Exclusive Breastfeeding and Developmental and Behavioral Status in Early Childhood
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Selected Measures of Developmental and Behavioral Status
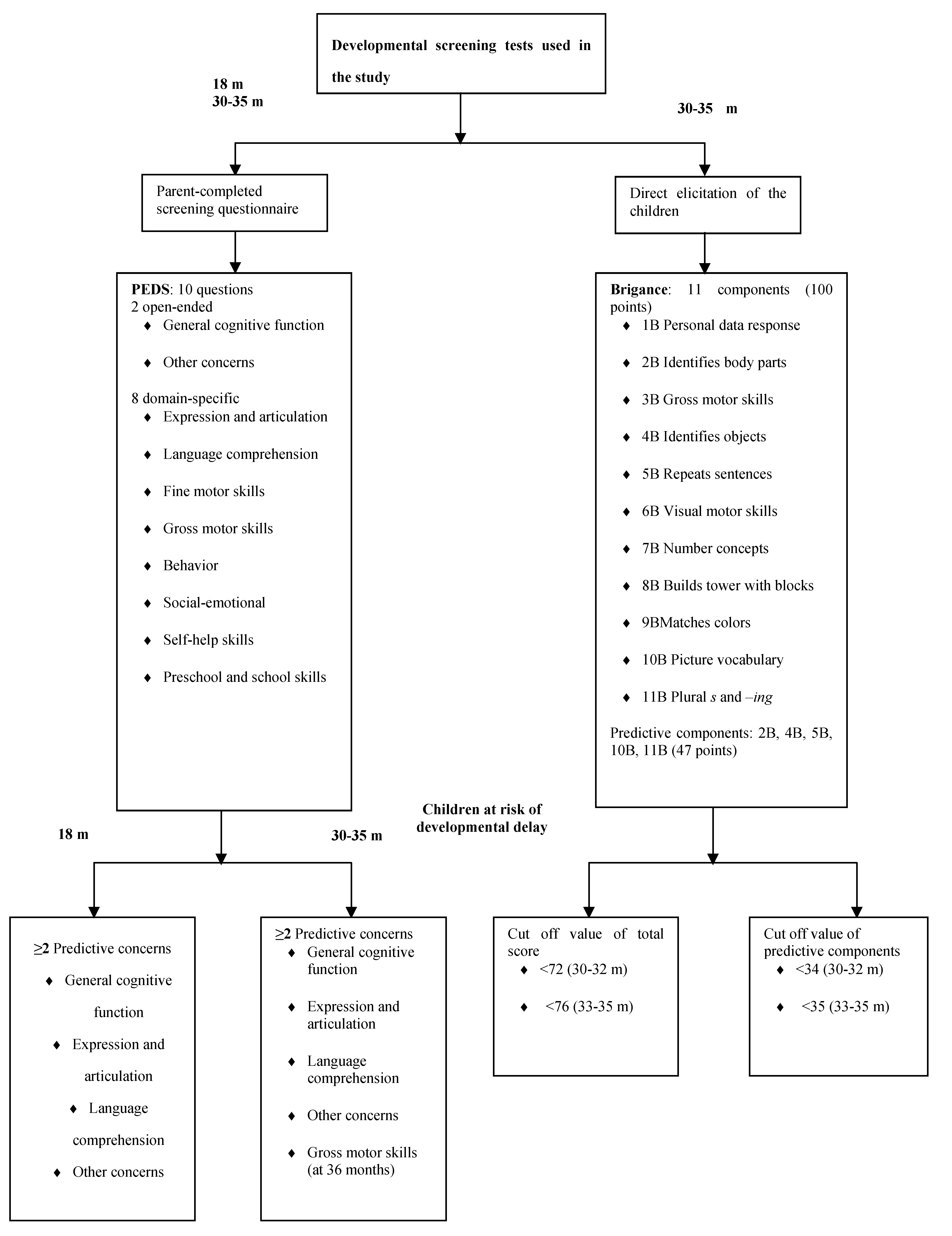
2.3. Statistical Analysis
3. Results
3.1. Sample Size and Characteristics of Participants
| Variables | Group CF | Group EBF |
|---|---|---|
| Boys | 17 (49%) * | 13 (42%) * |
| Birth weight (g) | 3687 (432) | 3733 (526) |
| Length at birth (cm) | 51.3 (1.8) | 51.7 (1.9) |
| Head circumference at birth (cm) | 35.8 (1.3) † | 35.9 (1.4) |
| Gain in head circumference from birth–18 months (cm) | 12.6 (1.2) ‡ | 12.6 (1.7) § |
| Age when Brigance Screens-II was performed (months) | 32.3 (1.6) | 32.8 (1.6) |
| Gestational length (days) | 280.5 (9.3) | 280.8 (7.1) |
| Maternal age (years) | 29.4 (4.4) | 31.2 (4.8) |
| Maternal education ║ | 22 (63%) * | 16 (52%) * |
| Vaginal delivery | 33 (94%) * | 23 (74%) * |
| Parity | 2.0 (2.0) ¶ | 2.0 (1.0) ¶ |
| Father’s education ║ | 13 (38%) *‡ | 14 (45%) * |
3.2. Developmental and Behavioral Status
| Variables | Group CF | Group EBF | P-value |
|---|---|---|---|
| PEDS questionnaire | |||
| Parents with concerns according to PEDS at 18 months | 5 (17%) *; n = 29 | 11 (44%) *; n = 25 | 0.03 |
| Parents with concerns according to PEDS at 30–35 months | 14 (33%) *; n = 42 | 15 (42%) *; n = 36 | 0.45 |
| Brigance Screens-II | n = 35 | n = 31 | |
| Total score at 30–35 months | 86.0 (12.5) † | 86.5 (12.5) † | 0.82 |
| Total score above cut off value ‡ | 2 (6%) * | 4 (13%) * | 0.41 |
| Score of predictive factors combined above cut off value § | 7 (20%) * | 3 (10%) *║ | 0.32 |
| Components of the Brigance Screens-II | |||
| Gross motor skills | 6.0 (6.0) † | 6.0 (4.5) †║ | 0.44 |
| Fine motor skills | 19.0 (3.0) † | 19.0 (3.0) †║ | 0.89 |
| Expressive and receptive language | 40.5 (8.0) † | 42.0 (9.5) †║ | 0.81 |
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Horta, B.L.; Bahl, R.; Martinés, J.C.; Victora, C.G. Evidence on the Long-Term Effects of Breastfeeding: Systematic Reviews and Meta-Analyses; World Health Organization: Geneva, Switzerland, 2007; pp. 11–39. [Google Scholar]
- Schack-Nielsen, L.; Michaelsen, K.F. Advances in our understanding of the biology of human milk and its effects on the offspring. J. Nutr. 2007, 137, 503S–510S. [Google Scholar]
