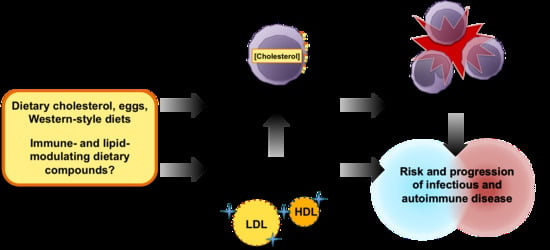
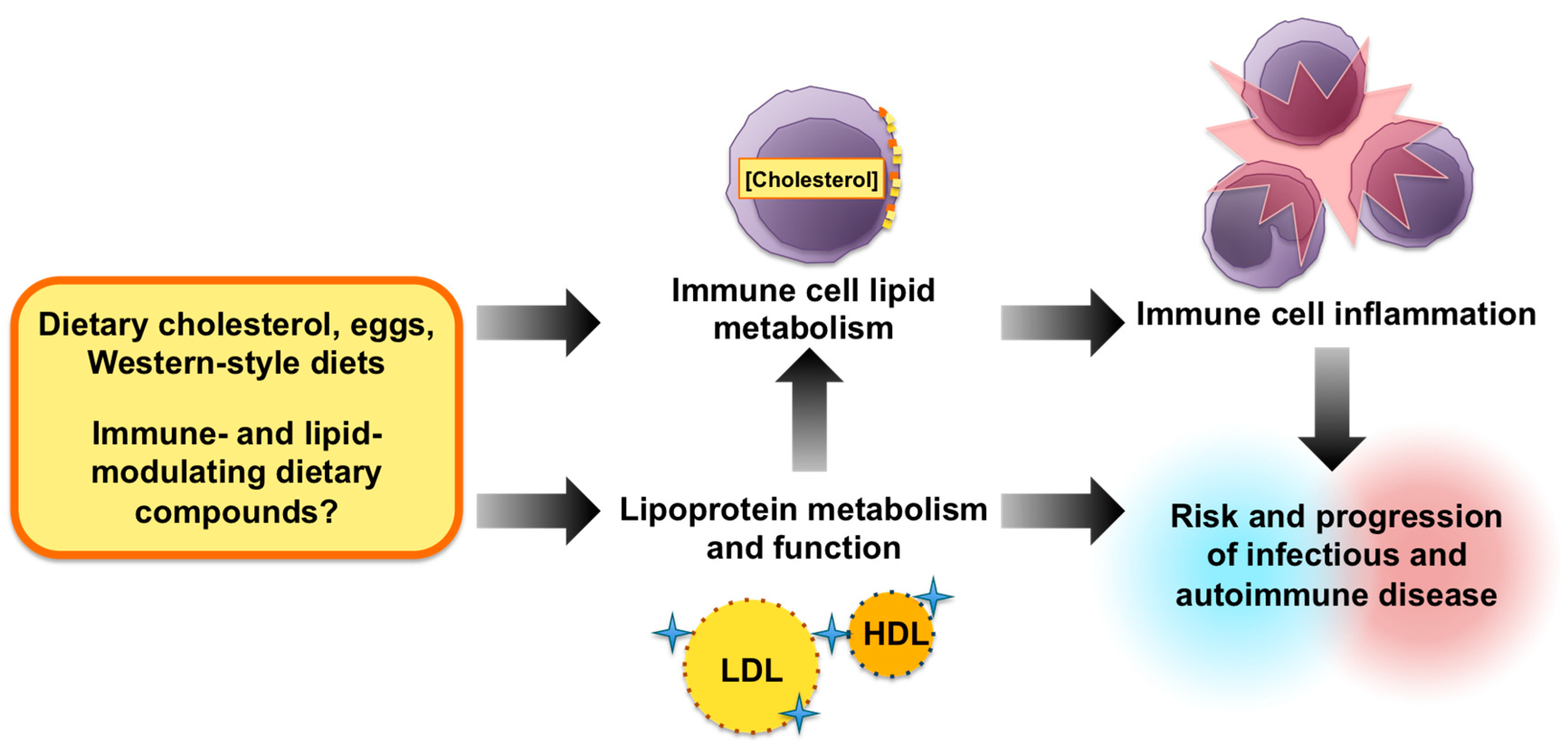
Impact of Dietary Cholesterol on the Pathophysiology of Infectious and Autoimmune Disease
Abstract
:1. Introduction
2. Role of Cholesterol in Immunity
2.1. Role of Cholesterol in the Pathophysiology of Infectious Disease
2.1.1. Role of Lipid Rafts and Lipoprotein Interactions in Bacterial Pathogenesis
2.1.2. Role of Lipid Rafts and Lipoprotein Interactions in Viral Infection
2.2. Role of Cholesterol in the Pathophysiology of Autoimmune Disease
2.3. Summary: Role of Cholesterol in Immunity
3. Effects of Dietary Cholesterol and Egg Intake on Lipoprotein Metabolism and Immune Inflammation
3.1. Effects of Dietary Cholesterol and Egg Intake on Leukocyte Lipid Rafts and Cholesterol Metabolism
3.2. Effects of Dietary Cholesterol and Egg Intake on Lipoprotein Metabolism and Function
3.3. Effects of Dietary Cholesterol and Egg Intake on Leukocyte Inflammation
3.4. Summary: Effects of Dietary Cholesterol and Egg Intake on Lipid Metabolism and Immune Inflammation
4. Dietary Cholesterol Effects in Infectious Disease
4.1. Tuberculosis
4.2. Pneumonia
4.3. Hepatitis C Virus
4.4. Human Immunodeficiency Virus (HIV)
5. Dietary Cholesterol Effects in Autoimmune Disease
5.1. Asthma
5.2. Rheumatoid Arthritis
5.3. Systemic Lupus Erythematous (SLE)
5.4. Multiple Sclerosis
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Meneghin, A.; Hogaboam, C.M. Infectious disease, the innate immune response, and fibrosis. J. Clin. Investig. 2007, 117, 530–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinchieri, G. Cancer Immunity: Lessons from Infectious Diseases. J. Infect. Dis. 2015, 212 (Suppl. 1), S67–S73. [Google Scholar] [CrossRef] [PubMed]
- Takakubo, Y.; Konttinen, Y.T. Immune-regulatory mechanisms in systemic autoimmune and rheumatic diseases. Clin. Dev. Immunol. 2012, 2012, 941346. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.; Siracusa, M.C.; Yap, G.S.; Gause, W.C. Innate cell communication kick-starts pathogen-specific immunity. Nat. Immunol. 2016, 17, 356–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed]
- Srikrishna, G.; Freeze, H.H. Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia 2009, 11, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Den Haan, J.M.; Arens, R.; van Zelm, M.C. The activation of the adaptive immune system: Cross-talk between antigen-presenting cells, T cells and B cells. Immunol. Lett. 2014, 162 Pt B, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, J.N.; Gilroy, D.W. Resolution of inflammation: A new therapeutic frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, M.D.; Remedios, K.A.; Abbas, A.K. Mechanisms of human autoimmunity. J. Clin. Investig. 2015, 125, 2228–2233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotts, J.E.; Matthay, M.A. Sepsis: Pathophysiology and clinical management. BMJ 2016, 353, i1585. [Google Scholar] [CrossRef] [PubMed]
- Castle, S.C. Clinical relevance of age-related immune dysfunction. Clin. Infect. Dis. 2000, 31, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.P.; Chiang, D.; Song, S.J.; Hoyte, E.G.; Huang, J.; Vanishsarn, C.; Nadeau, K.C. Regulatory T cell dysfunction in subjects with common variable immunodeficiency complicated by autoimmune disease. Clin. Immunol. 2009, 131, 240–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuura, E.; Atzeni, F.; Sarzi-Puttini, P.; Turiel, M.; Lopez, L.R.; Nurmohamed, M.T. Is atherosclerosis an autoimmune disease? BMC Med. 2014, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, A.; van Wijk, F. CD8(+) T cells in human autoimmune arthritis: The unusual suspects. Nat. Rev. Rheumatol. 2016, 12, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, J.; Gao, Y.D.; Guo, W. Th17 immunity in patients with allergic asthma. Int. Arch. Allergy Immunol. 2010, 151, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Paquissi, F.C. Immune Imbalances in Non-Alcoholic Fatty Liver Disease: From General Biomarkers and Neutrophils to Interleukin-17 Axis Activation and New Therapeutic Targets. Front. Immunol. 2016, 7, 490. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Chakraborty, T.; Chakraborty, K.; Pal, S.; Baral, R. Dysregulation in immune functions is reflected in tumor cell cytotoxicity by peripheral blood mononuclear cells from head and neck squamous cell carcinoma patients. Cancer Immun. 2008, 8, 10. [Google Scholar] [PubMed]
- Boynton, A.; Neuhouser, M.L.; Wener, M.H.; Wood, B.; Sorensen, B.; Chen-Levy, Z.; Kirk, E.A.; Yasui, Y.; Lacroix, K.; McTiernan, A.; et al. Associations between healthy eating patterns and immune function or inflammation in overweight or obese postmenopausal women. Am. J. Clin. Nutr. 2007, 86, 1445–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Moraes-Vieira, P.M.; Castoldi, A.; Aryal, P.; Yee, E.U.; Vickers, C.; Parnas, O.; Donaldson, C.J.; Saghatelian, A.; Kahn, B.B. Branched Fatty Acid Esters of Hydroxy Fatty Acids (FAHFAs) Protect against Colitis by Regulating Gut Innate and Adaptive Immune Responses. J. Biol. Chem. 2016, 291, 22207–22217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, C.J. Bioactive Egg Components and Inflammation. Nutrients 2015, 7, 7889–7913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Wang, L.; Wu, Z.; Yao, L.; Wu, Y.; Huang, L.; Liu, K.; Zhou, X.; Gou, D. Anthocyanin-rich fractions from red raspberries attenuate inflammation in both RAW264.7 macrophages and a mouse model of colitis. Sci. Rep. 2014, 4, 6234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, C.J.; Murphy, K.E.; Fernandez, M.L. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaqoob, P. The nutritional significance of lipid rafts. Annu. Rev. Nutr. 2009, 29, 257–282. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.J.; Lee, J.Y.; Blesso, C.N.; Carr, T.P.; Fernandez, M.L. Egg intake during carbohydrate restriction alters peripheral blood mononuclear cell inflammation and cholesterol homeostasis in metabolic syndrome. Nutrients 2014, 6, 2650–2667. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggio, R.; Viscomi, C.; Andreozzi, P.; D’Ettorre, G.; Viscogliosi, G.; Barbaro, B.; Gori, M.; Vullo, V.; Balsano, C. Normocaloric low cholesterol diet modulates Th17/Treg balance in patients with chronic hepatitis C virus infection. PLoS ONE 2014, 9, e112346. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.F.; Huang, S.L. Dietary cholesterol enhances pulmonary eosinophilic inflammation in a murine model of asthma. Int. Arch. Allergy Immunol. 2001, 125, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Gierman, L.M.; Kuhnast, S.; Koudijs, A.; Pieterman, E.J.; Kloppenburg, M.; van Osch, G.J.; Stojanovic-Susulic, V.; Huizinga, T.W.; Princen, H.M.; Zuurmond, A.M. Osteoarthritis development is induced by increased dietary cholesterol and can be inhibited by atorvastatin in APOE*3Leiden.CETP mice—A translational model for atherosclerosis. Ann. Rheum. Dis. 2014, 73, 921–927. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Serivces; U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th ed.; U.S. Department of Health and Human Serivces; U.S. Department of Agriculture: Rockville, MD, USA, 2015.
