Monitoring Healthy Metabolic Trajectories with Nutritional Metabonomics
Abstract
:1. Introduction
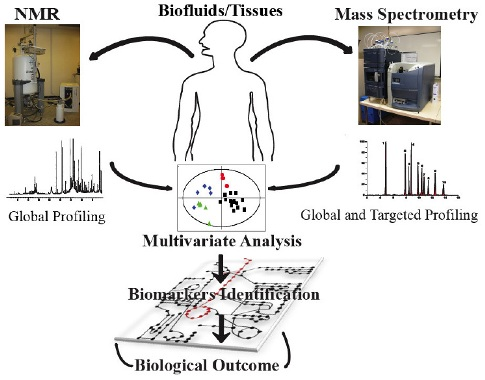
2. Metabonomics Analytical Profiling Techniques

2.1. Nutritional Metabonomics: Deciphering Food-Induced Metabolic Responses
2.2. Nutrimetabonomics: A Specific Tool to Decipher Gut Microbiota Contribution to the Host Metabolic Homeostasis Processes
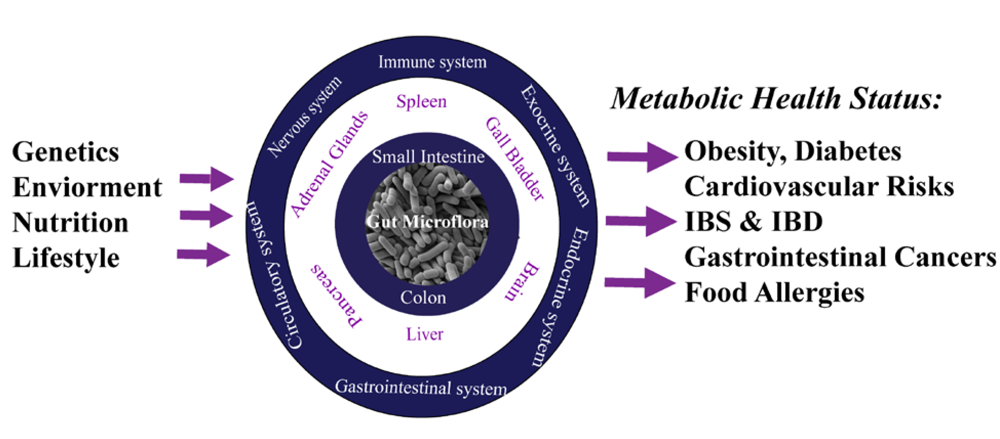
3. Conclusions
References
- Bruce, S.J.; Tavazzi, I.; Parisod, V.; Rezzi, S.; Kochhar, S.; Guy, P.A. Investigation of human blood plasma sample preparation for performing metabolomics using ultrahigh performance liquid chromatography/mass spectrometry. Anal. Chem. 2009, 81, 3285–3296. [Google Scholar]
- Skordi, E.; Yap, I.K.; Claus, S.P.; Martin, F.P.; Cloarec, O.; Lindberg, J.; Schuppe-Koistinen, I.; Holmes, E.; Nicholson, J.K. Analysis of time-related metabolic fluctuations induced by ethionine in the rat. J. Proteome. Res. 2007, 6, 4572–4581. [Google Scholar]
- Yap, I.K.; Li, J.V.; Saric, J.; Martin, F.P.; Davies, H.; Wang, Y.; Wilson, I.D.; Nicholson, J.K.; Utzinger, J.; Marchesi, J.R.; Holmes, E. Metabonomic and microbiological analysis of the dynamic effect of vancomycin-induced gut microbiota modification in the mouse. J. Proteome. Res. 2008, 7, 3718–3728. [Google Scholar] [CrossRef] [PubMed]
- Coen, M.; Lenz, E.M.; Nicholson, J.K.; Wilson, I.D.; Pognan, F.; Lindon, J.C. An integrated metabonomic investigation of acetaminophen toxicity in the mouse using NMR spectroscopy. Chem. Res. Toxicol. 2003, 16, 295–303. [Google Scholar]
- Coen, M.; Ruepp, S.U.; Lindon, J.C.; Nicholson, J.K.; Pognan, F.; Lenz, E.M.; Wilson, I.D. Integrated application of transcriptomics and metabonomics yields new insight into the toxicity due to paracetamol in the mouse. J. Pharm. Biomed. Anal. 2004, 35, 93–105. [Google Scholar]
- Waters, N.J.; Holmes, E.; Williams, C.M.; Waterfield, C.J.; Farrant, R.D.; Nicholson, J.K. NMR and Patter recognition studies on the time-related metabolic effects of alpha-Naphthylisothiocyanate on liver, urine and plasma in the rat: an integrative metabonomic approach. Chem. Res. Toxicol. 2001, 14, 1401–1412. [Google Scholar]
- Beckwith-Hall, B.M.; Nicholson, J.K.; Nicholls, A.W.; Foxall, P.J.; Lindon, J.C.; Connor, S.C.; Abdi, M.; Connelly, J.; Holmes, E. Nuclear magnetic resonance spectroscopic and principal components analysis investigations into biochemical effects of three model hepatotoxins. Chem. Res. Toxicol. 1998, 11, 260–272. [Google Scholar]
- Beckwith-Hall, B.M.; Thompson, N.A.; Nicholson, J.K.; Lindon, J.C.; Holmes, E. A metabonomic investigation of hepatotoxicity using diffusion-edited 1H NMR spectroscopy of blood serum. Analyst 2003, 128, 814–818. [Google Scholar]
- Beckonert, O.; Bollard, E.; Ebbels, T.M.D.; Keun, H.C.; Antti, H.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. NMR-based metabonomic toxicity classification: hierarchical cluster analysis and k-nearest-neighbour approaches. Anal. Chim. Acta 2003, 490, 3–15. [Google Scholar]