- Ip, S.; Chung, M.; Raman, G.; Chew, P.; Magula, N.; DeVine, D.; Trikalinos, T.; Lau, J. Breastfeeding and maternal and infant health outcomes in developed countries. Evid. Rep. Technol. Assess. 2007, 153, 1–186. [Google Scholar]
- Kramer, M.S.; Aboud, F.; Mironova, E.; Vanilovich, I.; Platt, R.W.; Matush, L.; Igumnov, S.; Fombonne, E.; Bogdanovich, N.; Ducruet, T.; et al. Breastfeeding and child cognitive development: New evidence from a large randomized trial. Arch. Gen. Psychiatry 2008, 65, 578–584. [Google Scholar] [CrossRef]
- Jedrychowski, W.; Perera, F.; Jankowski, J.; Butscher, M.; Mroz, E.; Flak, E.; Kaim, I.; Lisowska-Miszczyk, I.; Skarupa, A.; Sowa, A. Effect of exclusive breastfeeding on the development of children’s cognitive function in the Krakow prospective birth cohort study. Eur. J. Pediatr. 2012, 171, 151–158. [Google Scholar] [CrossRef]
- Rao, M.R.; Hediger, M.L.; Levine, R.J.; Naficy, A.B.; Vik, T. Effect of breastfeeding on cognitive development of infants born small for gestational age. Acta Paediatr. 2002, 91, 267–274. [Google Scholar] [CrossRef]
- Oddy, W.H.; Kendall, G.E.; Li, J.; Jacoby, P.; Robinson, M.; de Klerk, N.H.; Silburn, S.R.; Zubrick, S.R.; Landau, L.I.; Stanley, F.J. The long-term effects of breastfeeding on child and adolescent mental health: A pregnancy cohort study followed for 14 years. J. Pediatr. 2010, 156, 568–574. [Google Scholar] [CrossRef]
- Halpern, R.; Giugliani, E.R.; Victora, C.G.; Barros, F.C.; Horta, B.L. Risk factors for suspicion of developmental delays at 12 months of age. J. Pediatr. (Rio.J.) 2000, 76, 421–428. [Google Scholar] [CrossRef]
- Paterson, J.; Iusitini, L.; Gao, W. Child developmental assessment at two-years of age: Data from the Pacific Islands Families Study. Pac. Health Dialog. 2011, 17, 51–63. [Google Scholar]
- Kramer, M.S.; Fombonne, E.; Igumnov, S.; Vanilovich, I.; Matush, L.; Mironova, E.; Bogdanovich, N.; Tremblay, R.E.; Chalmers, B.; Zhang, X.; et al. Effects of prolonged and exclusive breastfeeding on child behavior and maternal adjustment: Evidence from a large, randomized trial. Pediatrics 2008, 121, e435–e440. [Google Scholar] [CrossRef]
- Kramer, M.S.; Kakuma, R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst. Rev. 2012, 8, CD003517. [Google Scholar]
