.; Fukushima, M.; Bird, A.R. Resistant starch as a prebiotic and symbiotic: State of the art. Proc. Nutr. Soc. 2003, 62, 171–176. [Google Scholar]
- Fuentes-Zaragoza, E.; Sánchez-Zapata, E.; Sendra, E.; Sayas, E.; Navarro, C.; Fernández-López, J.; Pérez-Alvarez, J.A. Resistant starch as prebiotic: A review. Starch Stärke 2011, 63, 406–415. [Google Scholar] [CrossRef]
- Englyst, H.; Wiggins, H.S.; Cummings, J.H. Determination of the non-starch polysaccharides in plant foods by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst 1982, 107, 307–318. [Google Scholar] [CrossRef]
- Bird, A.R.; Brown, I.L.; Topping, D.L. Starches, resistant starches, the gut microflora and human health. Curr. Issues Intest. Microbiol. 2000, 1, 25–37. [Google Scholar]
- Niba, L.L. Resistant starch: A potential functional food ingredient. Nutr. Food Sci. 2002, 32, 62–67. [Google Scholar]
- Nugent, A.P. Health properties of resistant starch. Nutr. Bull. 2005, 30, 27–54. [Google Scholar] [CrossRef]
- Zhou, Z.; Cao, X.; Zhou, J.Y.H. Effect of resistant starch structure on short-chain fatty acids production by human gut microbiota fermentation in vitro. Starch Stärke 2013, 65, 509–516. [Google Scholar]
- Langkilde, A.M.; Ekwall, H.; Björck, I.; Asp, N.G.; Andersson, H. Retrograded high-amylose corn starch reduces cholic acid excretion from the small bowel in ileostomy subjects. Eur. J. Clin. Nutr. 1998, 52, 790–795. [Google Scholar]
- Jenkins, D.J.A.; Kendall, C.W.C. Resistant starches. Curr. Opin. Gastroenterol. 2000, 16, 178–183. [Google Scholar] [CrossRef]
- Penn-Marshall, M.; Holtzman, G.I.; Barbeau, W.E. African Americans may have to consume more than 12 grams a day of resistant starch to lower their risk for type 2 diabetes. J. Med. Food 2010, 13, 999–1004. [Google Scholar] [CrossRef]
- Kwak, J.H.; Paik, J.K.; Kim, H.I.; Kim, O.Y.; Shin, D.Y.; Kim, H.J.; Lee, J.H.; Lee, J.H. Dietary treatment with rice containing resistant starch improves markers of endothelial function with reduction of postprandial blood glucose and oxidative stress in patients with prediabetes or newly diagnosed type 2 diabetes. Atherosclerosis 2012, 224, 457–464. [Google Scholar] [CrossRef]
- Topping, D.L.; Morell, M.K.; King, R.A.; Li, Z.; Bird, A.R.; Noakes, M. Resistant starch and health—Himalaya 292, a novel barley cultivar to deliver benefits to consumers. Starch Stärke 2003, 55, 539–545. [Google Scholar]
- Zheng, J.; Enright, F.; Keenan, M.; Finley, J.; Zhou, J.; Ye, J.; Greenway, F.; Senevirathne, R.N.; Gissendanner, C.R.; Manaois, R.; et al. Resistant starch, fermented resistant starch, and short-chain fatty acids reduce intestinal fat deposition in Caenorhabditis elegans. J. Agric. Food Chem. 2010, 58, 4744–4748. [Google Scholar] [CrossRef]
- Fuentes-Zaragoza, E.; Riquelme-Navarrete, M.J.; Sánchez-Zapata, E.; Pérez-Álvarez, J.A. Resistant starch as functional ingredient: A review. Food Res. Int. 2010, 43, 931–942. [Google Scholar] [CrossRef]
- Polesi, L.F.; Sarmento, S.B.S. Structural and physicochemical characterization of RS prepared using hydrolysis and heat treatments of chickpea starch. Starch Stärke 2011, 63, 226–235. [Google Scholar] [CrossRef]
- Sha, X.S.; Xiang, Z.J.; Bin, L.; Jing, L.; Bin, Z.; Jiao, Y.J.; Kun, S.R. Preparation and physical characteristics of resistant starch (type 4) in acetylated indica rice. Food Chem. 2012, 134, 149–154. [Google Scholar] [CrossRef]
- Lee, K.Y.; Yoo, S.H.; Lee, H.G. The effect of chemically-modified resistant starch, RS type-4, on body weight and blood lipid profiles of high fat diet-induced obese mice. Starch Stärke 2012, 64, 78–85. [Google Scholar] [CrossRef]
