Long-Chain Omega-3 Oils–An Update on Sustainable Sources
Abstract
:1. Introduction
1.1. Long-Chain Omega-3 Oils–Definitions and Health Benefits
2. Current Sources of LC Omega-3
2.1. Suggested Intake of LC Omega-3
2.2. Contaminant Concerns
| Food | mg/150 g (wet weight 2) |
|---|---|
| Wild Australian seafood | |
| Fish | 350 |
| Shellfish | 225 |
| Prawns | 180 |
| Lobster | 160 |
| Farmed Australian fish | |
| Striped perch | 3,700 |
| Atlantic salmon | 2,985 |
| Barramundi | 2,960 |
| Silver perch | 1,200 |
| Other food groups | |
| Turkey | 40 |
| Beef | 40 |
| Chicken | 40 |
| Pork | 40 |
| Lamb | 30 |
| Marketing Name | Scientific Name | Oil (g/100g) | Total LC omega-3 1 (mg/150 g) |
|---|---|---|---|
| Slender tuna | Allothunnus fallai | 16.5 | 5,640 |
| Swordfish | Xiphias gladius | 7.7 | 1,530 |
| Escolar 2 | Ruvettus pretiosus | 17.8 | 1,530 |
| Banded morwong | Cheilodactylus spectablis | 3.2 | 1,230 |
| Alfonsino | Beryx splendens | 5.2 | 1,195 |
| Whitebait | Lovettia sealii | 2.6 | 1,100 |
| Escolar 2 | Lepidocybium flavobrunneum | 19.2 | 1,075 |
| Big-eye trevally | Caranx sexfasciatus | 4.7 | 1,065 |
| Whitebait | Galaxias maculatus | 3.3 | 1,030 |
| Blue mackerel | Scomber australasicus | 3.8 | 760 |
| Australian bonito | Sarda australis | 1.5 | 650 |
| Gemfish | Rexea solandri | 2.6 | 640 |
| Rudderfish | Centrolophus niger | 14.4 | 620 |
| Spanish mackerel | Scomberomorus commerson | 3 | 575 |
| Sweep | Scorpis lineolatus | 1.3 | 555 |
| Australian herring | Arripis georgianus | 1.7 | 540 |
| Western blue grouper | Achoerodus gouldii | 3.6 | 540 |
| Bigspine boarfish | Pentaceros decacanthus | 1.5 | 530 |
| Eastern Australian salmon | Arripis trutta | 1.1 | 505 |
| Spotted mackerel | Scomberomorus munroi | 1.2 | 500 |
| School mackerel | Scomberomorus queenslandicus | 1.1 | 490 |
| Grey mackerel | Scomberomorus semifasciatus | 1.1 | 490 |
| Tailor | Pomatomus saltatrix | 1.3 | 490 |
| Threadfin emperor | Lethrinus genivittatus | 2.6 | 490 |
| Bight redfish | Centroberyx gerrardi | 0.5 | 485 |
| Pilchard | Sardinops neopilchardus | 1.2 | 470 |
| Blue eye trevalla | Hyperoglyphe antarctica | 1.3 | 470 |
2.3. Sustainability of Current Sources of LC Omega-3
| Authority / Group | mg/day |
|---|---|
| Omega Workshop, Adelaide, Australia, 2002 [20] | 300–400 |
| SACN/COT, UK 2004 [21] | 450 |
| National Heart Foundation, 2008 [22] | 500 |
| American Dietetic Association and Dietitians of Canada, 2007 [23] | 500 |
| FAO/WHO Expert Consultation, 2008 [24] | 250–2,000* |
| American Heart Association, 2002 [25] | |
| Coronary Heart Disease sufferers | 1,000 |
| Those seeking to reduce triacylglycerols (blood fats) | 2,000–4,000 |
| Australia and New Zealand (suggested dietary targets), 2006 [26] | |
| Female | 430 |
| Male | 610 |
3. Fish farming: Current Status and Looking to the Future