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; de Jesus, J.M.; Houston Miller, N.; Hubbard, V.S.; Lee, I.M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. American College of Cardiology/American Heart Association Task Force on Practice, G. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129 (Suppl. 2), S76–S99. [Google Scholar] [PubMed]
- Crane, J.M.; Tamm, L.K. Role of cholesterol in the formation and nature of lipid rafts in planar and spherical model membranes. Biophys. J. 2004, 86, 2965–2979. [Google Scholar] [CrossRef]
- Eich, C.; Manzo, C.; de Keijzer, S.; Bakker, G.J.; Reinieren-Beeren, I.; Garcia-Parajo, M.F.; Cambi, A. Changes in membrane sphingolipid composition modulate dynamics and adhesion of integrin nanoclusters. Sci. Rep. 2016, 6, 20693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensinger, S.J.; Bradley, M.N.; Joseph, S.B.; Zelcer, N.; Janssen, E.M.; Hausner, M.A.; Shih, R.; Parks, J.S.; Edwards, P.A.; Jamieson, B.D.; et al. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell 2008, 134, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Westcott, M.M.; Bi, X.; Liu, M.; Gowdy, K.M.; Seo, J.; Cao, Q.; Gebre, A.K.; Fessler, M.B.; Hiltbold, E.M.; et al. Myeloid cell-specific ABCA1 deletion protects mice from bacterial infection. Circ. Res. 2012, 111, 1398–1409. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lee, J.Y.; Timmins, J.M.; Brown, J.M.; Boudyguina, E.; Mulya, A.; Gebre, A.K.; Willingham, M.C.; Hiltbold, E.M.; Mishra, N.; et al. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J. Biol. Chem. 2008, 283, 22930–22941. [Google Scholar] [CrossRef] [PubMed]
- Surls, J.; Nazarov-Stoica, C.; Kehl, M.; Olsen, C.; Casares, S.; Brumeanu, T.D. Increased membrane cholesterol in lymphocytes diverts T-cells toward an inflammatory response. PLoS ONE 2012, 7, e38733. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Gebre, A.K.; Parks, J.S.; Hedrick, C.C. ATP-binding cassette transporter G1 negatively regulates thymocyte and peripheral lymphocyte proliferation. J. Immunol. 2010, 184, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Catapano, A.L.; Norata, G.D. HDL in infectious diseases and sepsis. Handb. Exp. Pharmacol. 2015, 224, 483–508. [Google Scholar] [PubMed]
- Lin, C.J.; Lai, C.K.; Kao, M.C.; Wu, L.T.; Lo, U.G.; Lin, L.C.; Chen, Y.A.; Lin, H.; Hsieh, J.T.; Lai, C.H.; et al. Impact of cholesterol on disease progression. Biomedicine 2015, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Boesze-Battaglia, K.; Brown, A.; Walker, L.; Besack, D.; Zekavat, A.; Wrenn, S.; Krummenacher, C.; Shenker, B.J. Cytolethal distending toxin-induced cell cycle arrest of lymphocytes is dependent upon recognition and binding to cholesterol. J. Biol. Chem. 2009, 284, 10650–10658. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Lai, C.K.; Lin, Y.J.; Hung, C.L.; Chu, C.H.; Feng, C.L.; Chang, C.S.; Su, H.L. Characterization of putative cholesterol recognition/interaction amino acid consensus-like motif of Campylobacter jejuni cytolethal distending toxin C. PLoS ONE 2013, 8, e66202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kachlany, S.C. Aggregatibacter actinomycetemcomitans leukotoxin: From threat to therapy. J. Dent. Res. 2010, 89, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Chang, Y.C.; Du, S.Y.; Wang, H.J.; Kuo, C.H.; Fang, S.H.; Fu, H.W.; Lin, H.H.; Chiang, A.S.; Wang, W.C. Cholesterol depletion reduces Helicobacter pylori CagA translocation and CagA-induced responses in AGS cells. Infect. Immun. 2008, 76, 3293–3303. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Wang, H.J.; Chang, Y.C.; Hsieh, W.C.; Lin, H.J.; Tang, C.H.; Sheu, J.J.; Lin, C.J.; Yang, M.S.; Tseng, S.F.; et al. Helicobacter pylori CagA-mediated IL-8 induction in gastric epithelial cells is cholesterol-dependent and requires the C-terminal tyrosine phosphorylation-containing domain. FEMS Microbiol. Lett. 2011, 323, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korhonen, J.T.; Puolakkainen, M.; Haveri, A.; Tammiruusu, A.; Sarvas, M.; Lahesmaa, R. Chlamydia pneumoniae entry into epithelial cells by clathrin-independent endocytosis. Microb. Pathog. 2012, 52, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Jutras, I.; Abrami, L.; Dautry-Varsat, A. Entry of the lymphogranuloma venereum strain of Chlamydia trachomatis into host cells involves cholesterol-rich membrane domains. Infect. Immun. 2003, 71, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Rikihisa, Y. Obligatory intracellular parasitism by Ehrlichia chaffeensis and Anaplasma phagocytophilum involves caveolae and glycosylphosphatidylinositol-anchored proteins. Cell. Microbiol. 2003, 5, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Owen, J.S.; Wilson, M.D.; Li, H.; Griffiths, G.L.; Thomas, M.J.; Hiltbold, E.M.; Fessler, M.B.; Parks, J.S. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J. Lipid Res. 2010, 51, 3196–3206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triantafilou, M.; Mouratis, M.A.; Lepper, P.M.; Haston, R.M.; Baldwin, F.; Lowes, S.; Ahmed, M.A.; Schumann, C.; Boyd, O.; Triantafilou, K. Serum proteins modulate lipopolysaccharide and lipoteichoic acid-induced activation and contribute to the clinical outcome of sepsis. Virulence 2012, 3, 136–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levels, J.H.; Abraham, P.R.; van Barreveld, E.P.; Meijers, J.C.; van Deventer, S.J. Distribution and kinetics of lipoprotein-bound lipoteichoic acid. Infect. Immun. 2003, 71, 3280–3284. [Google Scholar] [CrossRef] [PubMed]
- Munford, R.S.; Dietschy, J.M. Effects of specific antibodies, hormones, and lipoproteins on bacterial lipopolysaccharides injected into the rat. J. Infect. Dis. 1985, 152, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Pajkrt, D.; Doran, J.E.; Koster, F.; Lerch, P.G.; Arnet, B.; van der Poll, T.; ten Cate, J.W.; van Deventer, S.J. Antiinflammatory effects of reconstituted high-density lipoprotein during human endotoxemia. J. Exp. Med. 1996, 184, 1601–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roveran Genga, K.; Lo, C.; Cirstea, M.; Zhou, G.; Walley, K.R.; Russell, J.A.; Levin, A.; Boyd, J.H. Two-year follow-up of patients with septic shock presenting with low HDL: The effect upon acute kidney injury, death and estimated glomerular filtration rate. J. Intern. Med. 2017, 281, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ai, J.; Zheng, Z.; Howatt, D.A.; Daugherty, A.; Huang, B.; Li, X.A. High density lipoprotein protects against polymicrobe-induced sepsis in mice. J. Biol. Chem. 2013, 288, 17947–17953. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.M.; Parker, T.S.; Donnelly, T.M.; Walsh, A.; Rubin, A.L. In vivo protection against endotoxin by plasma high density lipoprotein. Proc. Natl. Acad. Sci. USA 1993, 90, 12040–12044. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.J.; Mo, Z.C.; Tang, S.L.; Ouyang, X.P.; He, P.P.; Lv, Y.C.; Yao, F.; Tan, Y.L.; Xie, W.; Shi, J.F.; et al. Chlamydia pneumoniae negatively regulates ABCA1 expression via TLR2-Nuclear factor-kappa B and miR-33 pathways in THP-1 macrophage-derived foam cells. Atherosclerosis 2014, 235, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Cheng, B.; Wu, X.; Wu, Q.; Qi, B.; Wu, J.; He, P. Chlamydia pneumoniae disrupts lipid metabolism in human umbilical vein endothelial cells. Mol. Med. Rep. 2014, 10, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, H.J.; Heezius, E.C.; Dallinga, G.M.; van Strijp, J.A.; Verhoef, J.; van Kessel, K.P. Lipoprotein metabolism in patients with severe sepsis. Crit. Care Med. 2003, 31, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.S.; Nierhaus, A.; Holzmann, M.; Mudersbach, E.; Bauer, A.; Robbe, L.; Zahrte, C.; Geffken, M.; Peine, S.; Schwedhelm, E.; et al. Decreased serum concentrations of sphingosine-1-phosphate in sepsis. Crit. Care 2015, 19, 372. [Google Scholar] [CrossRef] [PubMed]
