Changes in the Amino Acid Composition of Bogue (Boops boops) Fish during Storage at Different Temperatures by 1H-NMR Spectroscopy
Abstract
:1. Introduction
2. Experimental Section
2.1. Sampling
2.2. Sample Preparation for 1H-NMR Analysis
2.3. 1H-NMR Measurements
3. Results and Discussion
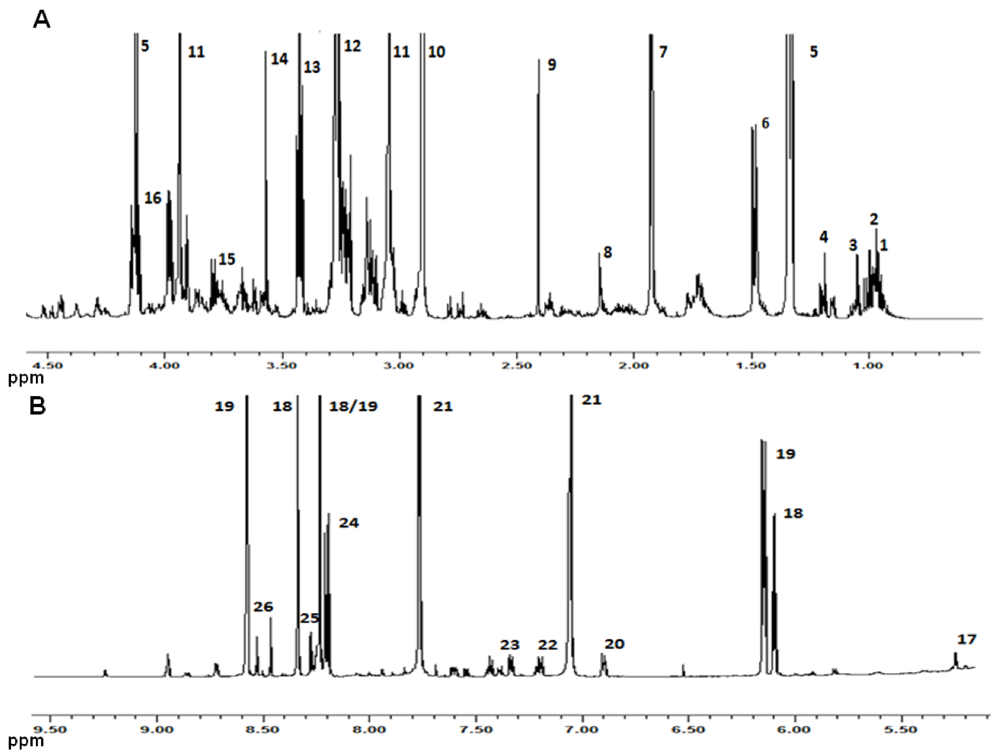
| Compound | Assignment | 1H (ppm) | Multiplicity |
|---|---|---|---|
| Isoleucine (Ile) 1 | δ-CH3 | 0.94 | t |
| Leucine (Leu) 2 | δ′-CH3 | 0.96 | d |
| Valine (Val) 3 | γ-CH3-γ′-CH3 | 1.00–1.05 | dd |
| Ethanol 4 | CH3 | 1.19 | t |
| Lactate (La) 5 | β-CH3 | 1.33 | d |
| Alanine (Ala) 6 | β-CH3 | 1.49 | d |
| Acetate 7 | CH3 | 1.93 | s |
| Methionine (Met) 8 | S–CH3 | 2.14 | s |
| Succinate 9 | α,β-CH2 | 2.41 | s |
| Trimethylamine (N-TMA) 10 | N–CH3 | 2.90 | s |
| Creatine/Phosphocreatine 11 | N–CH3 and N=C | 3.04 | s |
| Oxide Trimethylamine (N-TMAO) 12 | N–CH3 | 3.27 | S |
| Taurin (Tau) 13 | N–CH2 | 3.42 | t |
| Glycine (Gly) 14 | α-CH | 3.56 | s |
| Glutamate (Glu) 15 | α-CH | 3.75 | t |
| Creatine/Phosphocreatine 11 | N–CH2 | 3.94 | s |
| Serine (Ser) 16 | β-CH | 3.98 | dd |
| α-Glucose (α-GLC) 17 | CH-1 | 5.24 | d |
| Inosine (HxR) 18 | CH-1′, ribose | 6.10 | d |
| Inosine 5′-monophosphate (IMP) 19 | CH-1′, ribose | 6.14 | d |
| Tyrosine (Tyr) 20 | C3,5H, ring | 6.88 | d |
| Histidine (His) 21 | C2H ring/C4H ring | 7.06/7.77 | s |
| Tryptophan (Trp) 22 | C5H ring | 7.19 | t |
| Phenylalanine (Phe) 23 | CH-2,6 | 7.32 | m |
| Hypoxanthine (Hx) 24 | CH-8 | 8.19 | s |
| Hypoxanthine (Hx) 24 | CH-2 | 8.21 | s |
| Inosine (HxR) 18 | CH-8 | 8.233 | s |
| Inosine 5′-monophosphate (IMP) 19 | CH-8 | 8.236 | s |
| Adenosine 5′-triphosphate (ATP) 25 | CH-8 | 8.27 | s |
| Adenosine 5′-diphosphate (ADP) 25 | |||
| Adenosine 5′-monophosphate (AMP) 25 | |||
| Inosine (HxR) 18 | CH2, ring | 8.33 | s |
| Formate (Fo) 26 | CH | 8.46 | s |
| Inosine 5′-monophosphate (IMP) 19 | CH2, ring | 8.57 | s |

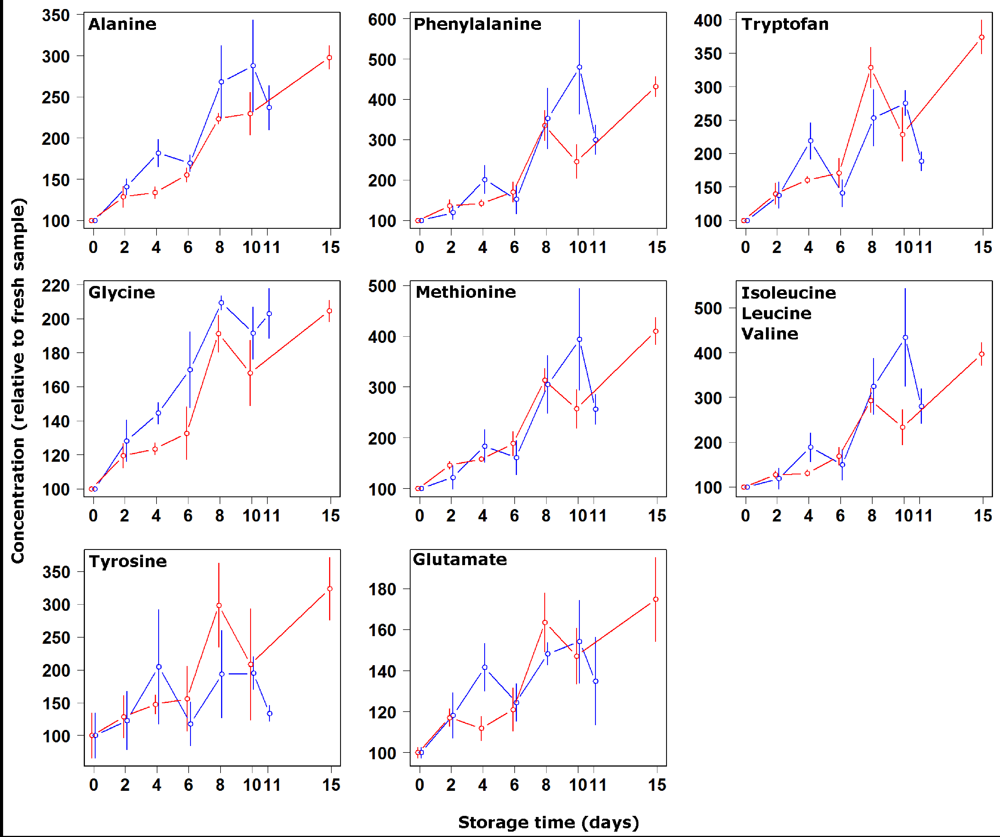
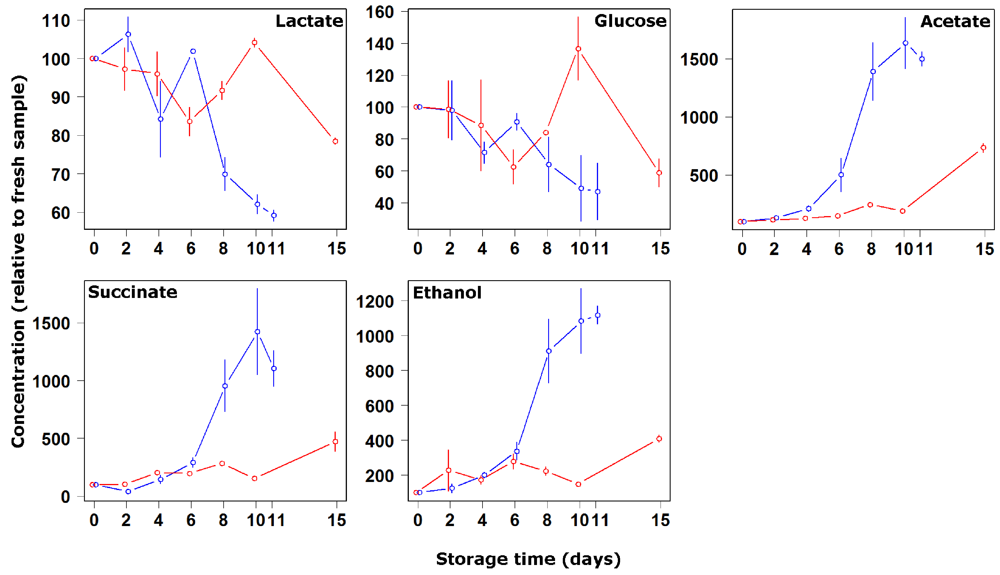
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Bramstedt, F. Amino acid composition of fresh fish and influence of storage and processing. In Fish in Nutrition; Heen, E., Kreuzer, R., Eds.; Fishing News (Books) Ltd.: London, UK, 1962; pp. 61–67. [Google Scholar]