- Chiu, W.C.; Liao, H.F.; Chang, P.J.; Chen, P.C.; Chen, Y.C. Duration of breast feeding and risk of developmental delay in Taiwanese children: A nationwide birth cohort study. Paediatr. Perinat. Epidemiol. 2011, 25, 519–527. [Google Scholar] [CrossRef]
- Whitehouse, A.J.; Robinson, M.; Li, J.; Oddy, W.H. Duration of breastfeeding and language ability in middle childhood. Paediatr. Perinat. Epidemiol. 2011, 25, 44–52. [Google Scholar] [CrossRef]
- Quinn, P.J.; O’Callaghan, M.; Williams, G.M.; Najman, J.M.; Andersen, M.J.; Bor, W. The effect of breastfeeding on child development at 5 years: A cohort study. J. Paediatr. Child Health 2001, 37, 465–469. [Google Scholar] [CrossRef]
- Tozzi, A.E.; Bisiacchi, P.; Tarantino, V.; Chiarotti, F.; D’Elia, L.; de Mei, B.; Romano, M.; Gesualdo, F.; Salmaso, S. Effect of duration of breastfeeding on neuropsychological development at 10 to 12 years of age in a cohort of healthy children. Dev. Med. Child Neurol. 2012, 54, 843–848. [Google Scholar] [CrossRef]
- Dee, D.L.; Li, R.; Lee, L.C.; Grummer-Strawn, L.M. Associations between breastfeeding practices and young children’s language and motor skill development. Pediatrics 2007, 119, S92–S98. [Google Scholar] [CrossRef]
- Vestergaard, M.; Obel, C.; Henriksen, T.B.; Sørensen, H.T.; Skajaa, E.; Ostergaard, J. Duration of breastfeeding and developmental milestones during the latter half of infancy. Acta Paediatr. 1999, 88, 1327–1332. [Google Scholar] [CrossRef]
- Thorsdottir, I.; Gunnarsdottir, I.; Kvaran, M.A.; Gretarsson, S.J. Maternal body mass index, duration of exclusive breastfeeding and children’s developmental status at the age of 6 years. Eur. J. Clin. Nutr. 2005, 59, 426–431. [Google Scholar] [CrossRef]
- Fewtrell, M.S.; Morgan, J.B.; Duggan, C.; Gunnlaugsson, G.; Hibberd, P.L.; Lucas, A.; Kleinman, R.E. Optimal duration of exclusive breastfeeding: What is the evidence to support current recommendations? Am. J. Clin. Nutr. 2007, 85, 635S–638S. [Google Scholar]
- World Health Organization (WHO). Infant and Young Child Nutrition. Global Strategy on Infant and Young Child Feeding. Report by the Secretariat. In Proceedings of the 55th World Health Assembly A55/15 Provisional Agenda Item 13.10, Geneva, Switzerland, 16 April 2002.