- Shu, X.; Backes, G.; Rasmussen, S.K. Genome-wide association study of resistant starch (RS) phenotypes in a barley variety collection. J. Agric. Food Chem. 2012, 60, 10302–10311. [Google Scholar]
- Hale, W.H.; Cuitun, L.; Saba, W.J.; Taylor, B.; Theurer, B. Effect of steam processing and flaking milo and barley on performance and digestion by steers. J. Anim. Sci. 1966, 25, 392–396. [Google Scholar]
- Schmidt, J.; Toth, T.; Fabian, J. Rumen fermentation and starch degradation by Holstein steers fed sodium hydroxide or formaldehyde treated wheat. Acta Vet. Hung. 2006, 54, 201–212. [Google Scholar] [CrossRef]
- Miron, J.; Ben-Ghedalia, D.; Solomon, R. Digestibility by dairy cows of monosaccharide components in diets containing either ground sorghum or sorghum grain treated with sodium hydroxide. J. Dairy Sci. 1997, 80, 144–151. [Google Scholar]
- Kennedy, S.; Rice, D.A. Renal lesions in cattle fed sodium hydroxide-treated Barley. Vet. Pathol. 1987, 24, 265–271. [Google Scholar]
- Dehghan-Banadaky, M.; Amanlob, H.; Nikkhah, A.; Danesh-Mesgaran, M.; Emamic, M.R. Rumen and post-abomasal disappearance in lactating cows of amino acids and other components of barley grain treated with sodium hydroxide, formaldehyde or urea. Anim. Feed Sci. Technol. 2008, 142, 306–316. [Google Scholar] [CrossRef]
- McNiven, M.A.; Weisbjerg, M.R.; Hvelplund, T. Influence of roasting or sodium hydroxide treatment of barley on digestion in lactating cows. J. Dairy Sci. 1995, 78, 1106–1115. [Google Scholar] [CrossRef]
- Cameron, R.E.; Roberts, S.A. The effects of concentration and sodium hydroxide on the rheological properties of potato starch gelatinization. Carbohydr. Polym. 2002, 50, 133–143. [Google Scholar] [CrossRef]
- Martínez, X.P.; Sánchez, M.R.; López, J.; Manjarrez, E.V.-A.; Padilla, E.G.; Ávila, H.R.V. Desarrollo folicular y tasa ovulatoria en cabras criollas después de un periodo corto de consumo de trigo protegido de la degradación ruminal (Follicular development and ovulation rate in Creole goats after short-term consumption of wheat protected from ruminal degradation). Tec. Pecu. Mex. 2008, 46, 449–462. [Google Scholar]
- Leroy, J.L.M.R.; Opsomer, G.; Van Soom, A.; Goovaerts, I.G.F.; Bols, P.E.J. Reduced fertility in high-yielding dairy cows: Are the oocyte and embryo in danger? Part II: Mechanisms linking nutrition and reduced oocyte and embryo quality in high-yielding cows. Reprod. Dom. Anim. 2008, 43, 623–632. [Google Scholar]
- Garnsworthy, P.C.; Gong, J.G.; Armstrong, D.G.; Mann, G.E.; Sinclair, K.D.; Webb, R. Effect of site of starch digestion on metabolic hormones and ovarian function in dairy cows. Livest. Sci. 2009, 125, 161–168. [Google Scholar]
- Fluharty, F.L.; Loerch, S.C. Chemical treatment of ground corn to limit ruminal starch digestion. Can. J. Anim. Sci. 1989, 69, 173–180. [Google Scholar]
- Oke, B.O.; Loerch, S.C.; Redman, D.R. Effects of dietary level and formaldehyde treatment of corn on nutrient digestion and rnetabolism in sheep. Can. J. Anim. Sci. 1991, 71, 1197–1205. [Google Scholar]
- Colkesen, M.; Kamalak, A.; Canbolat, O.; Gurbuz, Y.; Ozkan, C.O. Effect of cultivar and formaldehyde treatment of barley grain on rumen fermentation characteristics using in vitro gas production. S.Afr. J. Anim. Sci. 2005, 35, 206–212. [Google Scholar]
- Ortega-Cerrilla, M.E.; Finlayson, H.J.; Armstrong, D.G. The effect of chemical treatment of barley on starch digestion in ruminants. Anim. Feed Sci. Technol. 1999, 77, 73–81. [Google Scholar] [CrossRef]