- Holmes, E.; Nicholson, J.K.; Nicholls, A.; Lindon, J.C.; Connor, S.C.; Polly, S.; Connelly, J. The identification of novel biomarkers of renal toxicity using automatic data reduction techniques and PCA of proton NMR spectra of urine. Chemom. Intell. Lab. Syst. 1998, 44, 245–255. [Google Scholar]
- Dunn, W.B.; Bailey, N.J.; Johnson, H.E. Measuring the metabolome: current analytical technologies. Analyst 2005, 130, 606–625. [Google Scholar]
- Ellis, D.I.; Dunn, W.B.; Griffin, J.L.; Allwood, J.W.; Goodacre, R. Metabolic fingerprinting as a diagnostic tool. Pharmacogenomics 2007, 8, 1243–1266. [Google Scholar]
- Holmes, E.; Loo, R.L.; Stamler, J.; Bictash, M.; Yap, I.K.S.; Chan, Q.; Ebbels, T.; De Iorio, M.; Brown, I.J.; Veselkov, K.A.; Daviglus, M.L.; Kesteloot, H.; Ueshima, H.; Zhao, L.; Nicholson, J.K.; Elliott, P. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature 2008, 453, 396–400. [Google Scholar]
- Rezzi, S.; Martin, F.P.; Kochhar, S. Defining personal nutrition and metabolic health through metabonomics. Ernst. Schering Found. Symp. Proc. 2007, 4, 251–264. [Google Scholar]
- Rezzi, S.; Ramadan, Z.; Martin, F.P.; Fay, L.B.; van, B.P.; Lindon, J.C.; Nicholson, J.K.; Kochhar, S. Human metabolic phenotypes link directly to specific dietary preferences in healthy individuals. J. Proteome. Res. 2007, 6, 4469–4477. [Google Scholar]
- Rezzi, S.; Martin, F.P.; Shanmuganayagam, D.; Colman, R.J.; Nicholson, J.K.; Weindruch, R. Metabolic shifts due to long-term caloric restriction revealed in nonhuman primates. Exp. Gerontol. 2009, 44, 356–362. [Google Scholar]
- Makinen, V.P.; Forsblom, C.; Thorn, L.M.; Waden, J.; Gordin, D.; Heikkila, O.; Hietala, K.; Kyllonen, L.; Kyto, J.; Rosengard-Barlund, M.; Saraheimo, M.; Tolonen, N.; Parkkonen, M.; Kaski, K.; la-Korpela, M.; Groop, P.H. Metabolic phenotypes, vascular complications, and premature deaths in a population of 4,197 patients with type 1 diabetes. Diabetes 2008, 57, 2480–2487. [Google Scholar] [PubMed]
- Makinen, V.P.; Soininen, P.; Forsblom, C.; Parkkonen, M.; Ingman, P.; Kaski, K.; Groop, P.H.; la-Korpela, M. 1H NMR metabonomics approach to the disease continuum of diabetic complications and premature death. Mol. Syst. Biol. 2008, 4, 167. [Google Scholar]
- Martin, F.P.; Rezzi, S.; Philippe, D.; Tornier, L.; Messlik, A.; Holzlwimmer, G.; Baur, P.; Quintanilla-Fend, L.; Loh, G.; Blaut, M.; Blum, S.; Kochhar, S.; Haller, D. Metabolic assessment of gradual development of moderate experimental colitis in IL-10 deficient mice. J. Proteome. Res. 2009, 8, 2376–2387. [Google Scholar]
- Martin, F.P.; Verdu, E.F.; Wang, Y.; Dumas, M.E.; Yap, I.K.; Cloarec, O.; Bergonzelli, G.E.; Corthesy-Theulaz, I.; Kochhar, S.; Holmes, E.; Lindon, J.C.; Collins, S.M.; Nicholson, J.K. Transgenomic metabolic interactions in a mouse disease model: interactions of Trichinella spiralis infection with dietary Lactobacillus paracasei supplementation. J. Proteome. Res. 2006, 5, 2185–2193. [Google Scholar] [PubMed]
