Oats in the Diet of Children with Celiac Disease: Preliminary Results of a Double-Blind, Randomized, Placebo-Controlled Multicenter Italian Study
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Study Design and Diets
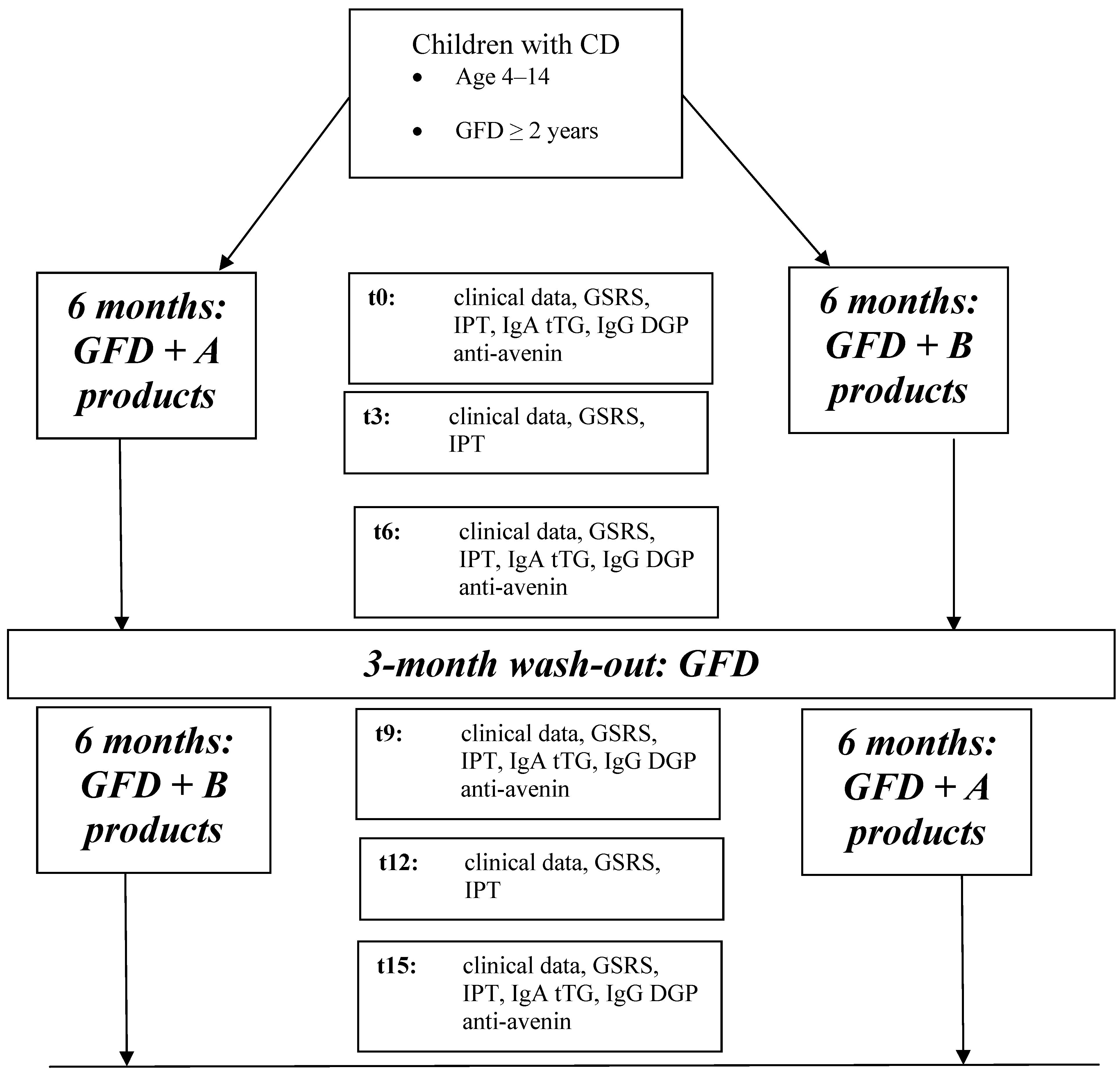
2.3. Methods
2.4. Ethics
2.5. Statistical Analysis
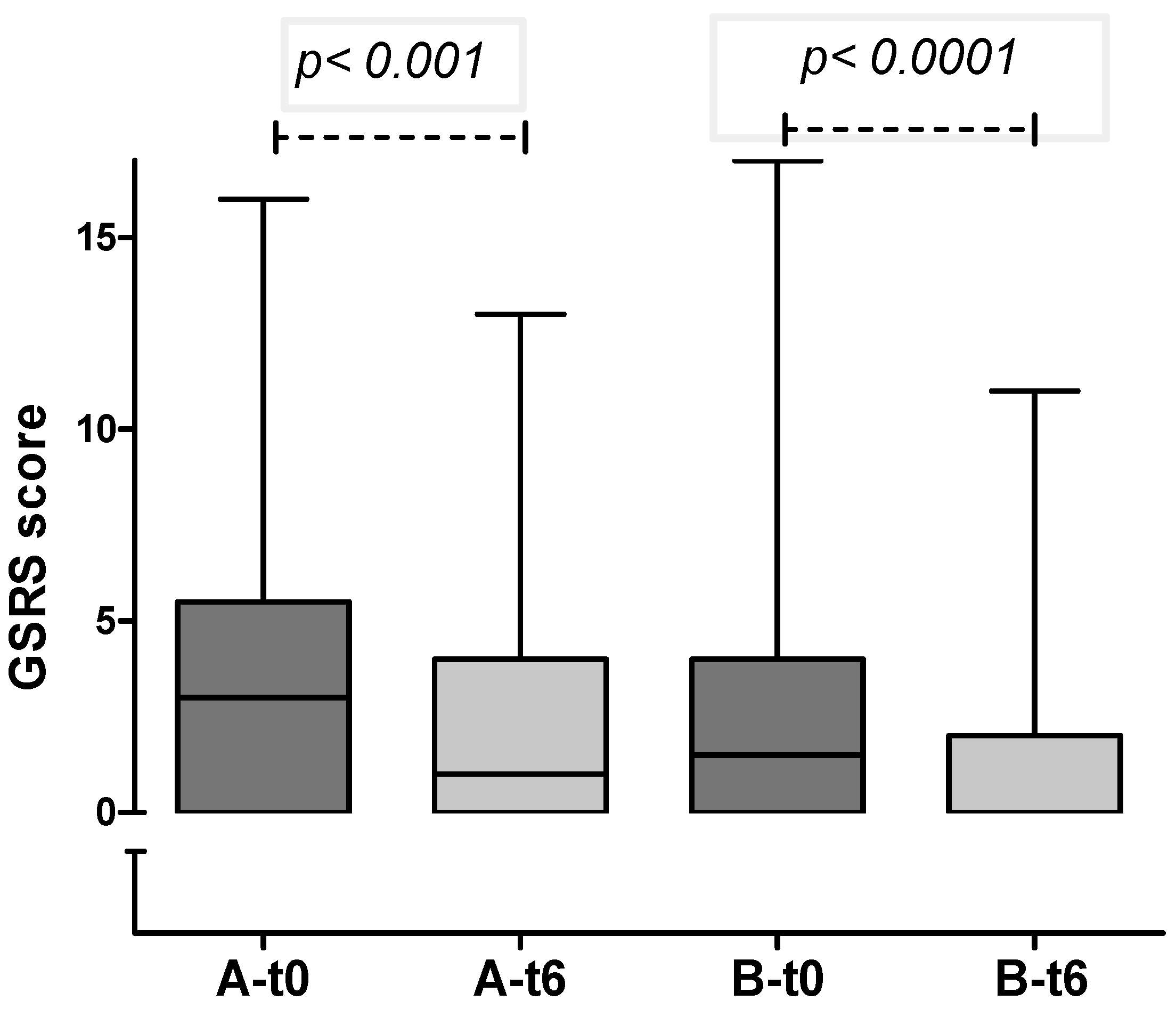
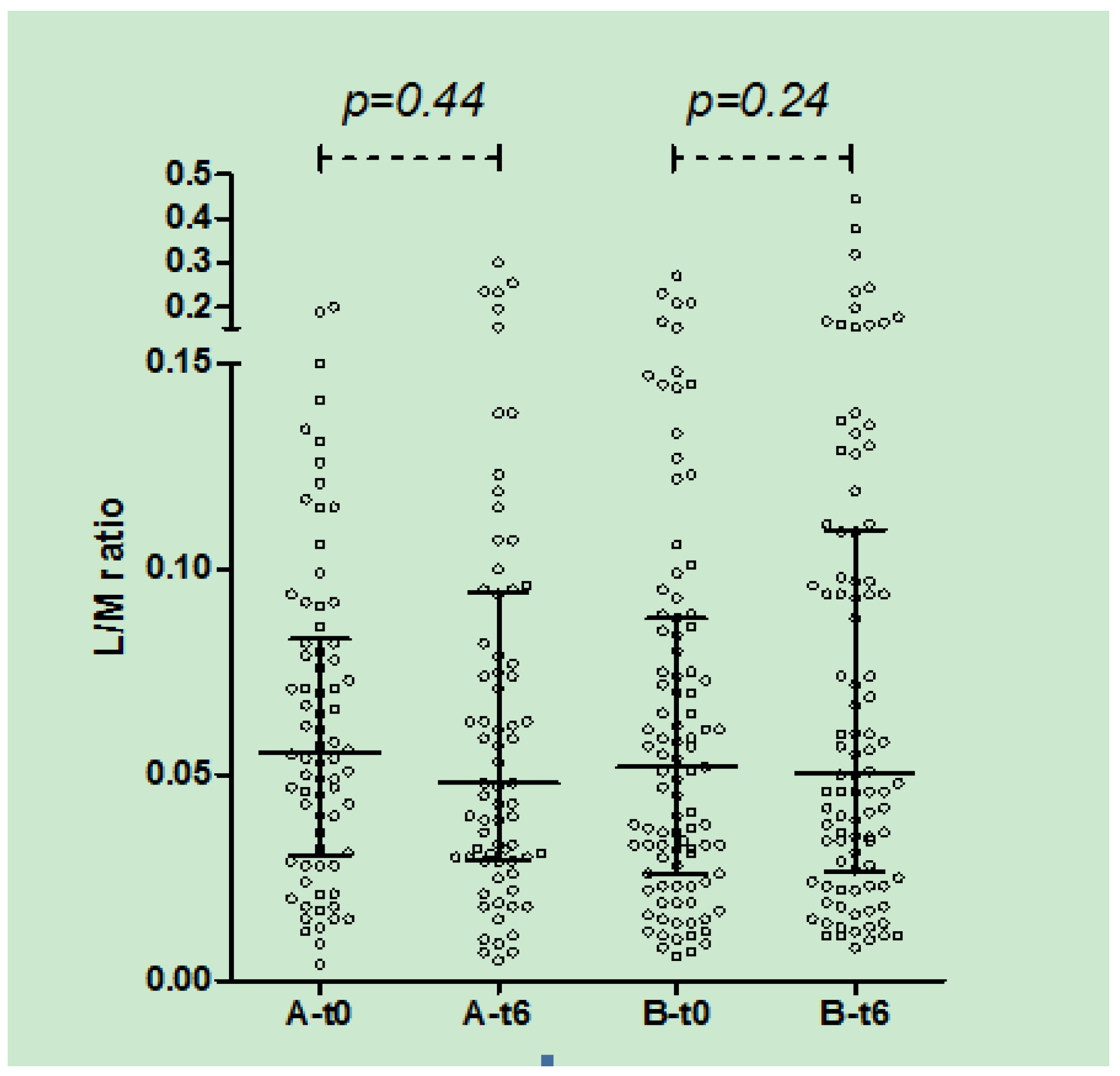
3. Results
| Group A (N = 75) | Group B (N = 96) | p | |
|---|---|---|---|
| Gender distribution (M:F) | 1:2.5 | 1:1.9 | 0.50 |
| Age at diagnosis Median (IQR) | 3.48 (1.98–6.36) | 2.84 (1.83–6.03) | 0.38 |
| Age at enrollment Median (IQR) | 8.76 (7.07–11.38) | 9.35 (7.24–12.01) | 0.54 |
| Duration of diet Median (IQR) | 4.25 (2.11–6.04) | 4.49 (1.25–6.55) | 0.06 |
| GSRS score at enrollment Median (IQR) | 3 (0–5.25) | 2 (0–4.5) | 0.36 |
| L/M at enrollment Median (IQR) | 0.055 (0.030–0.083) | 0.052 (0.026–0.088) | 0.62 |
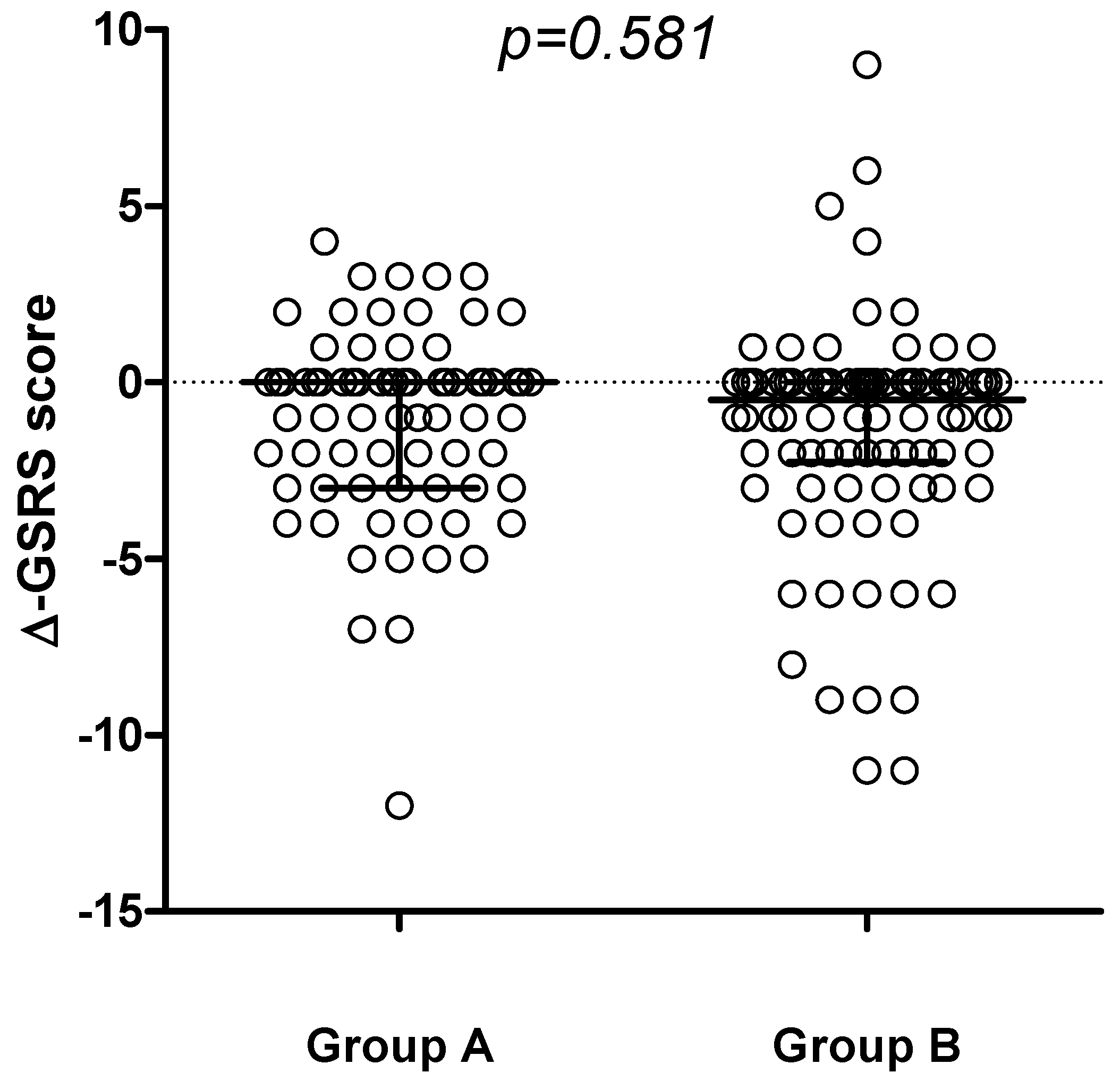
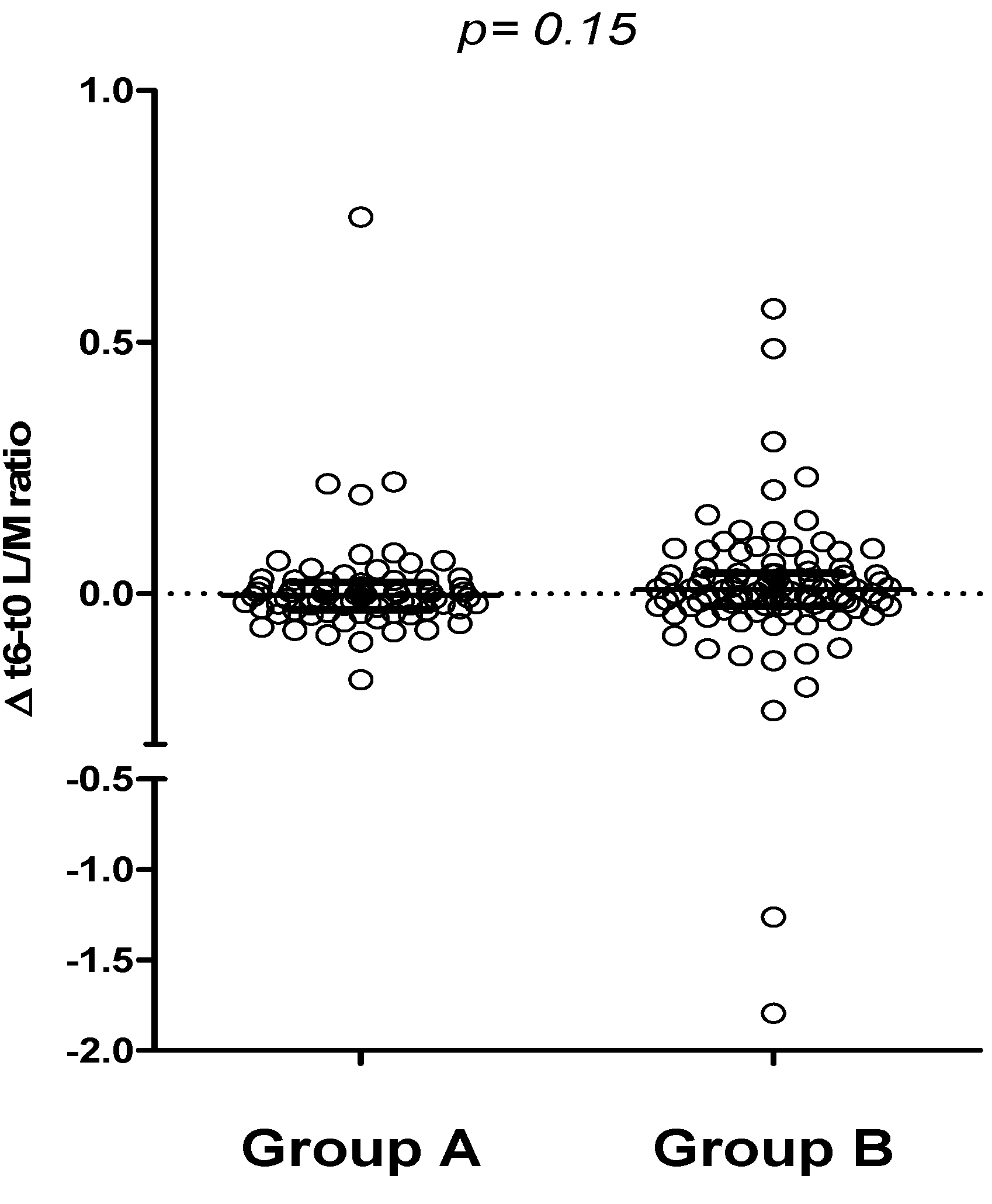

4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Janatuinen, E.K.; Pikkarainen, P.H.; Kamppainen, T.A.; Kosma, V.M.; Jarvinen, R.M.; Uusitupa, M.I.; Julkunen, R.J. A comparison of diets with and without oats in adults with celiac disease. N. Engl. J. Med. 1995, 333, 1033–1037. [Google Scholar] [CrossRef]