- Connell, J.J.; Howgate, P.F. The amino-acid composition of some british food fishes. J. Sci. Food Agric. 1959, 10, 241–244. [Google Scholar]
- Mukundan, M.K.; Antony, P.D.; Nair, M.R. A review on autolysis in fish. Fish Res. 1986, 4, 259–269. [Google Scholar] [CrossRef]
- Voskresensky, N.A. Fish as Food; Academic Press: New York, NY, USA, 1965; Volume 3, pp. 107–131. [Google Scholar]
- Gram, L.; Huss, H.H. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 1996, 33, 121–137. [Google Scholar] [CrossRef]
- Mendes, R.; Quinta, R.; Nunes, M.L. Changes in baseline levels of nucleotides during ice storage of fish and crustaceans from the portuguese coast. Eur. Food Res. Technol. 2001, 212, 141–146. [Google Scholar] [CrossRef]
- Márquez-Ríos, E.; Morán-Palacio, E.F.; Lugo-Sánchez, M.E.; Ocano-Higuera, V.M.; Pacheco-Aguilar, R. Postmortem biochemical behavior of giant squid (Dosidicus gigas) mantle muscle stored in ice and its relation with quality parameters. J. Food Sci. 2007, 72, C356–C362. [Google Scholar] [CrossRef]
- Saito, T.; Arai, K.; Matsuyoshi, M. A new method for estimating the freshness of fish. Bull. Jpn. Soc. Sci. Fish. 1959, 24, 749–750. [Google Scholar] [CrossRef]
- Capozzi, F.; Cremonini, M.A. Nuclear magnetic resonance spectroscopy in food analysis. In Handbook of Food Analysis Instruments; Otles, S., Ed.; CRC Press: Boca Roton, FL, USA, 2008; pp. 281–318. [Google Scholar]
- Picone, G.; Balling Engelsen, S.; Savorani, F.; Testi, S.; Badiani, A.; Capozzi, F. Metabolomics as a powerful tool for molecular quality assessment of the fish Sparus aurata. Nutrients 2011, 3, 212–227. [Google Scholar] [CrossRef]
- Savorani, F.; Picone, G.; Badiani, A.; Fagioli, P.; Capozzi, F.; Engelsen, S.B. Metabolic profiling and aquaculture differentiation of gilthead sea bream by 1H-NMR metabonomics. Food Chem. 2010, 120, 907–914. [Google Scholar] [CrossRef]
- Nørgaard, L.; Bro, R.; Westad, F.; Engelsen, S.B. A modification of canonical variates analysis to handle highly collinear multivariate data. J. Chemom. 2006, 20, 425–435. [Google Scholar] [CrossRef]
- Capozzi, F.; Ciampa, A.; Picone, G.; Placucci, G.; Savorani, F. Normalization is a Necessary Step in NMR Data Processing: Finding the Right Scale Factors. In Magnetic Resonance in Food Science: An Exciting Future; Renou, J.P., Belton, P.S., Webb, G.A., Eds.; CRC Press: Cambridge, UK, 2011; pp. 147–160. [Google Scholar]