- Robinson, P.H.; Kennelly, J.J. Influence of ammoniation of high moisture barley on its in situ rumen degradation and influence on rumen fermentation in dairy cows. Can. J. Anim. Sci. 1988, 68, 839–851. [Google Scholar] [CrossRef]
- Robinson, P.H.; Kennelly, J.J. Influence of ammoniation of high moisture barley on digestibility, kinetics of rumen ingesta turnover, and milk production in dairy cows. Can. J. Anim. Sci. 1989, 69, 195–203. [Google Scholar]
- Iqbal, S.; Terrill, S.J.; Zebeli, Q.; Mazzolari, A.; Dunn, S.M.; Yang, W.Z.; Ametaj, B.N. Treating barley grain with lactic acid and heat prevented sub-acute ruminal acidosis and increased milk fat content in dairy cows. Anim. Feed Sci. Technol. 2012, 172, 141–149. [Google Scholar]
- Iqbal, S.; Zebeli, Q.; Mazzolari, A.; Dunn, S.M.; Ametaj, B.N. Barley grain-based diet treated with lactic acid and heat modulated plasma metabolites and acute phase response in dairy cows. J. Anim. Sci. 2012, 90, 3143–3152. [Google Scholar]
- Yu, J.; Wang, W.H.; Menghe, B.L.G.; Jiri, M.T.; Wang, H.M.; Liu, W.J.; Bao, Q.H.; Lu, O.; Zhang, J.C.; Wang, F.; et al. Diversity of lactic acid bacteria associated with traditional fermented dairy products in Mongolia. J. Dairy Sci. 2011, 94, 3229–3241. [Google Scholar]
- Rhee, S.J.; Lee, J.E.; Lee, C.H. Importance of lactic acid bacteria in Asian fermented foods. Microb. Cell Fact. 2011, 10, S5:1–S5:13. [Google Scholar]
- Arendt, E.K.; Moroni, A.; Zannini, E. Medical nutrition therapy: Use of sourdough lactic acid bacteria as a cell factory for delivering functional biomolecules and food ingredients in gluten free bread. Microb. Cell Fact. 2011, 10, S15:1–S15:9. [Google Scholar]
- Liljeberg, H.G.M.; Lönner, C.H.; Björck, I.M.E. Sourdough fermentation or addition of organic acids or corresponding salts to bread improves nutritional properties of starch in healthy humans. J. Nutr. 1995, 125, 1503–1511. [Google Scholar]
- Berry, C.S. Resistant starch-formation and measurement of starch that survives exhaustive digestion with amylolytic enzymes during the determination of dietary fiber. J. Cereal Sci. 1986, 4, 301–314. [Google Scholar]
- Liljeberg, H.; Akerberg, A.; Björck, I. Resistant starch formation in bread as influenced by choice of ingredients or baking conditions. Food Chem. 1996, 56, 389–394. [Google Scholar]
- Hallström, E.; Sestili, F.; Lafiandra, D.; Björck, I.; Östman, E. A novel wheat variety with elevated content of amylose increases resistant starch formation and may beneficially influence glycaemia in healthy subjects. Food Nutr. Res. 2011, 55, 7074:1–7074:8. [Google Scholar]
- Östman, E.M.; Liljeberg-Elmstahl, H.G.M.; Björck, I.M.E. Barley bread containing lactic acid improves glucose tolerance at a subsequent meal in healthy men and women. J. Nutr. 2002, 132, 1173–1175. [Google Scholar]
- Östman, E.M.; Elmstahl, H.G.M.; Molin, G.; Lundquist, I.; Björck, I.M.E. A diet based on wheat bread baked with lactic acid improves glucosetolerance in hyperinsulinaemic Zucker (fa/fa) rats. J. Cereal Sci. 2005, 42, 300–308. [Google Scholar]
- Offner, A.; Bach, A.; Sauvant, D. Quantitative review of in situ starch degradation in the rumen. Anim. Feed Sci. Technol. 2003, 106, 81–93. [Google Scholar]
- Ljøkel, K.; Harstad, O.M.; Prestløkken, E.; Skrede, A. In situ digestibility of starch in barley grain (Hordeum vulgare) and peas (Pisum sativum L.) in dairy cows: Influence of heat treatment and glucose addition. Anim. Feed Sci. Technol. 2003, 105, 105–116. [Google Scholar]
- Jalč, D.; Kišidayová, S.; Nerud, F. Effect of plant oils and organic acids on rumen fermentation in vitro. Folia Microbiol. 2002, 47, 171–178. [Google Scholar] [CrossRef]