- Kumaraswamy, S.B.; Linder, A.; Akesson, P.; Dahlback, B. Decreased plasma concentrations of apolipoprotein M in sepsis and systemic inflammatory response syndromes. Crit. Care 2012, 16, R60. [Google Scholar] [CrossRef] [PubMed]
- Danthi, P.; Chow, M. Cholesterol removal by methyl-beta-cyclodextrin inhibits poliovirus entry. J. Virol. 2004, 78, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pekosz, A.; Lamb, R.A. Influenza virus assembly and lipid raft microdomains: A role for the cytoplasmic tails of the spike glycoproteins. J. Virol. 2000, 74, 4634–4644. [Google Scholar] [CrossRef] [PubMed]
- Frost, F.J.; Petersen, H.; Tollestrup, K.; Skipper, B. Influenza and COPD mortality protection as pleiotropic, dose-dependent effects of statins. Chest 2007, 131, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Kwong, J.C.; Li, P.; Redelmeier, D.A. Influenza morbidity and mortality in elderly patients receiving statins: A cohort study. PLoS ONE 2009, 4, e8087. [Google Scholar] [CrossRef] [PubMed]
- Fleming, D.M.; Verlander, N.Q.; Elliot, A.J.; Zhao, H.; Gelb, D.; Jehring, D.; Nguyen-Van-Tam, J.S. An assessment of the effect of statin use on the incidence of acute respiratory infections in England during winters 1998–1999 to 2005–2006. Epidemiol. Infect. 2010, 138, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- McLean, H.Q.; Chow, B.D.; VanWormer, J.J.; King, J.P.; Belongia, E.A. Effect of Statin Use on Influenza Vaccine Effectiveness. J. Infect. Dis. 2016, 214, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Ulivieri, C.; Fanigliulo, D.; Benati, D.; Pasini, F.L.; Baldari, C.T. Simvastatin impairs humoral and cell-mediated immunity in mice by inhibiting lymphocyte homing, T-cell activation and antigen cross-presentation. Eur. J. Immunol. 2008, 38, 2832–2844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrbod, P.; Omar, A.R.; Hair-Bejo, M.; Haghani, A.; Ideris, A. Mechanisms of action and efficacy of statins against influenza. Biomed. Res. Int. 2014, 2014, 872370. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.L.; Grant, A.; Mukhamedova, N.; Pushkarsky, T.; Jennelle, L.; Dubrovsky, L.; Gaus, K.; Fitzgerald, M.L.; Sviridov, D.; Bukrinsky, M. HIV-1 Nef mobilizes lipid rafts in macrophages through a pathway that competes with ABCA1-dependent cholesterol efflux. J. Lipid Res. 2012, 53, 696–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreux, M.; Pietschmann, T.; Granier, C.; Voisset, C.; Ricard-Blum, S.; Mangeot, P.E.; Keck, Z.; Foung, S.; Vu-Dac, N.; Dubuisson, J.; et al. High density lipoprotein inhibits hepatitis C virus-neutralizing antibodies by stimulating cell entry via activation of the scavenger receptor BI. J. Biol. Chem. 2006, 281, 18285–18295. [Google Scholar] [CrossRef] [PubMed]
- Mujawar, Z.; Rose, H.; Morrow, M.P.; Pushkarsky, T.; Dubrovsky, L.; Mukhamedova, N.; Fu, Y.; Dart, A.; Orenstein, J.M.; Bobryshev, Y.V.; et al. Human immunodeficiency virus impairs reverse cholesterol transport from macrophages. PLoS Biol. 2006, 4, e365. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Badralmaa, Y.; Yang, J.; Lempicki, R.; Hazen, A.; Natarajan, V. Retinoic acid and liver X receptor agonist synergistically inhibit HIV infection in CD4+ T cells by up-regulating ABCA1-mediated cholesterol efflux. Lipids Health Dis. 2012, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Morrow, M.P.; Grant, A.; Mujawar, Z.; Dubrovsky, L.; Pushkarsky, T.; Kiselyeva, Y.; Jennelle, L.; Mukhamedova, N.; Remaley, A.T.; Kashanchi, F.; et al. Stimulation of the liver X receptor pathway inhibits HIV-1 replication via induction of ATP-binding cassette transporter A1. Mol. Pharmacol. 2010, 78, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Agnello, V.; Abel, G.; Elfahal, M.; Knight, G.B.; Zhang, Q.X. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc. Natl. Acad. Sci. USA 1999, 96, 12766–12771. [Google Scholar] [CrossRef] [PubMed]
- Scarselli, E.; Ansuini, H.; Cerino, R.; Roccasecca, R.M.; Acali, S.; Filocamo, G.; Traboni, C.; Nicosia, A.; Cortese, R.; Vitelli, A. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 2002, 21, 5017–5025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimetti, F.; Weibel, G.K.; Duong, M.; Rothblat, G.H. Measurement of cholesterol bidirectional flux between cells and lipoproteins. J. Lipid Res. 2006, 47, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.M.; Varbo, A.; Tybjaerg-Hansen, A.; Frikke-Schmidt, R.; Nordestgaard, B.G. U-shaped relationship of HDL and risk of infectious disease: Two prospective population-based cohort studies. Eur. Heart J. 2018, 39, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- McGillicuddy, F.C.; de la Llera Moya, M.; Hinkle, C.C.; Joshi, M.R.; Chiquoine, E.H.; Billheimer, J.T.; Rothblat, G.H.; Reilly, M.P. Inflammation impairs reverse cholesterol transport in vivo. Circulation 2009, 119, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Gadkar, K.; Lu, J.; Sahasranaman, S.; Davis, J.; Mazer, N.A.; Ramanujan, S. Evaluation of HDL-modulating interventions for cardiovascular risk reduction using a systems pharmacology approach. J. Lipid Res. 2016, 57, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Nambiar, M.P.; Warke, V.G.; Fisher, C.U.; Mitchell, J.; Delaney, N.; Tsokos, G.C. Alterations in lipid raft composition and dynamics contribute to abnormal T cell responses in systemic lupus erythematosus. J. Immunol. 2004, 172, 7821–7831. [Google Scholar] [CrossRef] [PubMed]
- Mak, A.; Kow, N.Y. The pathology of T cells in systemic lupus erythematosus. J. Immunol. Res. 2014, 2014, 419029. [Google Scholar] [CrossRef] [PubMed]
- Aprahamian, T.; Bonegio, R.; Rizzo, J.; Perlman, H.; Lefer, D.J.; Rifkin, I.R.; Walsh, K. Simvastatin treatment ameliorates autoimmune disease associated with accelerated atherosclerosis in a murine lupus model. J. Immunol. 2006, 177, 3028–3034. [Google Scholar] [CrossRef] [PubMed]
- Jury, E.C.; Isenberg, D.A.; Mauri, C.; Ehrenstein, M.R. Atorvastatin restores Lck expression and lipid raft-associated signaling in T cells from patients with systemic lupus erythematosus. J. Immunol. 2006, 177, 7416–7422. [Google Scholar] [CrossRef] [PubMed]
- Artola, R.T.; Mihos, C.G.; Santana, O. Effects of Statin Therapy in Patients with Systemic Lupus Erythematosus. South. Med. J. 2016, 109, 705–711. [Google Scholar] [PubMed]
- De Jong, H.J.; Klungel, O.H.; van Dijk, L.; Vandebriel, R.J.; Leufkens, H.G.; van der Laan, J.W.; Cohen Tervaert, J.W.; van Loveren, H. Use of statins is associated with an increased risk of rheumatoid arthritis. Ann. Rheum. Dis. 2012, 71, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Jick, S.S.; Choi, H.; Li, L.; McInnes, I.B.; Sattar, N. Hyperlipidaemia, statin use and the risk of developing rheumatoid arthritis. Ann. Rheum. Dis. 2009, 68, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Al-Janadi, N.; al-Dalaan, A.; al-Balla, S.; Raziuddin, S. CD4+ T cell inducible immunoregulatory cytokine response in rheumatoid arthritis. J. Rheumatol. 1996, 23, 809–814. [Google Scholar] [PubMed]
