Perioperative Immunonutrition in Well-Nourished Patients Undergoing Surgery for Head and Neck Cancer: Evaluation of Inflammatory and Immunologic Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Protocol
2.2. Nutrition
| Oral Impact | Enteral Impact | Isosource Standard | |
|---|---|---|---|
| Energy (kcal/L) | 1000 | 1000 | 1200 |
| Protein (g/L) | 56 | 56 | 43 |
| Carbohydrate (g/L) | 134 | 130 | 170 |
| Fat (g/L) | 28 | 28 | 39 |
| Arginine (g/L) | 12.6 | 12.5 | 0 |
| Nucleotides (g/L) | 1.3 | 1.2 | 0 |
| EPA + DHA (g/L) | 3.3 | 1.7 | 0 |
| Fibre (g) | 10 | 0 | 0 |
2.3. Total Body Protein
2.4. Biochemistry
2.4.1. Fatty Acids
2.4.2. Inflammatory Markers
2.4.3. Immune Status
2.5. Clinical Outcome
2.6. Statistical Analysis
3. Results
| Pt | Sex | Age | Staging | Diagnosis | Procedure | OP | LOS | |
|---|---|---|---|---|---|---|---|---|
| Excision | Reconstruction | h | d | |||||
| Standard group | ||||||||
| A | F | 79 | Tx N1 Mx | Buccal melanoma | Wide local excision (WLE) | Forearm free flap | 8 | 24 |
| B | M | 71 | T2 N2 | SCC floor of mouth | WLE, marginal mandibulectomy | Pectoralis pedicle flap | 11 | 21 |
| C | M | 22 | T2 N0 M0 | SCC tongue | Hemiglossectomy | Forearm free flap | 8 | 22 |
| D | M | 17 | - a | Ameloblastoma mandible | Segmental mandibulectomy | Fibular free flap | 5.5 | 7 |
| Immunonutrition group | ||||||||
| E | M | 63 | T2 N0 M0 | SCC tongue | Hemiglossectomy | Forearm free flap | 7 | 10 |
| F | F | 68 | T4 N2 | SCC floor of mouth | Segmental mandibulectomy | Fibular free flap | 7.5 | 43 |
| G | M | 28 | T2 N1 | SCC tongue | Hemiglossectomy | Forearm free flap | 8 | 10 |
| H | M | 46 | - a | ACC floor of mouth | WLE | Forearm free flap | 8.5 | 9 |
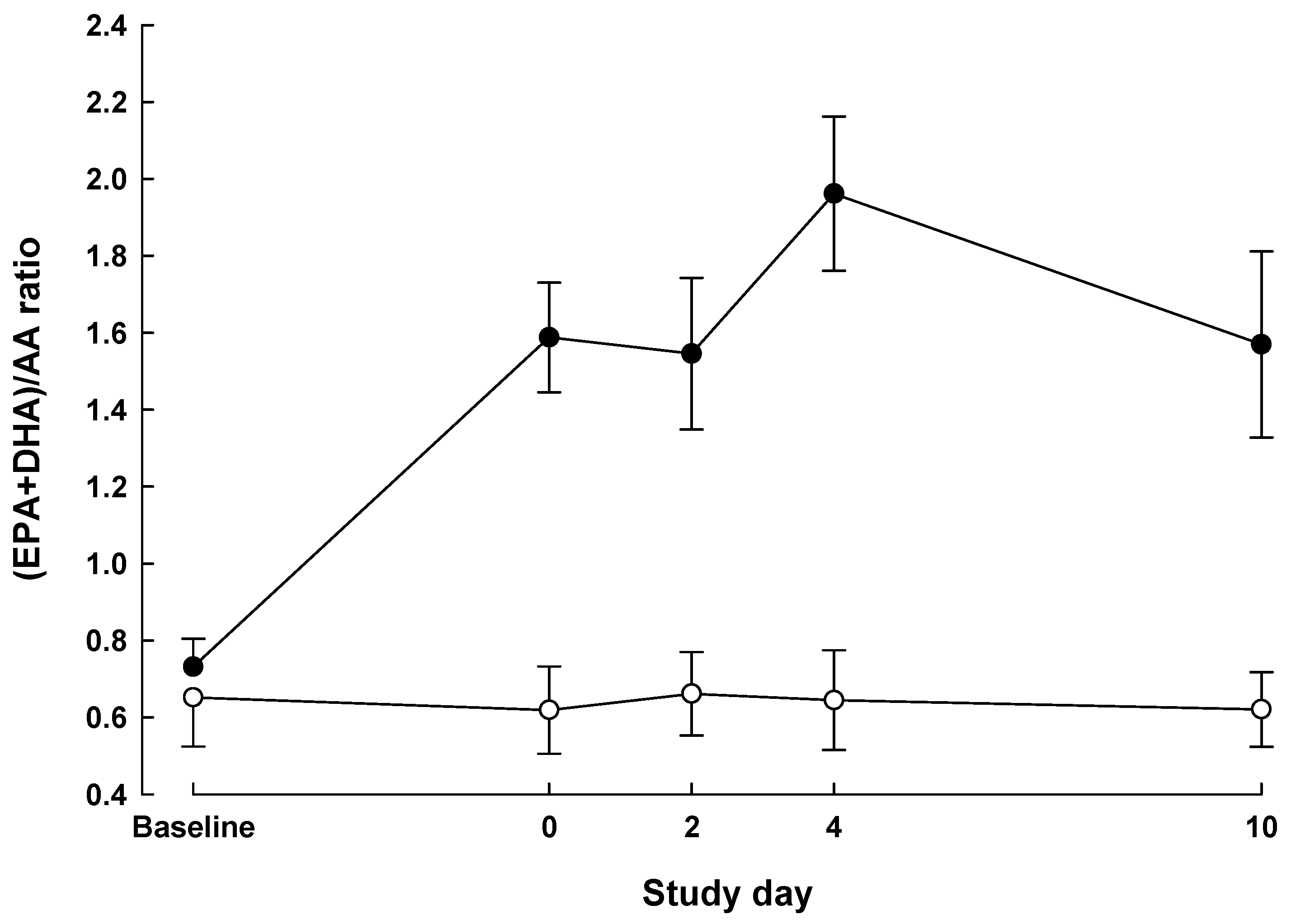
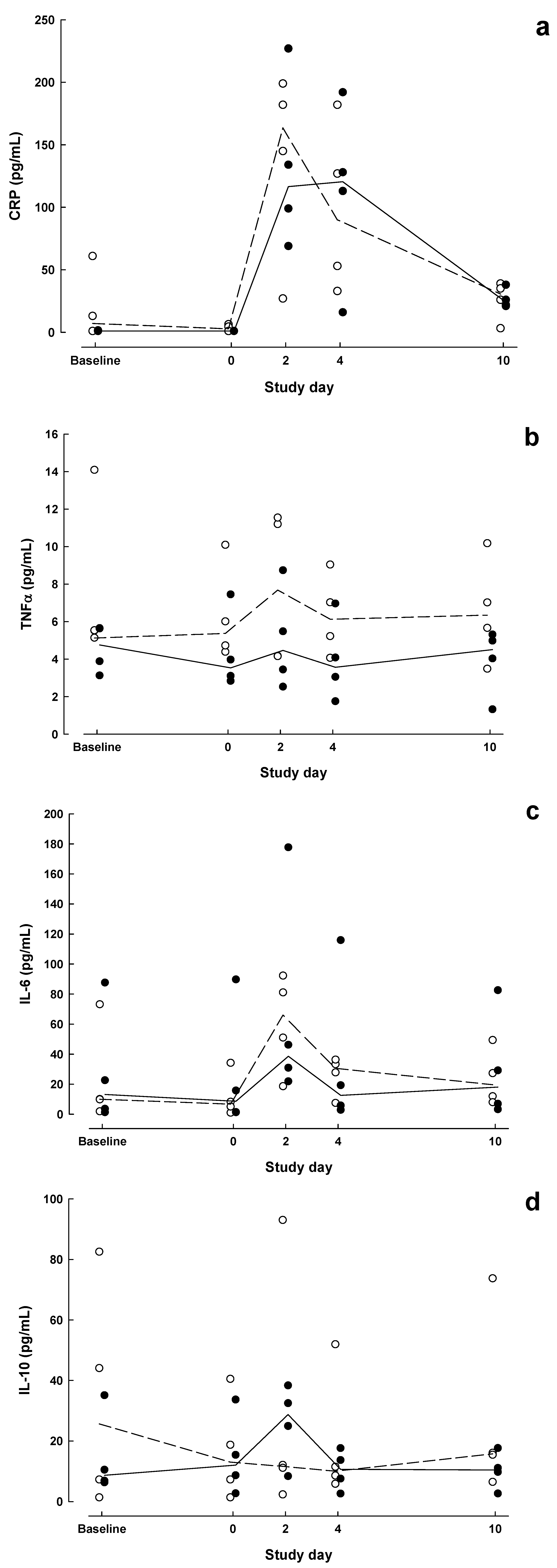
| Baseline | Day 0 | POD 2 | POD 4 | POD 10 | P value a | |||
|---|---|---|---|---|---|---|---|---|
| Group | Time | Group × Time | ||||||
| Immunoglobulin G (g/L) | ||||||||
| STD | 11.03 ± 1.26 | 9.40 ± 1.19 | 6.93 ± 0.69 c | 7.36 ± 1.03 | 9.50 ± 1.47 | 0.37 | 0.0047 | 0.56 |
| IMN | 13.08 ± 2.68 | 12.28 ± 2.03 | 8.15 ± 0.61 | 8.83 ± 0.91 | 10.25 ± 1.32 | |||