- Srinivasan, U.; Leonard, N.; Jones, E.; Kasarda, D.D.; Weir, D.G.; O’Farrelly, C.; Feighery, C. Absence of oats toxicity in adult coeliac disease. BMJ 1996, 313, 1300–1301. [Google Scholar] [CrossRef]
- Hardman, C.M.; Gariogh, J.J.; Leonard, J.N.; Thomas, H.J.; Walker, M.M.; Lortan, J.E.; Lister, A.; Fry, L. Absence of toxicity of oats in patients with dermatitis herpetiformis. N. Engl. J. Med. 1997, 337, 1884–1887. [Google Scholar] [CrossRef]
- Reunala, T.; Collin, P.; Holm, K.; Pikkarainen, P.; Miettinen, A.; Vuolteenaho, N.; Mäki, M. Tolerance to oats in dermatitis herpetiformis. Gut 1998, 43, 490–493. [Google Scholar] [CrossRef]
- Hoffenberg, E.J.; Haas, J.; Drescher, A.; Bamhurst, R.; Osberg, I.; Bao, F. A trial of oats in children with newly diagnosed celiac disease. J. Pediatr. 2000, 137, 361–366. [Google Scholar] [CrossRef]
- Janatuinen, E.K.; Kemppainen, T.H.; Julkunen, R.J.; Kosma, V.M.; Maki, M.; Heikkinen, M.; Uusitupa, M.I. No harm from five year ingestion of oats in coeliac disease. Gut 2002, 50, 332–335. [Google Scholar] [CrossRef]
- Storsrud, S.; Olsson, M.; Arvidsson Lenner, M.; Nilsson, L.A.; Nilsson, O.; Kilander, A. Adult coeliac patients do tolerate large amounts of oats. Eur. J. Clin. Nutr. 2003, 57, 163–169. [Google Scholar] [CrossRef]