- Takahashi, H.; Soderstrom, K.; Nilsson, E.; Kiessling, R.; Patarroyo, M. Integrins and other adhesion molecules on lymphocytes from synovial fluid and peripheral blood of rheumatoid arthritis patients. Eur. J. Immunol. 1992, 22, 2879–2885. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.E.; Xu, Y.J.; Xu, F.; Cheng, C.; Peh, H.Y.; Tannenbaum, S.R.; Wong, W.S.; Ong, C.N. Metabolomics reveals altered metabolic pathways in experimental asthma. Am. J. Respir. Cell Mol. Biol. 2013, 48, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Fessler, M.B.; Massing, M.W.; Spruell, B.; Jaramillo, R.; Draper, D.W.; Madenspacher, J.H.; Arbes, S.J.; Calatroni, A.; Zeldin, D.C. Novel relationship of serum cholesterol with asthma and wheeze in the United States. J. Allergy Clin. Immunol. 2009, 124, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Huang, Y. Meta-analysis of the association between asthma and serum levels of high-density lipoprotein cholesterol and low-density lipoprotein cholesterol. Ann. Allergy Asthma Immunol. 2017, 118, 61–65. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Grossman, J.; FitzGerald, J.; Dahlin-Lee, E.; Wallace, D.J.; Thong, B.Y.; Badsha, H.; Kalunian, K.; Charles, C.; Navab, M.; et al. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006, 54, 2541–2549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holzer, M.; Wolf, P.; Curcic, S.; Birner-Gruenberger, R.; Weger, W.; Inzinger, M.; El-Gamal, D.; Wadsack, C.; Heinemann, A.; Marsche, G. Psoriasis alters HDL composition and cholesterol efflux capacity. J. Lipid Res. 2012, 53, 1618–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Lee, E.Y.; Park, J.K.; Song, Y.W.; Kim, J.R.; Cho, K.H. Patients with Rheumatoid Arthritis Show Altered Lipoprotein Profiles with Dysfunctional High-Density Lipoproteins that Can Exacerbate Inflammatory and Atherogenic Process. PLoS ONE 2016, 11, e0164564. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Grossman, J.; Skaggs, B.; Fitzgerald, J.; Sahakian, L.; Ragavendra, N.; Charles-Schoeman, C.; Watson, K.; Wong, W.K.; Volkmann, E.; et al. Dysfunctional proinflammatory high-density lipoproteins confer increased risk of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheum. 2009, 60, 2428–2437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avina-Zubieta, J.A.; Choi, H.K.; Sadatsafavi, M.; Etminan, M.; Esdaile, J.M.; Lacaille, D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008, 59, 1690–1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blesso, C.N.; Fernandez, M.L. Dietary Cholesterol, Serum Lipids, and Heart Disease: Are Eggs Working for or Against You? Nutrients 2018, 10, 426. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference; U.S. Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2018.
- Lai, M.; Chandrasekera, P.C.; Barnard, N.D. You are what you eat, or are you? The challenges of translating high-fat-fed rodents to human obesity and diabetes. Nutr. Diabetes 2014, 4, e135. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Hong, C.; Oka, K.; Salazar, J.V.; Diehl, C.; Witztum, J.L.; Diaz, M.; Castrillo, A.; Bensinger, S.J.; Chan, L.; et al. Cholesterol Accumulation in CD11c(+) Immune Cells Is a Causal and Targetable Factor in Autoimmune Disease. Immunity 2016, 45, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.J.; Blesso, C.N.; Lee, J.; Barona, J.; Shah, D.; Thomas, M.J.; Fernandez, M.L. Egg Consumption Modulates HDL Lipid Composition and Increases the Cholesterol-Accepting Capacity of Serum in Metabolic Syndrome. Lipids 2013, 48, 557–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blesso, C.N.; Andersen, C.J.; Barona, J.; Volk, B.; Volek, J.S.; Fernandez, M.L. Effects of Carbohydrate Restriction and Dietary Cholesterol Provided by Eggs on Clinical Risk Factors in Metabolic Syndrome. J. Clin. Lipidol. 2013, 7, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Ameer, F.; Munir, R.; Usman, H.; Rashid, R.; Shahjahan, M.; Hasnain, S.; Zaidi, N. Lipid-load in peripheral blood mononuclear cells: Impact of food-consumption, dietary-macronutrients, extracellular lipid availability and demographic factors. Biochimie 2017, 135, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Ikonen, E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 2008, 9, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Mutungi, G.; Torres-Gonzalez, M.; McGrane, M.M.; Volek, J.S.; Fernandez, M.L. Carbohydrate restriction and dietary cholesterol modulate the expression of HMG-CoA reductase and the LDL receptor in mononuclear cells from adult men. Lipids Health Dis. 2007, 6, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paramsothy, P.; Knopp, R.H.; Kahn, S.E.; Retzlaff, B.M.; Fish, B.; Ma, L.; Ostlund, R.E., Jr. Plasma sterol evidence for decreased absorption and increased synthesis of cholesterol in insulin resistance and obesity. Am. J. Clin. Nutr. 2011, 94, 1182–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blesso, C.N.; Andersen, C.J.; Barona, J.; Volek, J.S.; Fernandez, M.L. Whole egg consumption improves lipoprotein profiles and insulin sensitivity to a greater extent than yolk-free egg substitute in individuals with metabolic syndrome. Metabolism 2013, 62, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Lemos, B.S.; Medina-Vera, I.; Blesso, C.N.; Fernandez, M.L. Intake of 3 Eggs per Day When Compared to a Choline Bitartrate Supplement, Downregulates Cholesterol Synthesis without Changing the LDL/HDL Ratio. Nutrients 2018, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N.; Karmally, W.; Siddiqui, M.; Holleran, S.; Tall, A.R.; Rumsey, S.C.; Deckelbaum, R.J.; Blaner, W.S.; Ramakrishnan, R. A dose-response study of the effects of dietary cholesterol on fasting and postprandial lipid and lipoprotein metabolism in healthy young men. Arterioscler. Thromb. Vasc. Biol. 1994, 14, 576–586. [Google Scholar] [CrossRef]
- Missimer, A.; Fernandez, M.L.; DiMarco, D.M.; Norris, G.H.; Blesso, C.N.; Murillo, A.G.; Vergara-Jimenez, M.; Lemos, B.S.; Medina-Vera, I.; Malysheva, O.V.; et al. Compared to an Oatmeal Breakfast, Two Eggs/Day Increased Plasma Carotenoids and Choline without Increasing Trimethyl Amine N-Oxide Concentrations. J. Am. Coll. Nutr. 2018, 37, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Herron, K.L.; Vega-Lopez, S.; Conde, K.; Ramjiganesh, T.; Shachter, N.S.; Fernandez, M.L. Men classified as hypo- or hyperresponders to dietary cholesterol feeding exhibit differences in lipoprotein metabolism. J. Nutr. 2003, 133, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Herron, K.L.; Vega-Lopez, S.; Conde, K.; Ramjiganesh, T.; Roy, S.; Shachter, N.S.; Fernandez, M.L. Pre-menopausal women, classified as hypo- or hyperresponders, do not alter their LDL/HDL ratio following a high dietary cholesterol challenge. J. Am. Coll. Nutr. 2002, 21, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.M.; Zern, T.L.; Wood, R.J.; Shrestha, S.; Aggarwal, D.; Sharman, M.J.; Volek, J.S.; Fernandez, M.L. Maintenance of the LDL cholesterol:HDL cholesterol ratio in an elderly population given a dietary cholesterol challenge. J. Nutr. 2005, 135, 2793–2798. [Google Scholar] [CrossRef] [PubMed]