- World Health Organization (WHO). Nutrition: Information and attitudes among health personnel about early infant-feeding practices. WHO Wkly. Epidem. Rec. 1995, 70, 117–120. [Google Scholar]
- Wells, J.C.; Jonsdottir, O.H.; Hibberd, P.L.; Fewtrell, M.S.; Thorsdottir, I.; Eaton, S.; Lucas, A.; Gunnlaugsson, G.; Kleinman, R.E. Randomized controlled trial of 4 compared with 6 mo of exclusive breastfeeding in Iceland: Differences in breast-milk intake by stable-isotope probe. Am. J. Clin. Nutr. 2012, 96, 73–79. [Google Scholar] [CrossRef]
- Jonsdottir, O.H.; Thorsdottir, I.; Hibberd, P.L.; Fewtrell, M.S.; Wells, J.C.; Palsson, G.I.; Lucas, A.; Gunnlaugsson, G.; Kleinman, R.E. Timing of the introduction of complementary foods in infancy: A randomized controlled trial. Pediatrics 2012, 130, 1038–1045. [Google Scholar] [CrossRef]
- Directorate of Health. Brjóstagjöf og næring ungbarna á Íslandi sem fædd eru 2004–2008 (Breastfeeding and Infant Nutrition among Infants Born 2004–2008 in Iceland). 2012. Available online: http://www.landlaeknir.is/servlet/file/store93/item16573/version4/brjostagjof_og_naering_2004-2008_juni.2012.pdf (accessed on 13 August 2013).
- WHO. Indicators for Assessing Breastfeeding Practices: Report of an Informal Meeting; Division of Child and Adolescent Health, WHO/CDD/SER/91.14.ed., World Health Organization: Geneva, Switzerland, 1991; p. 14. [Google Scholar]
- Glascoe, F.P. Technical Report for the BRIGANCE SCREENS; Educational Testing Institute and Directorate of Health: Reykjavik, Iceland, 2008.
- Glascoe, F.P. PEDS, Mat Foreldra á Þroska Barna (Parental Perception on Their Child’s Developmental Outcomes); Educational Testing Institute and Directorate of Health: Reykjavik, Iceland, 2009.
- Gunnlaugsson, G.; Saemundsson, E. Að Finna Frávik í Þroska og Hegðun 5 Ára Barna (Detection of Deviation in Development and Behaviour of Five-Year Old Children). In Ungir Íslendingar í Ljósi Vísindanna; Children’s Ombudsman and University of Iceland: Reykjavik, Iceland, 2005; pp. 237–245. [Google Scholar]
- Glascoe, F.P. Using parents’ concerns to detect and address developmental and behavioral problems. J. Soc. Pediatr. Nurs. 1999, 4, 24–35. [Google Scholar] [CrossRef]
- Glascoe, F.P. Detecting and addressing developmental and behavioral problems in primary care. Pediatr. Nurs. 2000, 26, 251–257. [Google Scholar]
- Glascoe, F.P. Evidence-based approach to developmental and behavioural surveillance using parents’ concerns. Child. Care Health Dev. 2000, 26, 137–149. [Google Scholar] [CrossRef]
- Rydz, D.; Shevell, M.I.; Majnemer, A.; Oskoui, M. Developmental screening. J. Child Neurol. 2005, 20, 4–21. [Google Scholar] [CrossRef]
- Brothers, K.B.; Glascoe, F.P.; Robertshaw, N.S. PEDS: Developmental milestones—An accurate brief tool for surveillance and screening. Clin. Pediatr. (Phila.) 2008, 47, 271–279. [Google Scholar] [CrossRef]
- Glascoe, F.P. Screening for developmental and behavioral problems. Ment. Retard. Dev. Disabil. Res. Rev. 2005, 11, 173–179. [Google Scholar] [CrossRef]
- Glascoe, F.P. Are overreferrals on developmental screening tests really a problem? Arch. Pediatr. Adolesc. Med. 2001, 155, 54–59. [Google Scholar] [CrossRef]
- Glascoe, F.P. The brigance infant and toddler screen: Standardization and validation. J. Dev. Behav. Pediatr. 2002, 23, 145–150. [Google Scholar] [CrossRef]
- Directorate of Health; Primary Health Care Organisation of Greater Reykjavik Area. Ung- Og Smábarnavernd. Leiðbeiningar um Heilsuvernd Barna 0–5 Ára (Preventive Child Health Services: Guidelines for Preventive Health Services for Children 0–5 Years of Age). Gudmundsdóttir, S., Aradóttir, A.B., Gunnlaugsson, G., Eds.; The Organisations: Reykjavík, Iceland, 2010. Available online: http://www.landlaeknir.is/servlet/file/store93/item21268/2ungbarnavernd_leidbeiningar_ 12.05.13.pdf (accessed on 13 August 2013).