- Högberg, L.; Laurin, P.; Falth-Magnusson, K.; Grant, C.; Grodzinsky, E.; Jansson, G.; Ascher, H.; Browaldh, L.; Hammersjö, J.A.; Lindberg, E.; et al. Oats to children with newly diagnosed coeliac disease: A randomised double blind study. Gut 2004, 53, 649–654. [Google Scholar] [CrossRef]
- Holm, K.; Maki, M.; Vuolteenaho, N.; Mustalahti, K.; Ashorn, M.; Ruuska, T.; Kaukinen, K. Oats in the treatment of childhood coeliac disease: A 2-year controlled trial and a long-term clinical follow-up study. Aliment. Pharmacol. Ther. 2006, 23, 1463–1472. [Google Scholar] [CrossRef]
- Sey, M.S.; Parfitt, J.; Gregor, J. Prospective study of clinical and histological safety of pure and uncontaminated Canadian oats in the management of celiac disease. J. Parenter. Enteral. Nutr. 2011, 35, 459–464. [Google Scholar] [CrossRef]
- Cooper, S.E.; Kennedy, N.P.; Mohamed, B.M.; Abuzakouk, M.; Dunne, J.; Byrne, G.; McDonald, G.; Davies, A.; Edwards, C.; Kelly, J.; et al. Immunological indicators of coeliac disease activity are not altered by long-term oats challenge. Clin. Exp. Immunol. 2013, 171, 313–318. [Google Scholar] [CrossRef]
- Silano, M.; Di Benedetto, R.; Maialetti, F.; De Vincenzi, A.; Calcaterra, R.; Cornell, H.J.; De Vincenzi, M. Avenins from different cultivars of oats elicit response by coeliac peripheral lymphocytes. Scand. J. Gastroenterol. 2007, 42, 1302–1305. [Google Scholar] [CrossRef]