- Knopp, R.H.; Retzlaff, B.; Fish, B.; Walden, C.; Wallick, S.; Anderson, M.; Aikawa, K.; Kahn, S.E. Effects of insulin resistance and obesity on lipoproteins and sensitivity to egg feeding. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, R.; Goodrow-Kotyla, E.F.; Wooten, B.R.; Wilson, T.A.; Nicolosi, R.J. Consumption of 2 and 4 egg yolks/d for 5 wk increases macular pigment concentrations in older adults with low macular pigment taking cholesterol-lowering statins. Am. J. Clin. Nutr. 2009, 90, 1272–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutungi, G.; Ratliff, J.; Puglisi, M.; Torres-Gonzalez, M.; Vaishnav, U.; Leite, J.O.; Quann, E.; Volek, J.S.; Fernandez, M.L. Dietary cholesterol from eggs increases plasma HDL cholesterol in overweight men consuming a carbohydrate-restricted diet. J. Nutr. 2008, 138, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Pearce, K.L.; Clifton, P.M.; Noakes, M. Egg consumption as part of an energy-restricted high-protein diet improves blood lipid and blood glucose profiles in individuals with type 2 diabetes. Br. J. Nutr. 2011, 105, 584–592. [Google Scholar] [CrossRef] [PubMed]
- DiMarco, D.M.; Norris, G.H.; Millar, C.L.; Blesso, C.N.; Fernandez, M.L. Intake of up to 3 Eggs per Day Is Associated with Changes in HDL Function and Increased Plasma Antioxidants in Healthy, Young Adults. J. Nutr. 2017, 147, 323–329. [Google Scholar] [PubMed]
- Greene, C.M.; Waters, D.; Clark, R.M.; Contois, J.H.; Fernandez, M.L. Plasma LDL and HDL characteristics and carotenoid content are positively influenced by egg consumption in an elderly population. Nutr. Metab. 2006, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Mutungi, G.; Waters, D.; Ratliff, J.; Puglisi, M.; Clark, R.M.; Volek, J.S.; Fernandez, M.L. Eggs distinctly modulate plasma carotenoid and lipoprotein subclasses in adult men following a carbohydrate-restricted diet. J. Nutr. Biochem. 2010, 21, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Tannock, L.R.; O’Brien, K.D.; Knopp, R.H.; Retzlaff, B.; Fish, B.; Wener, M.H.; Kahn, S.E.; Chait, A. Cholesterol feeding increases C-reactive protein and serum amyloid A levels in lean insulin-sensitive subjects. Circulation 2005, 111, 3058–3062. [Google Scholar] [CrossRef] [PubMed]
- Vaisar, T.; Tang, C.; Babenko, I.; Hutchins, P.; Wimberger, J.; Suffredini, A.F.; Heinecke, J.W. Inflammatory remodeling of the HDL proteome impairs cholesterol efflux capacity. J. Lipid Res. 2015, 56, 1519–1530. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, M.N.; Valenzuela, F.; Robles, A.E.; Artalejo, E.; Aguilar, D.; Andersen, C.J.; Valdez, H.; Fernandez, M.L. One Egg per Day Improves Inflammation when Compared to an Oatmeal-Based Breakfast without Increasing Other Cardiometabolic Risk Factors in Diabetic Patients. Nutrients 2015, 7, 3449–3463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratliff, J.C.; Mutungi, G.; Puglisi, M.J.; Volek, J.S.; Fernandez, M.L. Eggs modulate the inflammatory response to carbohydrate restricted diets in overweight men. Nutr. Metab. 2008, 5, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emerson, S.R.; Kurti, S.P.; Harms, C.A.; Haub, M.D.; Melgarejo, T.; Logan, C.; Rosenkranz, S.K. Magnitude and Timing of the Postprandial Inflammatory Response to a High-Fat Meal in Healthy Adults: A Systematic Review. Adv. Nutr. 2017, 8, 213–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Missimer, A.; DiMarco, D.M.; Andersen, C.J.; Murillo, A.G.; Vergara-Jimenez, M.; Fernandez, M.L. Consuming Two Eggs per Day, as Compared to an Oatmeal Breakfast, Decreases Plasma Ghrelin while Maintaining the LDL/HDL Ratio. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Pihlajamaki, J.; Gylling, H.; Miettinen, T.A.; Laakso, M. Insulin resistance is associated with increased cholesterol synthesis and decreased cholesterol absorption in normoglycemic men. J. Lipid Res. 2004, 45, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Gunther, P.; Lauterbach, M.A.R.; Duewell, P.; Biswas, D.; Pelka, K.; Scholz, C.J.; Oosting, M.; Haendler, K.; Bassler, K.; et al. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell 2018, 172, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Mailer, R.K.W.; Gistera, A.; Polyzos, K.A.; Ketelhuth, D.F.J.; Hansson, G.K. Hypercholesterolemia Enhances T Cell Receptor Signaling and Increases the Regulatory T Cell Population. Sci. Rep. 2017, 7, 15655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martens, G.W.; Arikan, M.C.; Lee, J.; Ren, F.; Vallerskog, T.; Kornfeld, H. Hypercholesterolemia impairs immunity to tuberculosis. Infect. Immun. 2008, 76, 3464–3472. [Google Scholar] [CrossRef] [PubMed]
- Schafer, G.; Guler, R.; Murray, G.; Brombacher, F.; Brown, G.D. The role of scavenger receptor B1 in infection with Mycobacterium tuberculosis in a murine model. PLoS ONE 2009, 4, e8448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soh, A.Z.; Chee, C.B.; Wang, Y.T.; Yuan, J.M.; Koh, W.P. Dietary Cholesterol Increases the Risk whereas PUFAs Reduce the Risk of Active Tuberculosis in Singapore Chinese. J. Nutr. 2016, 146, 1093–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Guzman, C.; Vargas, M.H.; Quinonez, F.; Bazavilvazo, N.; Aguilar, A. A cholesterol-rich diet accelerates bacteriologic sterilization in pulmonary tuberculosis. Chest 2005, 127, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Madenspacher, J.H.; Draper, D.W.; Smoak, K.A.; Li, H.; Griffiths, G.L.; Suratt, B.T.; Wilson, M.D.; Rudel, L.L.; Fessler, M.B. Dyslipidemia induces opposing effects on intrapulmonary and extrapulmonary host defense through divergent TLR response phenotypes. J. Immunol. 2010, 185, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hong, Z. Cholesterol Supplement can Alleviate the Severity of Pulmonary Infection of Patients with Hypocholesterolemia. J. Food Nutr. Res. 2016, 4, 131–136. [Google Scholar]
- Yu, L.; Morishima, C.; Ioannou, G.N. Dietary cholesterol intake is associated with progression of liver disease in patients with chronic hepatitis C: Analysis of the Hepatitis C Antiviral Long-term Treatment against Cirrhosis trial. Clin. Gastroenterol. Hepatol. 2013, 11, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Morishima, C.; Ioannou, G.N. Sex difference in liver-related mortality and transplantation associated with dietary cholesterol in chronic hepatitis C virus infection. Br. J. Nutr. 2016, 115, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, K.G.; Carville, A.; Wachtman, L.; Goldin, B.R.; Yearley, J.; Li, W.; Woods, M.; Gualtieri, L.; Shannon, R.; Wanke, C. A diet high in saturated fat and cholesterol accelerates simian immunodeficiency virus disease progression. J. Infect. Dis. 2007, 196, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Glaziou, P.; Sismanidis, C.; Floyd, K.; Raviglione, M. Global epidemiology of tuberculosis. Cold Spring Harb. Perspect. Med. 2014, 5, a017798. [Google Scholar] [CrossRef] [PubMed]
- Grobler, L.; Nagpal, S.; Sudarsanam, T.D.; Sinclair, D. Nutritional supplements for people being treated for active tuberculosis. Cochrane Database Syst. Rev. 2011, 9, CD006086. [Google Scholar] [CrossRef] [PubMed]