4. New Alternate Sources of LC Omega-3
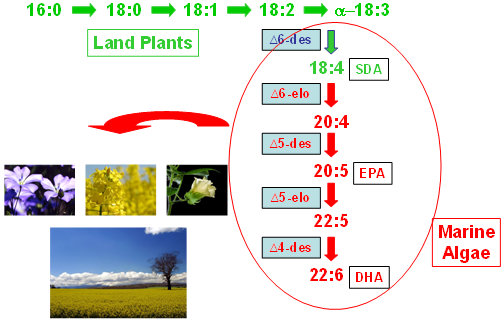
| Ref | SDA | EPA | ARA | DHA | |
|---|---|---|---|---|---|
| CSIRO: oilseeds (includes model plants) | [49] | 10 | |||
| [45] | 5 | 1 | |||
| [49] | 1 | 26 | 2 | ||
| [50] | 22 | ||||
| BASF: mustard | [46] | 15 | 7 | 1.5 | |
| Monsanto: soya bean | [51] | 20 | |||
| Dupont: soya bean | [47] | 20 | 3 | ||
| Farmed salmon | |||||
| Fed fish oil diet | [16,17] | 10 | 17 | ||
| Fed plant oil diet | [52] | 2.2 | 5 |
5. Conclusion
Acknowledgements
References
- Kinsella, J.E. Seafoods and fish oils in human health and disease; Marcel Dekker Inc.: New York, NY, USA, 1987; p. 317. [Google Scholar]
- Von Schacky, C. A review of omega-3 ethyl esters for cardiovascular prevention and treatment of increased blood triglyceride levels. Vasc. Health Risk Manag. 2006, 2, 251–262. [Google Scholar]
- Bang, H.O.; Dyerberg, J.E. Lipid metabolism and ischaemic heart disease in Greenland Eskimos. Adv. Nutr. Res. 1980, 3, 1–22. [Google Scholar]
- Bang, H.O.; Dyerberg, J.; Hjorne, N. The composition of food consumed by Greenland Eskimos. Acta Med. Scand. 1976, 200, 69–73. [Google Scholar]
- Carson, S.E. The role of PUFA in infant nutrition. INFORM 1995, 6, 940–946. [Google Scholar]
- Heird, W.C.; Prager, T.C.; Anderson, R.E. Docosahexaenoic acid and the development and function of the infant retina. Curr. Opin. Lipidol. 1997, 8, 12–16. [Google Scholar]
- Burdge, G.C.; Calder, P.C. Conversionof α-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005, 45, 581–597. [Google Scholar]
- Wang, C.; Harris, W.S.; Chung, M.; Lichtenstein, A.H.; Balk, E.M.; Kupelnick, B.; Jordan, H.S.; Lau, J. n-3 Fatty acids from fish or fish oil supplements, but notα-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am. J. Clin. Nutr. 2006, 84, 5–17. [Google Scholar] [PubMed]
- McLennan, P.L.; Bridle, T.M.; Abeywardena, M.Y.; Charnock, J.S. Dietary lipid modulation of ventricular fibrillation threshold in the marmoset monkey. Am. Heart J. 1992, 123, 1555–1561. [Google Scholar]
- McLennan, P.L.; Bridle, T.M.; Abeywardena, M.Y.; Charnock, J.S. Comparative efficacy of n-3 and n-6 polyunsaturated fatty acids in modulating ventricular fibrillation threshold in the marmoset monkey. Am. J. Clin. Nutr. 1993, 58, 666–669. [Google Scholar]
- Bays, H. Clinical overview of Omacor: a concentrated formulation of omega-3 polyunsaturated fatty acids. Am. J. Cardiol. 2006, 98, 71–76. [Google Scholar]
- Cleland, L.G.; James, M.J. Rheumatoid arthritis and the balance of dietary N-6 and N-3 essential fatty acids. British J. Rheumatology 1997, 36, 513–514. [Google Scholar]
- Parker, G.; Gibson, N.A.; Brotchie, H.; Heruc, G.; Rees, A.; Hadzi-Pavlovic, D. Omega-3 Fatty Acids and Mood Disorders. Am. J. Psychiatry 2006, 163, 969–978. [Google Scholar]