- Ballabio, C.; Uberti, F.; Manferdelli, S.; Vacca, E.; Boggini, G.; Redaelli, R.; Catassi, C.; Lionetti, E.; Penãs, E.; Restani, P. Molecular characterisation of 36 oat varieties and in vitro assessment of their suitability for coeliacs’ diet. J. Cereal Sci. 2011, 54, 110–115. [Google Scholar] [CrossRef]
- Comino, I.; Real, A.; de Lorenzo, L.; Cornell, H.; López-Casado, M.Á.; Barro, F.; Lorite, P.; Torres, M.I.; Cebolla, A.; Sousa, C. Diversity in oat potential immunogenicity: Basis for the selection of oat varieties with no toxicity in coeliac disease. Gut 2011, 60, 915–922. [Google Scholar] [CrossRef]
- Real, A.; Comino, I.; de Lorenzo, L.; Merchán, F.; Gil-Humanes, J.; Giménez, M.J.; López-Casado, M.Á.; Torres, M.I.; Cebolla, Á.; Sousa, C.; et al. Molecular and immunological characterization of gluten proteins isolated from oat cultivars that differ in toxicity for celiac disease. PLoS One 2012, 7, e48365. [Google Scholar] [CrossRef]
- Mujico, J.R.; Mitea, C.; Gilissen, L.J.W.J.; de Ru, A.; van Veelen, P.; Smulders, M.J.M.; Koning, F. Natural variation in avenin epitopes among oat varieties: Implications for celiac disease. J. Cereal Sci. 2011, 54, 8–12. [Google Scholar] [CrossRef]
- Rashid, M.; Butzner, D.; Burrows, V.; Zarkadas, M.; Case, S.; Molloy, M.; Warren, R.; Pulido, O.; Switzer, C. Consumption of pure oats by individuals with celiac disease: A position statement by the Canadian Celiac Association. Can. J. Gastroenterol. 2007, 21, 649–651. [Google Scholar]