| Immunoglobulin A (g/L) | ||||||||
| STD | 3.33 ± 0.09 | 2.88 ± 0.17 | 2.10 ± 0.14 b | 2.43 ± 0.26 | 3.43 ± 0.21 | 0.15 | 0.0001 | 0.35 |
| IMN | 2.85 ± 0.10 | 2.70 ± 0.11 | 1.93 ± 0.26 | 2.35 ± 0.28 | 2.80 ± 0.16 | |||
| Immunoglobulin M (g/L) | ||||||||
| STD | 1.34 ± 0.49 | 1.32 ± 0.43 | 0.80 ± 0.26 | 0.86 ± 0.27 | 1.67 ± 0.32 | 0.36 | 0.0028 | 0.17 |
| IMN | 0.90 ± 0.27 | 0.88 ± 0.31 | 0.57 ± 0.21 | 0.65 ± 0.22 | 0.89 ± 0.25 | |||
| Total lymphocytes (109/L) | ||||||||
| STD | 2.05 ± 0.56 | 2.05 ± 0.11 | 1.12 ± 0.30 | 1.23 ± 0.19 | 1.66 ± 0.34 | 0.98 | 0.026 | 0.56 |
| IMN | 1.89 ± 0.16 | 1.63 ± 0.25 | 1.43 ± 0.28 | 1.43 ± 0.10 | 1.80 ± 0.35 | |||
4. Discussion
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Marik, P.E.; Zaloga, G.P. Immunonutrition in high risk surgical patients: A systematic review and analysis of the literature. J. Parenter. Enteral Nutr. 2010, 34, 378–386. [Google Scholar] [CrossRef]
- Cerantola, Y.; Hübner, M.; Grass, F.; Demartines, N.; Schäfer, M. Immunonutrition in gastrointestinal surgery. Br. J. Surg. 2011, 98, 37–48. [Google Scholar] [CrossRef]
- Marimuthu, K.; Varadhan, K.K.; Ljungqvist, O.; Lobo, D.N. A meta-analysis of the effect of combinations of immune modulating nutrients on outcome in patients undergoing major open gastrointestinal surgery. Ann. Surg. 2012, 255, 1060–1068. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Y.; Guo, T.; Li, Y.; Cai, H. Perioperative immunonutrition for gastrointestinal cancer: A systematic review of randomized controlled trials. Surg. Oncol. 2012, 21, e87–e95. [Google Scholar] [CrossRef]
- Braga, M.; Gianotti, L.; Nespoli, L.; Radaelli, G.; di Carlo, V. Nutritional approach in malnourished surgical patients: A prospective randomized study. Arch. Surg. 2002, 137, 174–180. [Google Scholar] [CrossRef]
- Klek, S.; Sierzega, M.; Szybinski, P.; Szczepanek, K.; Scislo, L.; Walewska, E.; Kulig, J. The immunomodulating enteral nutrition in malnourished surgical patients—a prospective, randomized, double-blind clinical trial. Clin. Nutr. 2011, 30, 282–288. [Google Scholar] [CrossRef]