- Fine-Coulson, K.; Reaves, B.J.; Karls, R.K.; Quinn, F.D. The role of lipid raft aggregation in the infection of type II pneumocytes by Mycobacterium tuberculosis. PLoS ONE 2012, 7, e45028. [Google Scholar] [CrossRef] [PubMed]
- Deniz, O.; Gumus, S.; Yaman, H.; Ciftci, F.; Ors, F.; Cakir, E.; Tozkoparan, E.; Bilgic, H.; Ekiz, K. Serum total cholesterol, HDL-C and LDL-C concentrations significantly correlate with the radiological extent of disease and the degree of smear positivity in patients with pulmonary tuberculosis. Clin. Biochem. 2007, 40, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Gebremicael, G.; Amare, Y.; Challa, F.; Gebreegziabxier, A.; Medhin, G.; Wolde, M.; Kassa, D. Lipid Profile in Tuberculosis Patients with and without Human Immunodeficiency Virus Infection. Int. J. Chronic Dis. 2017, 2017, 3843291. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Weaver, A.; Wu, E.; Li, Y.; Gao, H.; Fan, W.; Wu, M. Lipid-based signaling modulates DNA repair response and survival against Klebsiella pneumoniae infection in host cells and in mice. Am. J. Respir. Cell Mol. Biol. 2013, 49, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Smoak, K.; Madenspacher, J.; Jeyaseelan, S.; Williams, B.; Dixon, D.; Poch, K.R.; Nick, J.A.; Worthen, G.S.; Fessler, M.B. Effects of liver X receptor agonist treatment on pulmonary inflammation and host defense. J. Immunol. 2008, 180, 3305–3312. [Google Scholar] [CrossRef] [PubMed]
- McCrae, K.C.; Weltman, B.; Alyward, S.; Shaw, R.A.; Sowa, M.G.; Unruh, H.W.; Rand, T.G.; Thliveris, J.A.; Scott, J.E. The effect of elevated dietary cholesterol on pulmonary surfactant function in adolescent mice. Pediatr. Pulmonol. 2008, 43, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Baritussio, A.; Enzi, G.; Inelmen, E.M.; Schiavon, M.; de Biasi, F.; Allegra, L.; Ursini, F.; Baldo, G. Altered surfactant synthesis and function in rats with diet-induced hyperlipidemia. Metabolism 1980, 29, 503–510. [Google Scholar] [CrossRef]
- Lambert, J.E.; Bain, V.G.; Ryan, E.A.; Thompson, A.B.; Clandinin, M.T. Elevated lipogenesis and diminished cholesterol synthesis in patients with hepatitis C viral infection compared to healthy humans. Hepatology 2013, 57, 1697–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, J.; Wang, C.; Sumpter, R., Jr.; Brown, M.S.; Goldstein, J.L.; Gale, M., Jr. Disruption of hepatitis C virus RNA replication through inhibition of host protein geranylgeranylation. Proc. Natl. Acad. Sci. USA 2003, 100, 15865–15870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujino, T.; Nakamuta, M.; Yada, R.; Aoyagi, Y.; Yasutake, K.; Kohjima, M.; Fukuizumi, K.; Yoshimoto, T.; Harada, N.; Yada, M.; et al. Expression profile of lipid metabolism-associated genes in hepatitis C virus-infected human liver. Hepatol. Res. 2010, 40, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Corey, K.E.; Kane, E.; Munroe, C.; Barlow, L.L.; Zheng, H.; Chung, R.T. Hepatitis C virus infection and its clearance alter circulating lipids: Implications for long-term follow-up. Hepatology 2009, 50, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Bruden, D.J.T.; McMahon, B.J.; Townshend-Bulson, L.; Gounder, P.; Gove, J.; Plotnik, J.; Homan, C.; Hewitt, A.; Barbour, Y.; Spradling, P.R.; et al. Risk of end-stage liver disease, hepatocellular carcinoma, and liver-related death by fibrosis stage in the hepatitis C Alaska Cohort. Hepatology 2017, 66, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.A.; Yan, P.; Lo Re, V., 3rd; Rimland, D.; Goetz, M.B.; Leaf, D.; Freiberg, M.S.; Klein, M.B.; Justice, A.C.; Sherman, K.E.; et al. Liver fibrosis progression in hepatitis C virus infection after seroconversion. JAMA Intern. Med. 2015, 175, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Bashiri, A.; Nesan, D.; Tavallaee, G.; Sue-Chue-Lam, I.; Chien, K.; Maguire, G.F.; Naples, M.; Zhang, J.; Magomedova, L.; Adeli, K.; et al. Cellular cholesterol accumulation modulates high fat high sucrose (HFHS) diet-induced ER stress and hepatic inflammasome activation in the development of non-alcoholic steatohepatitis. Biochim. Biophys. Acta 2016, 1861, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Goodspeed, L.; Wang, S.; Kim, J.; Zeng, L.; Ioannou, G.N.; Haigh, W.G.; Yeh, M.M.; Kowdley, K.V.; O’Brien, K.D.; et al. Dietary cholesterol exacerbates hepatic steatosis and inflammation in obese LDL receptor-deficient mice. J. Lipid Res. 2011, 52, 1626–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wouters, K.; van Gorp, P.J.; Bieghs, V.; Gijbels, M.J.; Duimel, H.; Lutjohann, D.; Kerksiek, A.; van Kruchten, R.; Maeda, N.; Staels, B.; et al. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology 2008, 48, 474–486. [Google Scholar] [CrossRef] [PubMed]
- De Ogburn, R.; Leite, J.O.; Ratliff, J.; Volek, J.S.; McGrane, M.M.; Fernandez, M.L. Effects of increased dietary cholesterol with carbohydrate restriction on hepatic lipid metabolism in Guinea pigs. Comp. Med. 2012, 62, 109–115. [Google Scholar]
- Turley, S.D.; Schwarz, M.; Spady, D.K.; Dietschy, J.M. Gender-related differences in bile acid and sterol metabolism in outbred CD-1 mice fed low- and high-cholesterol diets. Hepatology 1998, 28, 1088–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McHutchison, J.G.; Blatt, L.M.; de Medina, M.; Craig, J.R.; Conrad, A.; Schiff, E.R.; Tong, M.J. Measurement of serum hyaluronic acid in patients with chronic hepatitis C and its relationship to liver histology. Consensus Interferon Study Group. J. Gastroenterol. Hepatol. 2000, 15, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Bian, Z.; Zhao, L.; Liu, Y.; Liang, S.; Wang, Q.; Han, X.; Peng, Y.; Chen, X.; Shen, L.; et al. Interleukin-17 exacerbates hepatic steatosis and inflammation in non-alcoholic fatty liver disease. Clin. Exp. Immunol. 2011, 166, 281–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, Y.T.; Fan, X.P.; Fan, Y.C.; Zhao, J.; Wang, K. Change in the Treg/Th17 cell imbalance in hepatocellular carcinoma patients and its clinical value. Medicine 2017, 96, e7704. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, I.D.; Angdisen, J.; Moran, E.; Schulman, I.G. Differential regulation of gene expression by LXRs in response to macrophage cholesterol loading. Mol. Endocrinol. 2013, 27, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Ghilardi, N.; Ouyang, W. Targeting the development and effector functions of TH17 cells. Semin. Immunol. 2007, 19, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Senpuku, H.; Asano, T.; Matin, K.; Salam, M.A.; Tsuha, Y.; Horibata, S.; Shimazu, Y.; Soeno, Y.; Aoba, T.; Sata, T.; et al. Effects of human interleukin-18 and interleukin-12 treatment on human lymphocyte engraftment in NOD-scid mouse. Immunology 2002, 107, 232–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, R.; Sindhu, S.T.; Toma, E.; Morisset, R.; Ahmad, A. Elevated levels of circulating interleukin-18 in human immunodeficiency virus-infected individuals: Role of peripheral blood mononuclear cells and implications for AIDS pathogenesis. J. Virol. 2002, 76, 12448–12456. [Google Scholar] [CrossRef] [PubMed]