- Schaefer, E.J.; Bongard, V.; Beiser, A.S.; Lamon-Fava, S.; Robins, S.J.; Au, R.; Tucker, K.L.; Kyle, D.J.; Wilson, P.W.F.; Wolf, P.A. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch. Neurol. 2006, 63, 1545–1550. [Google Scholar]
- Freeman, M.P.; Hibbeln, J.R.; Wisner, K.L.; Davis, J.M.; Mischoulon, D.; Peet, M.; Keck, P.E.; Marangell, L.B.; Richardson, A.J.; Lake, J.; Stoll, A.L. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin. Psychiatry 2006, 67, 1954–1967. [Google Scholar]
- Nichols, P.D.; Virtue, P.; Mooney, B.D.; Elliott, N.G.; Yearsley, G.K. Seafood the Good Food. The oil content and composition of Australian commercial fishes, shellfishes and crustaceans. FRDC Project 95/122. Guide prepared for the Fisheries Research and Development Corporation, Australia; CSIRO: Hobart, Australia, 1998. [Google Scholar]
- Mooney, B.D.; Nichols, P.D; Elliott, N.G. Seafood the Good Food II. Oil profiles for further Australian seafoods, and influencing factors. FRDC Project 99/331. Guide prepared for the Fisheries Research and Development Corporation, Australia; CSIRO: Hobart, Australia, 2002. [Google Scholar]
- Howe, P.R.C.; Meyer, B.J.; Record, S.; Baghurst, K. Dietary intake of omega-3 polyunsaturated fatty acids: contribution of meat sources. Nutrition 2006, 22, 47–53. [Google Scholar]
- Hibbeln, J.R.; Davis, J.M; Steer, C.; Emmett, P.; Rogers, I.; Williams, C.; Golding, J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet 2007, 369, 578–585. [Google Scholar] [PubMed]
- Howe, P. The omega workshop: report and presentations. Food Australia 2002, 54, 505–512. [Google Scholar]
- SACN/COT. Scientific Advisory Committee on Nutrition (SACN) and Committee on Toxicity (COT), Advice on Fish Consumption: Benefits and Risks; TSO: Norwich, UK, 2004; p. 204.
- National Heart Foundation Australia. Position statement. Fish, fish oils, n-3 polyunsaturated fatty acids and cardiovascular health. 2008. Available online: http://www.heartfoundation.org.au/document/NHF/HW_FS_FishOils_PS_Final.pdf (accessed on 26 February 2010).
- American Dietetics Association and Dietitians of Canada. Position of the American Dietetics Association and Dietitians of Canada: Dietary fatty acids. J. Am. Dietetic Assoc. 2007, 107, 1599–1611.
- FAO/WHO. Interim summary of conclusions and dietary recommendations on total fat and fatty acids. In Proceedings of Joint FAO/WHO Expert Consultation on Fats and Fatty Acids in Human Nutriton, Geneva, Switzerland, November 10-14, 2008.
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. American Heart Association Committee. Fish consumption, fish oil, omega-3 fatty acids and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar]
- NHMRC, Nutrient Reference Values for Australia and New Zealand. Canberra, Commonwealth of Australia; NHMRC: Canberra, Australia, 2006.