- Calder, P.C. Immunonutrition in surgical and critically ill patients. Br. J. Nutr. 2007, 98, S133–S139. [Google Scholar]
- Gianotti, L.; Braga, M.; Nespoli, L.; Radaelli, G.; Beneduce, A.; di Carlo, V. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology 2002, 122, 1763–1770. [Google Scholar] [CrossRef]
- Klek, S.; Kulig, J.; Sierzega, M.; Szczepanek, K.; Szybiński, P.; Scislo, L.; Walewska, E.; Kubisz, A.; Szczepanik, A.M. Standard and immunomodulating enteral nutrition in patients after extended gastrointestinal surgery—a prospective, randomized, controlled clinical trial. Clin. Nutr. 2008, 27, 504–512. [Google Scholar] [CrossRef]
- Klek, S.; Kulig, J.; Sierzega, M.; Szybinski, P.; Szczepanek, K.; Kubisz, A.; Kowalczyk, T.; Gach, T.; Pach, R.; Szczepanik, A.M. The impact of immunostimulating nutrition on infectious complications after upper gastrointestinal surgery: A prospective, randomized, clinical trial. Ann. Surg. 2008, 248, 212–220. [Google Scholar] [CrossRef]
- Fujitani, K.; Tsujinaka, T.; Fujita, J.; Miyashiro, I.; Imamura, H.; Kimura, Y.; Kobayashi, K.; Kurokawa, Y.; Shimokawa, T.; Furukawa, H.; Osaka Gastrointestinal Cancer Chemotherapy Study Group. Prospective randomized trial of preoperative enteral immunonutrition followed by elective total gastrectomy for gastric cancer. Br. J. Surg. 2012, 99, 621–629. [Google Scholar] [CrossRef]
- Snyderman, C.H.; Kachman, K.; Molseed, L.; Wagner, R.; D’Amico, F.; Bumpous, J.; Rueger, R. Reduced postoperative infections with an immune-enhancing nutritional supplement. Laryngoscope 1999, 109, 915–921. [Google Scholar] [CrossRef]
- Casas-Rodera, P.; Gómez-Candela, C.; Benítez, S.; Mateo, R.; Armero, M.; Castillo, R.; Culebras, J.M. Immunoenhanced enteral nutrition formulas in head and neck cancer surgery: A prospective, randomized clinical trial. Nutr. Hosp. 2008, 23, 105–110. [Google Scholar]
- Linn, B.S.; Robinson, D.S.; Klimas, N.G. Effects of age and nutritional status on surgical outcomes in head and neck cancer. Ann. Surg. 1989, 207, 267–273. [Google Scholar]