- Martin, S.A. Manipulation of ruminal fermentation with organic acids: A review. J. Anim. Sci. 1998, 76, 3123–3132. [Google Scholar]
- Khampa, S.; Wanapat, M. Manipulation of rumen fermentation with organic acids supplementation in ruminants raised in the tropics. Pak. J. Nutr. 2007, 6, 20–27. [Google Scholar] [CrossRef]
- Castillo, C.; Benedito, J.L.; Mendez, J.; Pereira, V.; Lopez-Alonso, M.; Miranda, M.; Hernandez, J. Organic acids as a substitute for monensin in diets for beef cattle. Anim. Feed Sci. Technol. 2004, 115, 101–116. [Google Scholar]
- Makkar, H.P.S. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef]
- Cerrilla, M.E.O.; Martínez, G.M. Starch digestion and glucose metabolism in the ruminant: A review. Interciencia 2003, 28, 380–386. [Google Scholar]
- Herdt, T. Postabsorptive Nutrient Utilization. In Textbook of Veterinary Physiology,, 3rd; Cunningham, J.G., Ed.; W.B. Saunders Company: Philadelphia, PA, USA, 2002; pp. 305–308. [Google Scholar]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species (Review). Physiol. Rev. 1990, 70, 567–590. [Google Scholar]
- Saleem, F.; Ametaj, B.N.; Bouatra, S.; Mandal, R.; Zebeli, Q.; Dunn, S.M.; Wishart, D.S. Metabolomics approach to uncover the effects of grain diets on rumen health in dairy cows. J. Dairy Sci. 2012, 95, 6606–6623. [Google Scholar] [CrossRef]
- Zebeli, Q.; Aschenbach, J.R.; Tafaj, M.; Boguhn, J.; Ametaj, B.N.; Drochner, W. Invited review: Role of physically effective fiber and estimation of dietary fiber adequacy in high-producing dairy cattle. J. Dairy Sci. 2012, 95, 1041–1056. [Google Scholar]
- Kirat, D.; Masuoka, J.; Hayashi, H.; Iwano, H.; Yokota, H.; Taniyama, H.; Kato, S. Monocarboxylate transporter 1 (MCT1) plays a direct role in short-chain fatty acids absorption in caprine rumen. J. Physiol. 2006, 576, 635–647. [Google Scholar] [CrossRef]
- Koho, N.M.; Taponen, J.; Tiihonen, H.; Manninen, M.; Pösö, A.R. Effects of age and concentrate feeding on the expression of MCT 1 and CD147 in the gastrointestinal tract of goats and Hereford finishing beef bulls. Res. Vet. Sci. 2011, 90, 301–305. [Google Scholar] [CrossRef]
- Armentano, L.E. Ruminant hepatic metabolism of volatile fatty acids, lactate and pyruvate. J. Nutr. 1992, 122, 838–842. [Google Scholar]
- Harmon, D.L.; Yamka, R.M.; Elam, N.A. Factors affecting intestinal starch digestion in ruminants: A review. Can. J. Anim. Sci. 2004, 84, 309–318. [Google Scholar] [CrossRef]
- Reynolds, C.K. Production and metabolic effects of site of starch digestion in dairy cattle. Anim. Feed Sci. Technol. 2006, 130, 78–94. [Google Scholar] [CrossRef]
- Gressley, T.F.; Hall, M.B.; Armentano, L.E. Ruminant Nutrition Symposium: Productivity, digestion, and health responses to hindgut acidosis in ruminants. J. Anim. Sci. 2011, 89, 1120–1130. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Deckardt, K.; Khol-Parisini, A.; Zebeli, Q. Peculiarities of Enhancing Resistant Starch in Ruminants Using Chemical Methods: Opportunities and Challenges. Nutrients 2013, 5, 1970-1988. https://doi.org/10.3390/nu5061970
Deckardt K, Khol-Parisini A, Zebeli Q. Peculiarities of Enhancing Resistant Starch in Ruminants Using Chemical Methods: Opportunities and Challenges. Nutrients. 2013; 5(6):1970-1988. https://doi.org/10.3390/nu5061970
Chicago/Turabian StyleDeckardt, Kathrin, Annabella Khol-Parisini, and Qendrim Zebeli. 2013. "Peculiarities of Enhancing Resistant Starch in Ruminants Using Chemical Methods: Opportunities and Challenges" Nutrients 5, no. 6: 1970-1988. https://doi.org/10.3390/nu5061970