- American Academy of Pediatrics, Council on Children With Disabilities; Section on Developmental Behavioral Pediatrics; Bright Futures Steering Committee; Medical Home Initiatives for Children With Special Needs Project Advisory Committee. Identifying infants and young children with developmental disorders in the medical home: An algorithm for developmental surveillance and screening. Pediatrics 2006, 118, 405–420. [Google Scholar] [CrossRef]
- Glascoe, F.P.; Squires, J. Issues with the new developmental screening and surveillance policy statement. Pediatrics 2007, 119, 861–862, discussion 862–863. [Google Scholar] [CrossRef]
- Anderson, L.M.; Shinn, C.; Fullilove, M.T.; Scrimshaw, S.C.; Fielding, J.E.; Normand, J.; Carande-Kulis, V.G. The effectiveness of early childhood development programs: A systematic review. Am. J. Prev. Med. 2003, 24, 32–46. [Google Scholar] [CrossRef]
- Glascoe, F.P. Parents’ evaluation of developmental status: How well do parents’ concerns identify children with behavioral and emotional problems? Clin. Pediatr. (Phila.) 2003, 42, 133–138. [Google Scholar] [CrossRef]
- Sices, L.; Stancin, T.; Kirchner, L.; Bauchner, H. PEDS and ASQ developmental screening tests may not identify the same children. Pediatrics 2009, 124, e640–e647. [Google Scholar] [CrossRef]
- Limbos, M.M.; Joyce, D.P. Comparison of the ASQ and PEDS in screening for developmental delay in children presenting for primary care. J. Dev. Behav. Pediatr. 2011, 32, 499–511. [Google Scholar] [CrossRef]
- Kramer, M.S.; Chalmers, B.; Hodnett, E.D.; Sevkovskaya, Z.; Dzikovich, I.; Shapiro, S.; Collet, J.P.; Vanilovich, I.; Mezen, I.; Ducruet, T.; et al. Promotion of Breastfeeding Intervention Trial (PROBIT): A Randomized Trial in the Republic of Belarus. J. Am. Med. Assoc. 2001, 285, 413–420. [Google Scholar] [CrossRef]
- Zhou, S.J.; Baghurst, P.; Gibson, R.A.; Makrides, M. Home environment, not duration of breast-feeding, predicts intelligence quotient of children at four years. Nutrition 2007, 23, 236–241. [Google Scholar] [CrossRef]
- Quigley, M.A.; Hockley, C.; Carson, C.; Kelly, Y.; Renfrew, M.J.; Sacker, A. Breastfeeding is associated with improved child cognitive development: A population-based cohort study. J. Pediatr. 2012, 160, 25–32. [Google Scholar] [CrossRef]
- Anderson, J.W.; Johnstone, B.M.; Remley, D.T. Breast-feeding and cognitive development: A meta-analysis. Am. J. Clin. Nutr. 1999, 70, 525–535. [Google Scholar]
- Fewtrell, M.S.; Morley, R.; Abbott, R.A.; Singhal, A.; Isaacs, E.B.; Stephenson, T.; MacFadyen, U.; Lucas, A. Double-blind, randomized trial of long-chain polyunsaturated fatty acid supplementation in formula fed to preterm infants. Pediatrics 2002, 110, 73–82. [Google Scholar] [CrossRef]
- Slykerman, R.F.; Thompson, J.M.; Becroft, D.M.; Robinson, E.; Pryor, J.E.; Clark, P.M.; Wild, C.J.; Mitchell, E.A. Breastfeeding and intelligence of preschool children. Acta Paediatr. 2005, 94, 832–837. [Google Scholar] [CrossRef]
- Koletzko, B.; Brands, B.; Poston, L.; Godfrey, K.; Demmelmair, H. Early nutrition programming of long-term health. Proc. Nutr. Soc. 2012, 71, 371–378. [Google Scholar] [CrossRef]