- American Dietetic Association. Evidence-based nutrition practice guidelines on celiac disease. Available online: http://www.guideline.gov/content (accessed on 17 November 2013).
- Generoso, M.; De Rosa, M.; De Rosa, R.; De Magistris, L.; Secondulfo, M.; Fiandra, R.; Carratù, R.; Cartenì, M.J. Cellobiose and lactulose coupled with mannitol and determined using ion-exchange chromatography with pulsed amperometric detection, are reliable probes for investigation of intestinal permeability. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003, 783, 349–357. [Google Scholar] [CrossRef]
- Dimenas, E.; Glise, H.; Hallerback, B.; Hernqvist, H.; Svedlund, J.; Wiklund, I. Well-being and gastrointestinal symptoms among patients referred to endoscopy owing to suspected duodenal ulcer. Scand. J. Gastroenterol. 1995, 30, 1046–1052. [Google Scholar] [CrossRef]
- Dimenas, E.; Carlsson, G.; Glise, H.; Israelsson, B.; Wiklund, I. Relevance of norm values as part of the documentation of quality of life instruments for use in upper gastrointestinal disease. Scand. J. Gastroenterol. Suppl. 1996, 221, 8–13. [Google Scholar]
- Lohiniemi, S.; Mäki, M.; Kaukinen, K.; Laippala, P.; Collin, P. Gastrointestinal symptoms rating scale in coeliac disease patients on wheat starch-based gluten-free diets. Scand. J. Gastroenterol. 2000, 35, 947–949. [Google Scholar] [CrossRef]
- Hallert, C.; Grännö, C.; Grant, C.; Hultén, S.; Midhagen, G.; Ström, M.; Svensson, H.; Valdimarsson, T.; Wickström, T. Quality of life of adult coeliac patients treated for 10 years. Scand. J. Gastroenterol. 1998, 33, 933–938. [Google Scholar] [CrossRef]
- Thomson, M.; Fritscher-Ravens, A.; Hall, S.; Afzal, N.; Ashwood, P.; Swain, C.P. Endoluminal gastroplication in children with significant gastro-oesophageal reflux disease. Gut 2004, 53, 1745–1750. [Google Scholar] [CrossRef]
- Arentz-Hansen, H.; Fleckenstein, B.; Molberg, Ø.; Scott, H.; Koning, F.; Jung, G.; Roepstorff, P.; Lundin, K.E.; Sollid, L.M. The molecular basis for oat intolerance in patients with celiac disease. PLoS Med. 2004, 1, e1. [Google Scholar] [CrossRef]
- Vogelsang, H.; Schwarzenhofer, M.; Oberhuber, G. Changes in gastrointestinal permeability in celiac disease. Dig. Dis. 1998, 16, 333–336. [Google Scholar] [CrossRef]
- Duerksen, D.R.; Wilhelm-Boyles, C.; Parry, D.M. Intestinal permeability in long-term follow-up of patients with celiac disease on a gluten-free diet. Dig. Dis. Sci. 2005, 50, 85–90. [Google Scholar]