- Dunne, C. Adaptation of bacteria to the intestinal niche: probiotics and gut disorder. Inflamm. Bowel. Dis. 2001, 7, 136–145. [Google Scholar]
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar]
- Ley, R.; Turnbaugh, P.; Klein, S.; Gordon, J. Microbial ecology: human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar]
- Nicholson, J.K.; Holmes, E.; Wilson, I.D. Gut microorganisms, mammalian metabolism and personalized health care. Nat. Rev. Microbiol. 2005, 3, 431–438. [Google Scholar]
- Verdu, E.F.; Bercik, P.; Bergonzelli, G.E.; Huang, X.X.; Blennerhasset, P.; Rochat, F.; Fiaux, M.; Mansourian, R.; Corthesy-Theulaz, I.; Collins, S.M. Lactobacillus paracasei normalizes muscle hypercontractility in a murine model of postinfective gut dysfunction. Gastroenterology 2004, 127, 826–837. [Google Scholar]
- Dethlefsen, L.; Fall-Ngai, M.; Relman, D.A. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature 2007, 449, 811–818. [Google Scholar]
- Bik, E.M.; Eckburg, P.B.; Gill, S.R.; Nelson, K.E.; Purdom, E.A.; Francois, F.; Perez-Perez, G.; Blaser, M.J.; Relman, D.A. Molecular analysis of the bacterial microbiota in the human stomach. Proc. Natl. Acad. Sci. U S A 2006, 103, 732–737. [Google Scholar]
- Eckburg, P.B.; Relman, D.A. The role of microbes in Crohn's disease. Clin. Infect. Dis. 2007, 44, 256–262. [Google Scholar]
- Holmes, E.; Nicholson, J. Variation in gut microbiota strongly influences individual rodent phenotypes. Toxicol. Sci. 2005, 87, 1–2. [Google Scholar]
- Dumas, M.E.; Barton, R.H.; Toye, A.; Cloarec, O.; Blancher, C.; Rothwell, A.; Fearnside, J.; Tatoud, R.; Blanc, V.; Lindon, J.C.; Mitchell, S.C.; Holmes, E.; McCarthy, M.I.; Scott, J.; Gauguier, D.; Nicholson, J.K. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc. Natl. Acad. Sci. U S A 2006, 103, 12511–12516. [Google Scholar]
- Lezama, R.; Diaz-Tellez, A.; Ramos-Mandujano, G.; Oropeza, L.; Pasantes-Morales, H. Epidermal growth factor receptor is a common element in the signaling pathways activated by cell volume changes in isosmotic, hyposmotic or hyperosmotic conditions. Neurochem. Res. 2005, 30, 1589–1597. [Google Scholar]
- Turnbaugh, P.; Ley, R.; Mahowald, M.; Magrini, V.; Mardis, E.; Gordon, J. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar]
- Bjorksten, B.; Sepp, E.; Julge, K.; Voor, T.; Mikelsaar, M. Allergy development and the intestinal microflora during the first year of life. J. Allergy Clin. Immunol. 2001, 108, 516–520. [Google Scholar]
- Warren, J.R. Gastric pathology associated with Helicobacter pylori. Gastroenterol. Clin. North Am. 2000, 29, 705–751. [Google Scholar]
- Marshall, B. Helicobacter pylori: past, present and future. Keio J. Med. 2003, 52, 80–85. [Google Scholar]
- Pereira, S.; Marliss, E.B.; Morais, J.A.; Chevalier, S.; Gougeon, R. Insulin resistance of protein metabolism in type 2 diabetes. Diabetes 2008, 57, 56–63. [Google Scholar]