- Turck, D. Later effects of breastfeeding practice: The evidence. Nestle Nutr. Workshop Ser. Pediatr. Program. 2007, 60, 31–39, discussion 39–42. [Google Scholar] [CrossRef]
- Wu, T.C.; Chen, P.H. Health consequences of nutrition in childhood and early infancy. Pediatr. Neonatol. 2009, 50, 135–142. [Google Scholar] [CrossRef]
- Helland, I.B.; Smith, L.; Saarem, K.; Saugstad, O.D.; Drevon, C.A. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics 2003, 111, e39–e44. [Google Scholar] [CrossRef]
- Mitmesser, S.H.; Jensen, C.L. Roles of long-chain polyunsaturated fatty acids in the term infant: Developmental benefits. Neonatal Netw. 2007, 26, 229–234. [Google Scholar] [CrossRef]
- Koletzko, B.; Rodriguez-Palmero, M. Polyunsaturated fatty acids in human milk and their role in early infant development. J. Mammary Gland Biol. Neoplasia 1999, 4, 269–284. [Google Scholar] [CrossRef]
- Lanting, C.I.; Fidler, V.; Huisman, M.; Touwen, B.C.; Boersma, E.R. Neurological differences between 9-year-old children fed breast-milk or formula-milk as babies. Lancet 1994, 344, 1319–1322. [Google Scholar] [CrossRef]
- Butte, N.F.; Lopez-Alarcon, M.G.; Garza, C. Nutrient Adequacy of Exclusive Breastfeeding for the Term Infant during the First Six Months of Life; World Health Organisation: Geneva, Switzerland, 2002; pp. 8–14. [Google Scholar]
- Fewtrell, M.S.; Kennedy, K.; Singhal, A.; Martin, R.M.; Ness, A.; Hadders-Algra, M.; Koletzko, B.; Lucas, A. How muchloss to follow-up is acceptable in long-term randomised trials and prospective studies? Arch. Dis. Child. 2008, 93, 458–461. [Google Scholar] [CrossRef]
- Iannotti, L.L.; Tielsch, J.M.; Black, M.M.; Black, R.E. Iron supplementation in early childhood: Health benefits and risks. Am. J. Clin. Nutr. 2006, 84, 1261–1276. [Google Scholar]
- Gunnarsson, B.S.; Thorsdottir, I.; Palsson, G.; Gretarsson, S.J. Iron status at 1 and 6 years versus developmental scores at 6 years in a well-nourished affluent population. Acta Paediatr. 2007, 96, 391–395. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jonsdottir, O.H.; Thorsdottir, I.; Gunnlaugsson, G.; Fewtrell, M.S.; Hibberd, P.L.; Kleinman, R.E. Exclusive Breastfeeding and Developmental and Behavioral Status in Early Childhood. Nutrients 2013, 5, 4414-4428. https://doi.org/10.3390/nu5114414
Jonsdottir OH, Thorsdottir I, Gunnlaugsson G, Fewtrell MS, Hibberd PL, Kleinman RE. Exclusive Breastfeeding and Developmental and Behavioral Status in Early Childhood. Nutrients. 2013; 5(11):4414-4428. https://doi.org/10.3390/nu5114414
Chicago/Turabian StyleJonsdottir, Olof H., Inga Thorsdottir, Geir Gunnlaugsson, Mary S. Fewtrell, Patricia L. Hibberd, and Ronald E. Kleinman. 2013. "Exclusive Breastfeeding and Developmental and Behavioral Status in Early Childhood" Nutrients 5, no. 11: 4414-4428. https://doi.org/10.3390/nu5114414
APA StyleJonsdottir, O. H., Thorsdottir, I., Gunnlaugsson, G., Fewtrell, M. S., Hibberd, P. L., & Kleinman, R. E. (2013). Exclusive Breastfeeding and Developmental and Behavioral Status in Early Childhood. Nutrients, 5(11), 4414-4428. https://doi.org/10.3390/nu5114414