- Lindegaard, B.; Hansen, A.B.; Gerstoft, J.; Pedersen, B.K. High plasma level of interleukin-18 in HIV-infected subjects with lipodystrophy. J. Acquir. Immune Defic. Syndr. 2004, 36, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, D.; deOgburn, R.C.; Volek, J.S.; Fernandez, M.L. Cholesterol-induced inflammation and macrophage accumulation in adipose tissue is reduced by a low carbohydrate diet in guinea pigs. Nutr. Res. Pract. 2014, 8, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, S.A.; Gerndt, N.; Winchenbach, J.; Stumpf, S.K.; Hosang, L.; Odoardi, F.; Ruhwedel, T.; Bohler, C.; Barrette, B.; Stassart, R.; et al. Dietary cholesterol promotes repair of demyelinated lesions in the adult brain. Nat. Commun. 2017, 8, 14241. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.F.; Huang, S.L. Enhancing effect of dietary cholesterol and inhibitory effect of pravastatin on allergic pulmonary inflammation. J. Biomed. Sci. 2004, 11, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, R.R.; Rosenkranz, S.K.; Neessen, K.J. Dietary factors associated with lifetime asthma or hayfever diagnosis in Australian middle-aged and older adults: A cross-sectional study. Nutr. J. 2012, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Hafstrom, I.; Ringertz, B.; Spangberg, A.; von Zweigbergk, L.; Brannemark, S.; Nylander, I.; Ronnelid, J.; Laasonen, L.; Klareskog, L. A vegan diet free of gluten improves the signs and symptoms of rheumatoid arthritis: The effects on arthritis correlate with a reduction in antibodies to food antigens. Rheumatology 2001, 40, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- McDougall, J.; Bruce, B.; Spiller, G.; Westerdahl, J.; McDougall, M. Effects of a very low-fat, vegan diet in subjects with rheumatoid arthritis. J. Altern. Complement. Med. 2002, 8, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen-Kragh, J.; Haugen, M.; Borchgrevink, C.F.; Laerum, E.; Eek, M.; Mowinkel, P.; Hovi, K.; Forre, O. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet 1991, 338, 899–902. [Google Scholar] [CrossRef]
- Kjeldsen-Kragh, J.; Mellbye, O.J.; Haugen, M.; Mollnes, T.E.; Hammer, H.B.; Sioud, M.; Forre, O. Changes in laboratory variables in rheumatoid arthritis patients during a trial of fasting and one-year vegetarian diet. Scand. J. Rheumatol. 1995, 24, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Taille, C.; Grootenboer-Mignot, S.; Estellat, C.; Roy, C.; Ly Ka So, S.; Pretolani, M.; Aubier, M.; Crestani, B.; Chollet-Martin, S. Perip7lakin is a target for autoimmunity in asthma. Respir. Res. 2016, 17, 126. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, A.; Asero, R. Asthma and autoimmunity: A complex but intriguing relation. Expert Rev. Clin. Immunol. 2008, 4, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Leonard, P.; Sur, S. Interleukin-12: Potential role in asthma therapy. BioDrugs 2003, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Meyts, I.; Hellings, P.W.; Hens, G.; Vanaudenaerde, B.M.; Verbinnen, B.; Heremans, H.; Matthys, P.; Bullens, D.M.; Overbergh, L.; Mathieu, C.; et al. IL-12 contributes to allergen-induced airway inflammation in experimental asthma. J. Immunol. 2006, 177, 6460–6470. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.; Moreira, A.; Padrao, P.; Teixeira, V.H.; Carvalho, P.; Delgado, L.; Lopes, C.; Severo, M.; Moreira, P. Dietary patterns and asthma prevalence, incidence and control. Clin. Exp. Allergy 2015, 45, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Brigham, E.P.; Kolahdooz, F.; Hansel, N.; Breysse, P.N.; Davis, M.; Sharma, S.; Matsui, E.C.; Diette, G.; McCormack, M.C. Association between Western diet pattern and adult asthma: A focused review. Ann. Allergy Asthma Immunol. 2015, 114, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Charles-Schoeman, C.; Watanabe, J.; Lee, Y.Y.; Furst, D.E.; Amjadi, S.; Elashoff, D.; Park, G.; McMahon, M.; Paulus, H.E.; Fogelman, A.M.; et al. Abnormal function of high-density lipoprotein is associated with poor disease control and an altered protein cargo in rheumatoid arthritis. Arthritis Rheum. 2009, 60, 2870–2879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charles-Schoeman, C.; Lee, Y.Y.; Grijalva, V.; Amjadi, S.; FitzGerald, J.; Ranganath, V.K.; Taylor, M.; McMahon, M.; Paulus, H.E.; Reddy, S.T. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann. Rheum. Dis. 2012, 71, 1157–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, J.; Charles-Schoeman, C.; Miao, Y.; Elashoff, D.; Lee, Y.Y.; Katselis, G.; Lee, T.D.; Reddy, S.T. Proteomic profiling following immunoaffinity capture of high-density lipoprotein: Association of acute-phase proteins and complement factors with proinflammatory high-density lipoprotein in rheumatoid arthritis. Arthritis Rheum. 2012, 64, 1828–1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charles-Schoeman, C.; Yin Lee, Y.; Shahbazian, A.; Wang, X.; Elashoff, D.; Curtis, J.R.; Navarro-Millan, I.; Yang, S.; Chen, L.; Cofield, S.S.; et al. Improvement of High-Density Lipoprotein Function in Patients With Early Rheumatoid Arthritis Treated With Methotrexate Monotherapy or Combination Therapies in a Randomized Controlled Trial. Arthritis Rheumatol. 2017, 69, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Y.; Chih, H.M.; Lan, J.L.; Chang, H.Y.; Chen, W.W.; Chiang, E.P. Blood lipid profiles and peripheral blood mononuclear cell cholesterol metabolism gene expression in patients with and without methotrexate treatment. BMC Med. 2011, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gil, M.; Reyes, C.; Ramos, R.; Sanchez-Santos, M.T.; Prieto-Alhambra, D.; Spector, T.D.; Hart, D.J.; Arden, N.K. Serum Lipid Levels and Risk Of Hand Osteoarthritis: The Chingford Prospective Cohort Study. Sci. Rep. 2017, 7, 3147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, S.; Jaiswal, K.S.; Gupta, B. Managing Rheumatoid Arthritis with Dietary Interventions. Front. Nutr. 2017, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Agren, J.J.; Tvrzicka, E.; Nenonen, M.T.; Helve, T.; Hanninen, O. Divergent changes in serum sterols during a strict uncooked vegan diet in patients with rheumatoid arthritis. Br. J. Nutr. 2001, 85, 137–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkan, A.C.; Sjoberg, B.; Kolsrud, B.; Ringertz, B.; Hafstrom, I.; Frostegard, J. Gluten-free vegan diet induces decreased LDL and oxidized LDL levels and raised atheroprotective natural antibodies against phosphorylcholine in patients with rheumatoid arthritis: A randomized study. Arthritis Res. Ther. 2008, 10, R34. [Google Scholar] [CrossRef] [PubMed]
- Villalvilla, A.; Gomez, R.; Largo, R.; Herrero-Beaumont, G. Lipid transport and metabolism in healthy and osteoarthritic cartilage. Int. J. Mol. Sci. 2013, 14, 20793–20808. [Google Scholar] [CrossRef] [PubMed]