- Özogul, Y.; Özogul, F. Fatty acid profiles of commercially important fish species from the mediterranean, aegean and black seas. Food Chem. 2007, 100, 1634–1638. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Nychas, G.-J.E. Chemical and sensory changes associated with microbial flora of mediterranean boque (Boops boops) stored aerobically at 0, 3, 7, and 10 °C. Appl. Environ. Microbiol. 1999, 65, 698–706. [Google Scholar]
- Boland, F.E. Trimethylammine nitrogen in seadfood method colorimetric. J. Assoc. Off. Anal. Chem. 1971, 54, 9. [Google Scholar]
- Bordoni, A.; Picone, G.; Babini, E.; Vignali, M.; Danesi, F.; Valli, V.; Di Nunzio, M.; Laghi, L.; Capozzi, F. NMR comparison of in vitro digestion of Parmigiano reggiano cheese aged 15 and 30 months. Magn. Reson. Chem. 2011, 49, S61–S70. [Google Scholar]
- Burton, I.W.; Quilliam, M.A.; Walter, J.A. Quantitative 1H-NMR with external standards: Use in preparation of calibration solutions for algal toxins and other natural products. Anal. Chem. 2005, 77, 3123–3131. [Google Scholar] [CrossRef]
- Ndagijimana, M.; Laghi, L.; Vitali, B.; Placucci, G.; Brigidi, P.; Guerzoni, M.E. Effect of a synbiotic food consumption on human gut metabolic profiles evaluated by 1 h nuclear magnetic resonance spectroscopy. Int. J. Food Microbiol. 2009, 134, 147–153. [Google Scholar] [CrossRef]
- Craig, A.; Cloarec, O.; Holmes, E.; Nicholson, J.K.; Lindon, J.C. Scaling and normalization effects in NMR spectroscopic metabonomic data sets. Anal. Chem. 2006, 78, 2262–2267. [Google Scholar] [CrossRef]
- Kriat, M.; Confort-Gouny, S.; Vion-Dury, J.; Sciaky, M.; Viout, P.; Cozzone, P.J. Quantitation of metabolites in human blood serum by proton magnetic resonance spectroscopy. A comparative study of the use of formate and tsp as concentration standards. NMR Biomed. 1992, 5, 179–184. [Google Scholar] [CrossRef]
- Jiang, S.T.; Lee, T.C. Changes in free amino acids and protein denaturation of fish muscle during frozen storage. J. Agric. Food Chem. 1985, 33, 839–844. [Google Scholar] [CrossRef]
- Je, J.-Y.; Park, P.-J.; Jung, W.-K.; Kim, S.-K. Amino acid changes in fermented oyster (Crassostrea gigas) sauce with different fermentation periods. Food Chem. 2005, 91, 15–18. [Google Scholar] [CrossRef]
