Novel Experimental and Clinical Therapeutic Uses of Low-Molecular-Weight Heparin/Protamine Microparticles
Abstract
:1. Introduction
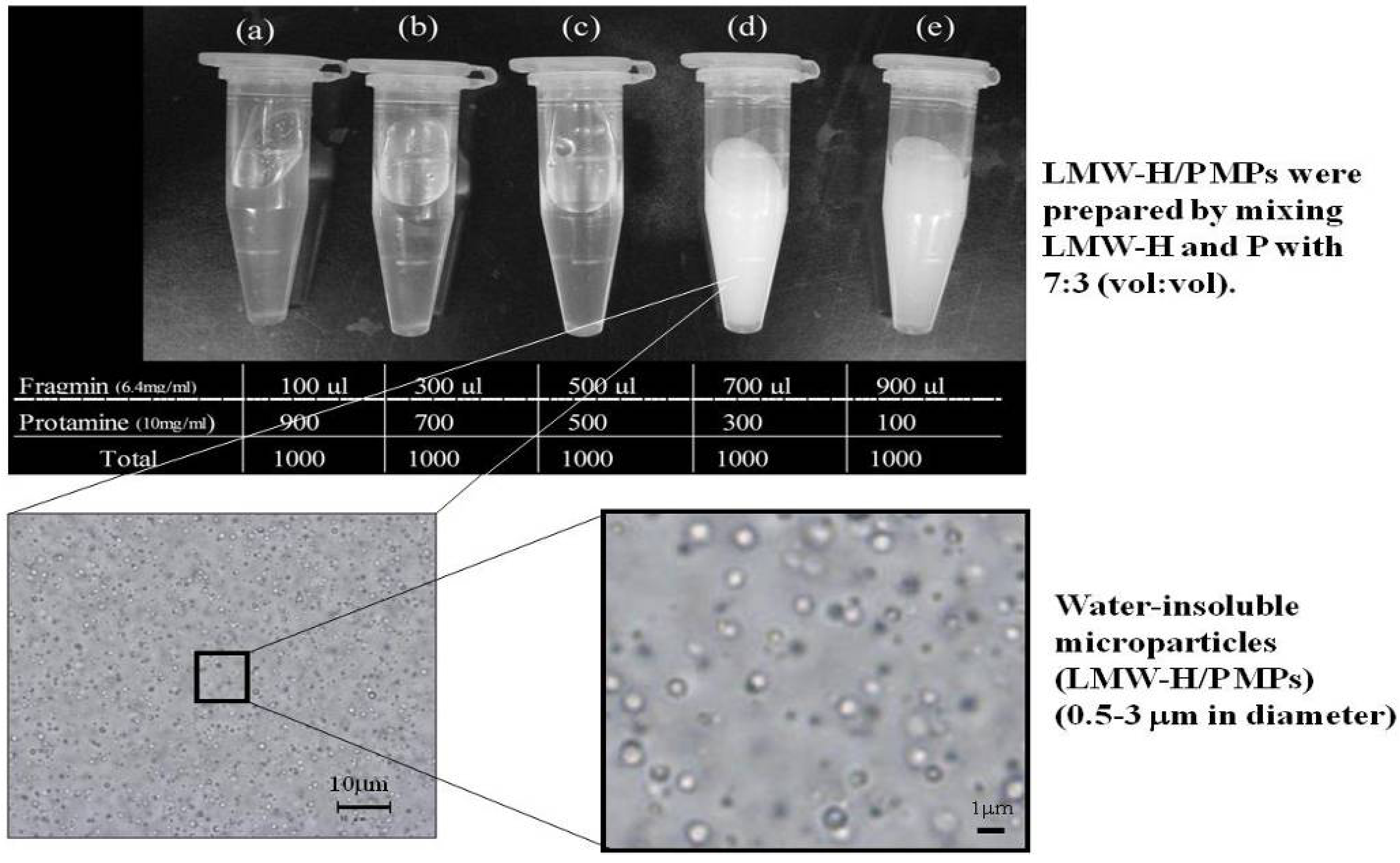