- Myers, R.; Worm, B. Rapid worldwide depletion of predatory fish communities. Nature 2003, 423, 280–283. [Google Scholar]
- Polacheck, T. Tuna longline catch rates in the Indian Ocean: Did industrial fishing result in a 90% rapid decline in the abundance of large predatory species? Marine Policy 2006, 30, 470–482. [Google Scholar] [CrossRef]
- Worm, B.; Barbier, E.B.; Beaumont, N.; Duffy, J.E.; Folke, C.; Halpern, B.S.; Jackson, J.B.C.; Lotze, H.K.; Micheli, F.; Palumbi, S.R.; Sala, E.; Selkoe, K.A.; Stachowicz, J.J.; Watson, R. Impacts of Biodiversity Loss on Ocean Ecosystem Services. Science 2006, 313, 787–790. [Google Scholar]
- Worm, B.; Hilborn, R.; Baum, J.K.; Branch, T.A.; Collie, J.S.; Costello, C.; Fogarty, M.J.; Fulton, E.A.; Hutchings, J.A.; Jennings, S.; Jensen, O.P.; Lotze, H.K.; Mace, P.M.; McClanahan, T.R.; Minto, C.; Palumbi, S.R.; Parma, A.M.; Ricard, D.; Rosenberg, A.A.; Watson, R.; Zeller, D. Rebuilding Global Fisheries. Science 2009, 325, 578–585. [Google Scholar] [PubMed]
- Kearney, B.; Foran, B.; Poldy, F.; Lowe, D. Modelling Australia’s fisheries to 2050: policy and management implications; Fisheries Research and Development Corporation: Canberra, Australia, 2003; p. 38. [Google Scholar]
- Pitcher, T.; Kalikoski, D.; Pramod, G.; Short, K. Not honouring the code. Nature 2009, 457, 658–659. [Google Scholar]
- Tacon, A.G.J. Aquaculture production trends analysis. In Review of the state of world aquaculture, FAO Fisheries Circular; FAO: Rome, Italy, 2003; pp. 5–29. [Google Scholar]
- Nichols, P.D. Fish oil sources. In Long-chain omega-3 specialty oils; Brevik, H., Ed.; Oily Press: PJ Barnes & Associates, Bridgewater, England, 2007; pp. 23–42. [Google Scholar]
- Miller, M.R.; Nichols, P.D.; Carter, C.G. Omega 3 oil sources for use in aquaculture-alternatives to the unsustainable harvest of wild fish. Nutr. Res. Rev. 2008, 21, 85–96. [Google Scholar]
- Naylor, R.L.; Hardy, R.W.; Bureau, D.P.; Chiu, A.; Elliott, M.; Farrell, A.P.; Forster, I.; Gatlin, D.M.; Goldburg, R.J.; Hua, K; Nichols, P.D. Feeding Aquaculture in an Era of Finite Resources. Proc. Natl. Acad. Sci. 2009, 106, 15103–15110. [Google Scholar]
- Glencross, B. Exploring the nutritional demand for essential fatty acids by aquaculture species. Rev. Aquaculture 2009, 1, 71–124. [Google Scholar]
- Turchini, G.M.; Torstensen, B.E.; Ng, W.-K. Fish oil replacement in finfish nutrition. Rev. Aquaculture 2009, 1, 10–57. [Google Scholar] [CrossRef]
- Wynn, J.; Behrens, P.; Sundararajan, A.; Hanson, J.; Apt, K. Production of single cell oils by dinoflagellates; AOCS Press: Champaign, IL, USA, 2005; pp. 86–98. [Google Scholar]
- Kyle, D.J. The future development of single cell oils. In Single Cell Oils; Cohen, Z., Ratledge, C., Eds.; AOCS Press: Champaign, IL, USA, 2005; pp. 239–248. [Google Scholar]
- Lewis, T.; Nichols, P.D; McMeekin, T.A. The biotechnological potential of thraustochytrids. Mar. Biotechnolog. 1999, 1, 580–587. [Google Scholar] [CrossRef]
- Ratledge, C. Single cell oils for the 21st century. In Single Cell Oils; Cohen, Z., Ratledge, C., Eds.; AOCS Press: Champaign, IL, USA, 2005; pp. 1–20. [Google Scholar]
- Graham, I.A.; Cirpus, P.; Rein, D.; Napier, J.A. The use of very long chain polyunsaturated fatty acids to ameliorate metabolic syndrome: transgenic plants as an alternative sustainable source to fish oils. Nutr. Bull. 2004, 29, 228–233. [Google Scholar]