- Felekis, D.; Eleftheriadou, A.; Papadakos, G.; Bosinakou, I.; Ferekidou, E.; Kandiloros, D.; Katsaragakis, S.; Charalabopoulos, K.; Manolopoulos, L. Effect of perioperative immuno-enhanced enteral nutrition on inflammatory response, nutritional status, and outcomes in head and neck cancer patients undergoing major surgery. Nutr. Cancer 2010, 62, 1105–1112. [Google Scholar] [CrossRef]
- Plank, L.D.; Gane, E.J.; Peng, S.; Muthu, C.; Mathur, S.; Gillanders, L.; McIlroy, K.; Donaghy, A.J.; McCall, J.L. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: A randomized 12-month trial. Hepatology 2008, 48, 557–566. [Google Scholar] [CrossRef]
- Plank, L.D.; Metzger, D.J.; McCall, J.L.; Barclay, K.L.; Gane, E.J.; Streat, S.J.; Munn, S.R.; Hill, G.L. Sequential changes in the metabolic response to orthotopic liver transplantation during the first year after surgery. Ann. Surg. 2001, 234, 245–255. [Google Scholar] [CrossRef]
- Burdge, G.C.; Wright, P.; Jones, A.E.; Wootton, S.A. A method for separation of phosphatidylcholine, triacylglycerol, non-esterified fatty acids and cholesterol esters from plasma by solid-phase extraction. Br. J. Nutr. 2000, 84, 781–787. [Google Scholar]
- Gamer, J.S.; Jarvis, W.R.; Embri, T.G.; Horan, T.C.; Hughes, J.M. CDC definitions for nosocomial infections. Am. J. Infect. Control 1988, 16, 128–140. [Google Scholar] [CrossRef]
- Braga, M.; Gianotti, L.; Radaelli, G.; Vignali, A.; Mari, G.; Gentilini, O.; di Carlo, V. Perioperative immunonutrition in patients undergoing cancer surgery. Results of a randomized double-blind phase 3 trial. Arch. Surg. 1999, 134, 428–433. [Google Scholar]
- De Luis, D.A.; Aller, R.; Izaola, O.; Cuellar, L.; Terroba, M.C. Postsurgery enteral nutrition in head and neck cancer patients. Eur. J. Clin. Nutr. 2002, 56, 1126–1129. [Google Scholar] [CrossRef]
- De Luis, D.A.; Izaola, O.; Cuellar, L.; Terroba, M.C.; Arranz, M.; Fernandez, N.; Aller, R. Effect of c-reactive protein and interleukins blood levels in postsurgery arginine-enhanced enteral nutrition in head and neck cancer patients. Eur. J. Clin. Nutr. 2003, 57, 96–99. [Google Scholar]