- Vilela, E.G.; Ferrari, M.L.; de Gama Torres, H.O.; Martins, F.P.; Goulart, E.M.; Lima, A.S.; da Cunha, A.S. Intestinal permeability and antigliadin antibody test for monitoring adult patients with celiac disease. Dig. Dis. Sci. 2007, 52, 1304–1309. [Google Scholar] [CrossRef]
- Catassi, C.; Fabiani, E.; Rätsch, I.M.; Bonucci, A.; Dotti, M.; Coppa, G.V.; Giorgi, P.L. Is the sugar intestinal permeability test a reliable investigation for coeliac disease screening? Gut 1997, 40, 215–217. [Google Scholar]
- Kelly, C.P.; Green, P.H.; Murray, J.A.; Dimarino, A.; Colatrella, A.; Leffler, D.A.; Alexander, T.; Arsenescu, R.; Leon, F.; Jiang, J.G.; et al. Larazotide Acetate in patients with celiac disease undergoing a gluten-challenge: A randomized placebo-controlled study. Aliment. Pharmacol. Ther. 2013, 37, 252–262. [Google Scholar] [CrossRef]
- Leffler, D.A.; Kelly, C.P.; Abdallah, H.Z.; Colatrella, A.M.; Harris, L.A.; Leon, F.; Arterburn, L.A.; Paterson, B.M.; Lan, Z.H.; Murray, J.A. A randomized, double-blind study of larazotide acetate to prevent the activation of celiac disease during gluten challenge. Am. J. Gastroenterol. 2012, 107, 1554–1562. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gatti, S.; Caporelli, N.; Galeazzi, T.; Francavilla, R.; Barbato, M.; Roggero, P.; Malamisura, B.; Iacono, G.; Budelli, A.; Gesuita, R.; et al. Oats in the Diet of Children with Celiac Disease: Preliminary Results of a Double-Blind, Randomized, Placebo-Controlled Multicenter Italian Study. Nutrients 2013, 5, 4653-4664. https://doi.org/10.3390/nu5114653