- Farnaghi, S.; Crawford, R.; Xiao, Y.; Prasadam, I. Cholesterol metabolism in pathogenesis of osteoarthritis disease. Int. J. Rheum. Dis. 2017, 20, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, S.T.; Craft, J. The pathogenesis of systemic lupus erythematosus-an update. Curr. Opin. Immunol. 2012, 24, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Szabo, M.Z.; Szodoray, P.; Kiss, E. Dyslipidemia in systemic lupus erythematosus. Immunol. Res. 2017, 65, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Voloshyna, I.; Teboul, I.; Littlefield, M.J.; Siegart, N.M.; Turi, G.K.; Fazzari, M.J.; Carsons, S.E.; DeLeon, J.; Reiss, A.B. Resveratrol counters systemic lupus erythematosus-associated atherogenicity by normalizing cholesterol efflux. Exp. Biol. Med. (Maywood) 2016, 241, 1611–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, M.; Adams-Huet, B.; Kavanaugh, A.; Coyle, Y.; Lipsky, P. Nutrient intake and diet quality in patients with systemic lupus erythematosus on a culturally sensitive cholesterol lowering dietary program. J. Rheumatol. 2004, 31, 71–75. [Google Scholar] [PubMed]
- Shah, M.; Kavanaugh, A.; Coyle, Y.; Adams-Huet, B.; Lipsky, P.E. Effect of a culturally sensitive cholesterol lowering diet program on lipid and lipoproteins, body weight, nutrient intakes, and quality of life in patients with systemic lupus erythematosus. J. Rheumatol. 2002, 29, 2122–2128. [Google Scholar] [PubMed]
- Hearth-Holmes, M.; Baethge, B.A.; Broadwell, L.; Wolf, R.E. Dietary treatment of hyperlipidemia in patients with systemic lupus erythematosus. J. Rheumatol. 1995, 22, 450–454. [Google Scholar] [PubMed]
- Wu, G.F.; Alvarez, E. The immunopathophysiology of multiple sclerosis. Neurol. Clin. 2011, 29, 257–278. [Google Scholar] [CrossRef] [PubMed]
- Saher, G.; Brugger, B.; Lappe-Siefke, C.; Mobius, W.; Tozawa, R.; Wehr, M.C.; Wieland, F.; Ishibashi, S.; Nave, K.A. High cholesterol level is essential for myelin membrane growth. Nat. Neurosci. 2005, 8, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Saher, G.; Rudolphi, F.; Corthals, K.; Ruhwedel, T.; Schmidt, K.F.; Lowel, S.; Dibaj, P.; Barrette, B.; Mobius, W.; Nave, K.A. Therapy of Pelizaeus-Merzbacher disease in mice by feeding a cholesterol-enriched diet. Nat. Med. 2012, 18, 1130–1135. [Google Scholar] [CrossRef] [PubMed]

| Experimental Model/Population | Dietary Conditions | Effect | Reference |
|---|---|---|---|
| Tuberculosis | |||
| ApoE-deficient mice | High cholesterol (1.25%) diet | ↓ Th1 response ↑ bacterial burden, lung inflammation, early onset mortality | [130] |
| Wild type and SR-BI knockout mice | High cholesterol (1.25%) diet | ↑ bacterial burden ↔ pulmonary histopathology, cytokine expression | [131] |
| Men and women in the Singapore Chinese Health Study, n = 63,257 | Observational study | ↑ increased risk of active TB | [132] |
| Active pulmonary TB patients, n = 21 | Cholesterol-rich diet (800 mg/day) vs. normocholesterolemic diet (250 mg/day) | ↓ positive sputum cultures, sputum production | [133] |
| Pneumonia | |||
| C57BL/6 mice | Cholesterol-rich (1.25%) Western Diet | ↓ pulmonary bacterial clearance, pulmonary PMN numbers and chemotaxis, LPS-induced pulmonary TNFα, MIP-2, NF-ĸB p65 subunit activation ↑ rate of clearance of pathogens from blood, serum TNFα, MIP-2 ↓ bacterial burden in spleen and liver ↔ survival rates | [134] |
| Pneumonia patients, n = 47 | 600 mg/day from egg yolks for 10 days | ↓ plasma CRP, IL-6 ↑ SAPSII and SGA scores | [135] |
| Experimental Model/Population | Dietary Conditions | Effect | Reference |
|---|---|---|---|
| Hepatitis C Virus | |||
| Chronic HCV patients, n = 608 | Observational study: 224–310 mg cholesterol/day; >310 mg cholesterol/day | ↑ risk for fibrosis and/or cirrhosis | [136] |
| Chronic HCV patients from the HALT-C Trial with advanced fibrosis or compensated cirrhosis, n = 657 | Observational study | ↑ risk of liver-related death and transplantation in women ↔ relationship between dietary cholesterol and disease progression in men | [137] |
| Chronic HCV patients, n = 30 | Normocaloric, low-cholesterol (185 mg/day) diet for 30 days | ↑ % Treg cells, PBMC mRNA expression of LXRα, LXRβ, SREBP-1c, ABCA1 ↓ % Th17 cells, serum IL-17, IL-22, TGFβ, HA | [26] |
| HIV/AIDS | |||
| SIV-infected macaque primates | High-fat (40% of energy)/high-cholesterol (1%) diet | ↑ peak viral loads, rate of disease progression, plasma IL-18, incidence of co-infections, body wasting, risk of SIV-related death | [138] |
| Experimental Model/Population | Dietary Conditions | Effect | Reference |
|---|---|---|---|
| Asthma | |||
| Ovalbumin-sensitized C57BL/6 mice | Cholesterol-rich (1% and 2%) diets | ↑ bronchoaveolar inflammation, eosinophil numbers, IL-5, cysteinyl leukotrienes | [27] |
| Ovalbumin-sensitized C57BL/6 mice | Cholesterol-rich (2%) diet | ↑ IL-5, PGE2, MCP-1, eosinophils numbers in bronchoalveolar lavage fluid ↑ IL-4, IFNγ production by pulmonary lymphocytes ↓ IL-12 | [169] |
| Men and women from The 45 and Up Study, n = 156,035 | Diets rich in meats, poultry, and seafood | ↑ odds of asthma/hayfever diagnosis | [170] |
| Men and women from The 45 and Up Study, n = 156,035 | Diets rich in cheese | ↑ odds of asthma/hayfever diagnosis in men ↓ odds of asthma/hayfever diagnosis in women | [170] |
| Rheumatoid arthritis | |||
| RA patients, n = 47 | Gluten-free, vegan diet for nine months vs. non-vegan diet | ↑ clinical improvement by ACR20 criteria | [171] |
| RA patients, n = 24 | Very low-fat (10% of energy) vegan diet | ↓ joint pain and swelling ↑ joint mobility | [172] |
| RA patients, n = 53 | 7–10 day fast → gluten-free vegan diet vs. standard non-vegan diet | ↓ number of tender and swollen joints, pain score, duration of morning stiffness, white blood cell count, CRP ↑ grip strength | [173] |
| RA patients, 53 | 7–10 day fast → gluten-free vegan diet → vegetarian diet vs. a standard non-vegan diet | ↓ leukocyte and platelet counts, total IgG and IgM rheumatoid factor, calprotectin, C3 and C4 complement proteins | [174] |
| APOE*3Leiden.CETP mice | Cholesterol-rich (0.1% and 0.3%) Western diets | ↑ joint inflammation, osteoarthritis development | [28] |
| Systemic Lupus Erythematous | |||
| ApoE/LXRβ-deficient mice | Cholesterol-rich (0.21%) Western diet | ↑ cholesterol accumulation in spleen and lymph nodes, T cell priming, B cell expansion, autoantibody production * Note: HDL-mediated efflux suppressed diet-induced B cell expansion and autoantibody production | [100] |
| Multiple sclerosis | |||
| EAE mouse model of MS | High cholesterol (5%) diet | ↓ spinal immune cell infiltration, mRNA expression of TNFα, IL-17, IFNγ, GM-CSF, MHCII ↔ clinical scoring of CNS lesions and disease pathology | [168] |
| Cuprizone-induced mouse model of MS | High cholesterol (2%) diet | ↔ demyelinization ↑ remyelinization, oligodendrocyte precursor cell proliferation, oligodendrocyte differentiation, motor function | [168] |
| Lysolechithin-induced mouse model of MS | High cholesterol (2%) diet | ↑ remyelinization | [168] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersen, C.J. Impact of Dietary Cholesterol on the Pathophysiology of Infectious and Autoimmune Disease. Nutrients 2018, 10, 764. https://doi.org/10.3390/nu10060764
Andersen CJ. Impact of Dietary Cholesterol on the Pathophysiology of Infectious and Autoimmune Disease. Nutrients. 2018; 10(6):764. https://doi.org/10.3390/nu10060764
Chicago/Turabian StyleAndersen, Catherine J. 2018. "Impact of Dietary Cholesterol on the Pathophysiology of Infectious and Autoimmune Disease" Nutrients 10, no. 6: 764. https://doi.org/10.3390/nu10060764
APA StyleAndersen, C. J. (2018). Impact of Dietary Cholesterol on the Pathophysiology of Infectious and Autoimmune Disease. Nutrients, 10(6), 764. https://doi.org/10.3390/nu10060764