- De Luis, D.A.; Izaola, O.; Cuellar, L.; Terroba, M.C.; Aller, R. Randomized clinical trial with an enteral arginine-enhanced formula in early postsurgical head and neck cancer patients. Eur. J. Clin. Nutr. 2004, 58, 1505–1508. [Google Scholar] [CrossRef]
- De Luis, D.A.; Arranz, M.; Aller, R.; Izaola, O.; Cuellar, L.; Terroba, M.C. Immunoenhanced enteral nutrition, effect on inflammatory markers in head and neck cancer patients. Eur. J. Clin. Nutr. 2005, 59, 145–147. [Google Scholar] [CrossRef]
- De Luis, D.A.; Izaola, O.; Cuellar, L.; Terroba, M.C.; Martin, T.; Aller, R. Clinical and biochemical outcomes after a randomized trial with a high dose of enteral arginine formula in postsurgical head and neck cancer patients. Eur. J. Clin. Nutr. 2007, 61, 200–204. [Google Scholar] [CrossRef]
- Riso, S.; Aluffi, P.; Brugnani, M.; Farinetti, F.; Pia, F.; D’Andrea, F. Postoperative enteral immunonutrition in head and neck cancer patients. Clin. Nutr. 2000, 19, 407–412. [Google Scholar] [CrossRef]
- Reynolds, J.V.; Daly, J.M.; Zhang, S.; Evantash, E.; Shou, J.; Sigal, R.; Ziegler, M.M. Immunomodulatory mechanisms of arginine. Surgery 1988, 104, 142–151. [Google Scholar]
- Barbul, A.; Lazarou, S.A.; Efron, D.T.; Wasserkrug, H.L.; Efron, G. Arginine enhances wound healing and lymphocyte immune responses in humans. Surgery 1990, 108, 331–337. [Google Scholar]
- Daly, J.M.; Reynolds, J.; Thom, A.; Kinsley, L.; Dietrick-Gallagher, M.; Shou, J.; Ruggieri, B. Immune and metabolic effects of arginine in the surgical patient. Ann. Surg. 1988, 208, 512–523. [Google Scholar] [CrossRef]
- Van Bokhorst-De Van Der Schueren, M.A.; Quak, J.J.; von Blomberg-van der Flier, B.M.; Kuik, D.J.; Langendoen, S.I.; Snow, G.B.; Green, C.J.; van Leeuwen, P.A.M. Effect of perioperative nutrition, with and without arginine supplementation, on nutritional status, immune function, postoperative morbidity, and survival in severely malnourished head and neck cancer patients. Am. J. Clin. Nutr. 2001, 73, 323–332. [Google Scholar]
- De Luis, D.A.; Izaola, O.; Aller, R.; Cuellar, L.; Terroba, M.C. A randomized clinical trial with oral Immunonutrition (ω3-enhanced formula vs. arginine-enhanced formula) in ambulatory head and neck cancer patients. Ann. Nutr. Metab. 2005, 49, 95–99. [Google Scholar]