- Gupta, P.; Andrew, H.; Kirschner, B.S.; Guandalini, S. Is lactobacillus GG helpful in children with Crohn's disease? Results of a preliminary, open-label study. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 453–457. [Google Scholar]
- Sartor, R.B. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology 2004, 126, 1620–1633. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.P.; Dumas, M.E.; Wang, Y.; Legido-Quigley, C.; Yap, I.K.; Tang, H.; Zirah, S.; Murphy, G.M.; Cloarec, O.; Lindon, J.C.; Sprenger, N.; Fay, L.B.; Kochhar, S.; van Bladeren, P.; Holmes, E.; Nicholson, J.K. A top-down systems biology view of microbiome-mammalian metabolic interactions in a mouse model. Mol. Syst. Biol. 2007, 3, 112. [Google Scholar]
- Martin, F.P.; Sprenger, N.; Yap, I.K.; Wang, Y.; Bibiloni, R.; Rochat, F.; Rezzi, S.; Cherbut, C.; Kochhar, S.; Lindon, J.C.; Holmes, E.; Nicholson, J.K. Panorganismal Gut Microbiome-Host Metabolic Crosstalk. J. Proteome. Res. 2009, 8, 2090–2105. [Google Scholar]
- Martin, F.P.; Wang, Y.; Sprenger, N.; Yap, I.K.; Rezzi, S.; Ramadan, Z.; Pere-Trepat, E.; Rochat, F.; Cherbut, C.; van, B.P.; Fay, L.B.; Kochhar, S.; Lindon, J.C.; Holmes, E.; Nicholson, J.K. Top-down systems biology integration of conditional prebiotic modulated transgenomic interactions in a humanized microbiome mouse model. Mol. Syst. Biol. 2008, 4, 205. [Google Scholar]
- Martin, F.P.; Wang, Y.; Sprenger, N.; Yap, I.K.; Lundstedt, T.; Lek, P.; Rezzi, S.; Ramadan, Z.; van Bladeren, P.; Fay, L.B.; Kochhar, S.; Lindon, J.C.; Holmes, E.; Nicholson, J.K. Probiotic modulation of symbiotic gut microbial-host metabolic interactions in a humanized microbiome mouse model. Mol. Syst. Biol. 2008, 4, 157. [Google Scholar]
- Collins, M.D.; Gibson, G.R. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am. J. Clin. Nutr. 1999, 69, 1052–1057. [Google Scholar] [PubMed]
- Montoliu, I.; Martin, F.P.; Collino, S.; Rezzi, S.; Kochhar, S. Multivariate modeling strategy for intercompartmental analysis of tissue and plasma 1H NMR spectrotypes. J. Proteome. Res. 2009, 8, 2397–2406. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Collino, S.; Martin, F.-P.J.; Kochhar, S.; Rezzi, S. Monitoring Healthy Metabolic Trajectories with Nutritional Metabonomics. Nutrients 2009, 1, 101-110. https://doi.org/10.3390/nu1010101
Collino S, Martin F-PJ, Kochhar S, Rezzi S. Monitoring Healthy Metabolic Trajectories with Nutritional Metabonomics. Nutrients. 2009; 1(1):101-110. https://doi.org/10.3390/nu1010101
Chicago/Turabian StyleCollino, Sebastiano, François-Pierre J. Martin, Sunil Kochhar, and Serge Rezzi. 2009. "Monitoring Healthy Metabolic Trajectories with Nutritional Metabonomics" Nutrients 1, no. 1: 101-110. https://doi.org/10.3390/nu1010101
APA StyleCollino, S., Martin, F.-P. J., Kochhar, S., & Rezzi, S. (2009). Monitoring Healthy Metabolic Trajectories with Nutritional Metabonomics. Nutrients, 1(1), 101-110. https://doi.org/10.3390/nu1010101