- Venegas-Caleron, M.; Sayanova, O.; Napier, J.A. An alternative to fish oils: Metabolic engineering of oil-seed crops to produce omega-3 long chain polyunsaturated fatty acids. Prog. Lipid Res. 2010, 49, 108–119. [Google Scholar]
- Robert, S.S.; Singh, S.P.; Zhou, X.-R.; Petrie, J.R.; Blackburn, S.I.; Mansour, P.M.; Nichols, P.D.; Liu, Q.; Green, A.G. Metabolic engineering of Arabidopsis to produce nutritionally important DHA in seed oil. Func. Plant Biol. 2005, 32, 473–479. [Google Scholar] [CrossRef]
- Wu, G.; Truksa, M.; Datla, N.; Vrinten, P.; Bauer, J.; Zank, T.; Cirpus, P.; Heinz, E.; Qiu, X. Stepwise engineering to produce high yields of very long-chain polyunsaturated fatty acids in plants. Nat. Biotechnol. 2005, 23, 1013–1017. [Google Scholar]
- Finney, A.J. Metabolic engineering in plants for human health and nutrition. Curr. Opin. Biotechnol. 2006, 17, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Bimbo, A.P. Current and future sources of raw materials for the long-chain omega-3 fatty acid market. Lipid Tech. 2007, 19, 176–179. [Google Scholar]
- Petrie, J.P.; Shrestha, P.; Mansour, M.P.; Nichols, P.D.; Liu, Q.; Singh, S.P. Metabolic engineering of omega-3 long-chain polyunsaturated fatty acids in plants using an acyl-CoA Δ6-desaturase with ω3-preference from the marine microalga. Micromonas pusilla. Metab. Eng. 2010, 12, 2330–2400. [Google Scholar]
- Petrie, J.; Liu, Q.; Mansour, M.; Nichols, P.; Singh, S. Engineering of long-chain polyunsaturated fatty acids in plant oils. International Society for Fats and 28th World Congress on Oils 2009, Sydney, Australia, September 2009.
- Harris, W.S.; Lemke, S.L.; Hansen, S.N.; Goldstein, D.A.; DiRienzo, M.A.; Su, H.; Nemeth, M.A.; Taylor, M.L.; Ahmed, G.; George, C. Stearidonic acid-enriched soybean oil increased the omega-3 index an emerging cardiovascular risk marker. Lipids 2008, 43, 805–811. [Google Scholar]
- Miller, M.R.; Bridle, A.R.; Nichols, P.D.; Carter, C.G. Increased elongase and desaturase gene expression with stearidonic acid enriched diet does not enhance long-chain (n-3) content of seawater Atlantic salmon. (Salmo salar L.). J. Nutr. 2008, 138, 2179–2185. [Google Scholar]
- Cox, D.N.; Evans, G.; Lease, H.J. Australian consumers' preferences for conventional and novel sources of long chain omega-3 fatty acids: A conjoint study. Food Qual. Pref. 2008, 19, 306–314. [Google Scholar]
- Cox, D.N.; Evans, G.; Lease, H.J. Predictors of Australian consumers’ intentions to consume conventional and novel sources of long chain omega-3 fatty acids. Pub. Health Nutr. 2008, 11, 8–16. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nichols, P.D.; Petrie, J.; Singh, S. Long-Chain Omega-3 Oils–An Update on Sustainable Sources. Nutrients 2010, 2, 572-585. https://doi.org/10.3390/nu2060572
Nichols PD, Petrie J, Singh S. Long-Chain Omega-3 Oils–An Update on Sustainable Sources. Nutrients. 2010; 2(6):572-585. https://doi.org/10.3390/nu2060572
Chicago/Turabian StyleNichols, Peter D., James Petrie, and Surinder Singh. 2010. "Long-Chain Omega-3 Oils–An Update on Sustainable Sources" Nutrients 2, no. 6: 572-585. https://doi.org/10.3390/nu2060572
APA StyleNichols, P. D., Petrie, J., & Singh, S. (2010). Long-Chain Omega-3 Oils–An Update on Sustainable Sources. Nutrients, 2(6), 572-585. https://doi.org/10.3390/nu2060572