- Van Bokhorst-de van der Schueren, M.A.E.; von Blomberg-van der Flier, B.M.E.; Riezebos, R.K.; Scholten, P.E.T.; Quak, J.J.; Snow, G.B.; van Leeuwen, P.A.M. Differences in immune status between well-nourished and malnourished head and neck cancer patients. Clin. Nutr. 1998, 17, 107–111. [Google Scholar] [CrossRef]
- Peng, S.; Plank, L.D.; McCall, J.L.; Gillanders, L.K.; McIlroy, K.; Gane, E.J. Body composition, muscle function and energy expenditure in patients with liver cirrhosis: A comprehensive study. Am. J. Clin. Nutr. 2007, 85, 1257–1266. [Google Scholar]
- Browning, L.M.; Walker, C.G.; Mander, A.P.; West, A.L.; Madden, J.; Gambell, J.M.; Young, S.; Wang, L.; Jebb, S.A.; Calder, P.C. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am. J. Clin. Nutr. 2012, 96, 748–758. [Google Scholar] [CrossRef]
- Calder, P.C. Mechanisms of action of (n-3) fatty acids. J. Nutr. 2012, 142, 592S–599S. [Google Scholar] [CrossRef]
- Castell, J.V.; Gomez-Lechon, M.J.; David, M.; Andus, T.; Geiger, T.; Trullenque, R.; Fabra, R.; Heinrich, P.C. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989, 242, 237–239. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Turnock, A.; Calder, P.C.; West, A.L.; Izzard, M.; Morton, R.P.; Plank, L.D. Perioperative Immunonutrition in Well-Nourished Patients Undergoing Surgery for Head and Neck Cancer: Evaluation of Inflammatory and Immunologic Outcomes. Nutrients 2013, 5, 1186-1199. https://doi.org/10.3390/nu5041186
Turnock A, Calder PC, West AL, Izzard M, Morton RP, Plank LD. Perioperative Immunonutrition in Well-Nourished Patients Undergoing Surgery for Head and Neck Cancer: Evaluation of Inflammatory and Immunologic Outcomes. Nutrients. 2013; 5(4):1186-1199. https://doi.org/10.3390/nu5041186
Chicago/Turabian StyleTurnock, Amy, Philip C. Calder, Annette L. West, Mark Izzard, Randall P. Morton, and Lindsay D. Plank. 2013. "Perioperative Immunonutrition in Well-Nourished Patients Undergoing Surgery for Head and Neck Cancer: Evaluation of Inflammatory and Immunologic Outcomes" Nutrients 5, no. 4: 1186-1199. https://doi.org/10.3390/nu5041186