- Ababouch, L.; Afilal, M.E.; Benabdeljelil, H.; Busta, F.F. Quantitative changes in bacteria, amino acids and biogenic amines in sardine (Sardina pilchardus) stored at ambient temperature (25–28 °C) and in ice. Int. J. Food Sci. Technol. 1991, 26, 297–306. [Google Scholar]
- Özden, Ö. Changes in amino acid and fatty acid composition during shelf-life of marinated fish. J. Sci. Food Agric. 2005, 85, 2015–2020. [Google Scholar] [CrossRef]
- Kiesvaara, M. On the Soluble Nitrogen Fraction of Barrel-Salted Herring and Semi-Preserves During Ripening; Valtion Teknillinen Tutkimuskeskus: Espoo, Finland, 1975. [Google Scholar]
- Love, R.M. The Chemical Biology of Fishes: Advances 1968–1977, with a Supplementary Key to the Chemical Literature; Academic Press: San Diego, CA, USA, 1980. [Google Scholar]
- Herbert, R.A.; Shewan, J.M. Precursors of the volatile sulphides in spoiling north sea cod (Gadus morhua). J. Sci. Food Agric. 1975, 26, 1195–1202. [Google Scholar] [CrossRef]
- Giannakourou, M.C.; Koutsoumanis, K.; Nychas, G.J.E.; Taoukis, P.S. Development and assessment of an intelligent shelf life decision system for quality optimization of the food chill chain. J. Food Prot. 2001, 64, 1051–1057. [Google Scholar]
- Dainty, R.H. Chemical/biochemical detection of spoilage. Int. J. Food Microbiol. 1996, 33, 19–33. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ciampa, A.; Picone, G.; Laghi, L.; Nikzad, H.; Capozzi, F. Changes in the Amino Acid Composition of Bogue (Boops boops) Fish during Storage at Different Temperatures by 1H-NMR Spectroscopy. Nutrients 2012, 4, 542-553. https://doi.org/10.3390/nu4060542
Ciampa A, Picone G, Laghi L, Nikzad H, Capozzi F. Changes in the Amino Acid Composition of Bogue (Boops boops) Fish during Storage at Different Temperatures by 1H-NMR Spectroscopy. Nutrients. 2012; 4(6):542-553. https://doi.org/10.3390/nu4060542
Chicago/Turabian StyleCiampa, Alessandra, Gianfranco Picone, Luca Laghi, Homa Nikzad, and Francesco Capozzi. 2012. "Changes in the Amino Acid Composition of Bogue (Boops boops) Fish during Storage at Different Temperatures by 1H-NMR Spectroscopy" Nutrients 4, no. 6: 542-553. https://doi.org/10.3390/nu4060542