Gatti S, Caporelli N, Galeazzi T, Francavilla R, Barbato M, Roggero P, Malamisura B, Iacono G, Budelli A, Gesuita R, et al. Oats in the Diet of Children with Celiac Disease: Preliminary Results of a Double-Blind, Randomized, Placebo-Controlled Multicenter Italian Study. Nutrients. 2013; 5(11):4653-4664. https://doi.org/10.3390/nu5114653
Chicago/Turabian StyleGatti, Simona, Nicole Caporelli, Tiziana Galeazzi, Ruggiero Francavilla, Maria Barbato, Paola Roggero, Basilio Malamisura, Giuseppe Iacono, Andrea Budelli, Rosaria Gesuita, and et al. 2013. "Oats in the Diet of Children with Celiac Disease: Preliminary Results of a Double-Blind, Randomized, Placebo-Controlled Multicenter Italian Study" Nutrients 5, no. 11: 4653-4664. https://doi.org/10.3390/nu5114653
APA StyleGatti, S., Caporelli, N., Galeazzi, T., Francavilla, R., Barbato, M., Roggero, P., Malamisura, B., Iacono, G., Budelli, A., Gesuita, R., Catassi, C., & Lionetti, E. (2013). Oats in the Diet of Children with Celiac Disease: Preliminary Results of a Double-Blind, Randomized, Placebo-Controlled Multicenter Italian Study. Nutrients, 5(11), 4653-4664. https://doi.org/10.3390/nu5114653