2. Protein-Delivery Microparticles
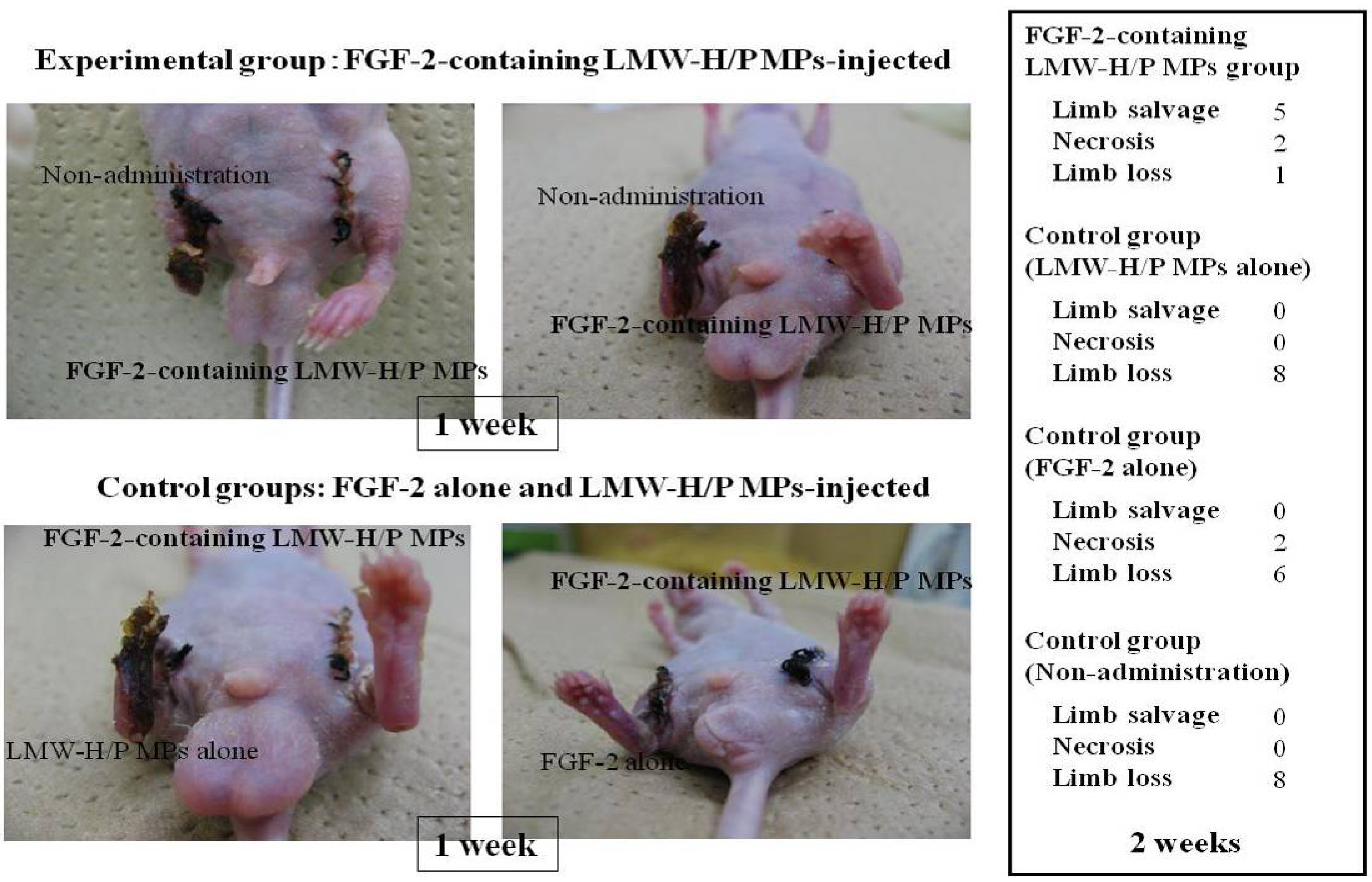
2.1. Preparation of FGF-2-Containing LMW-H/P MPs and Their Applications
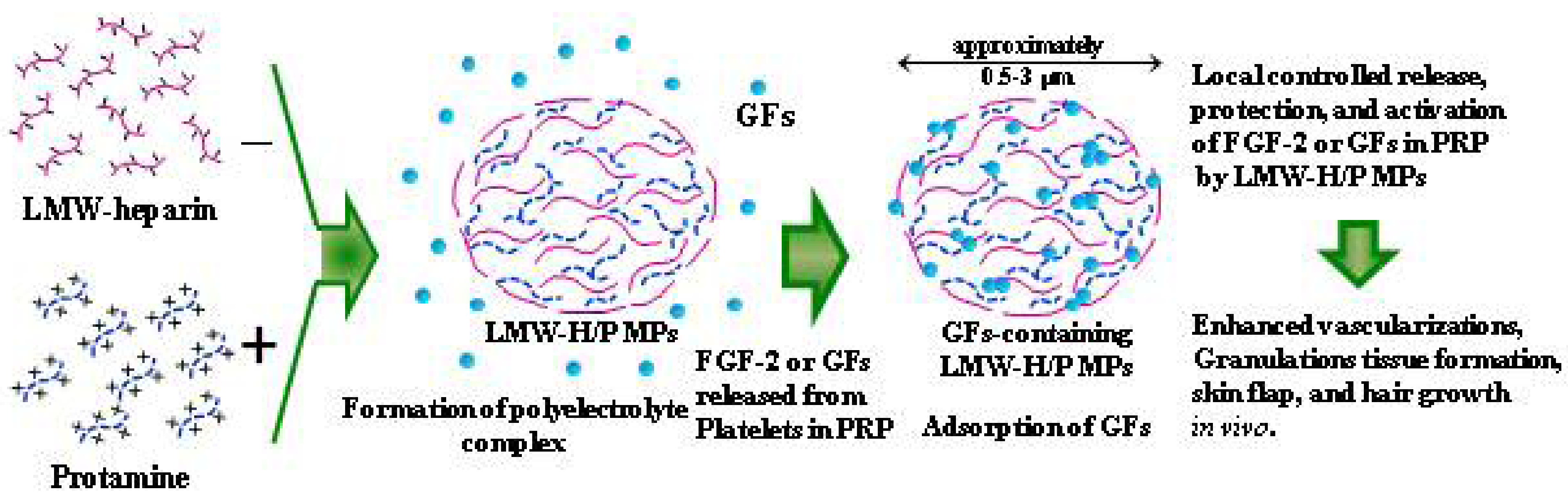
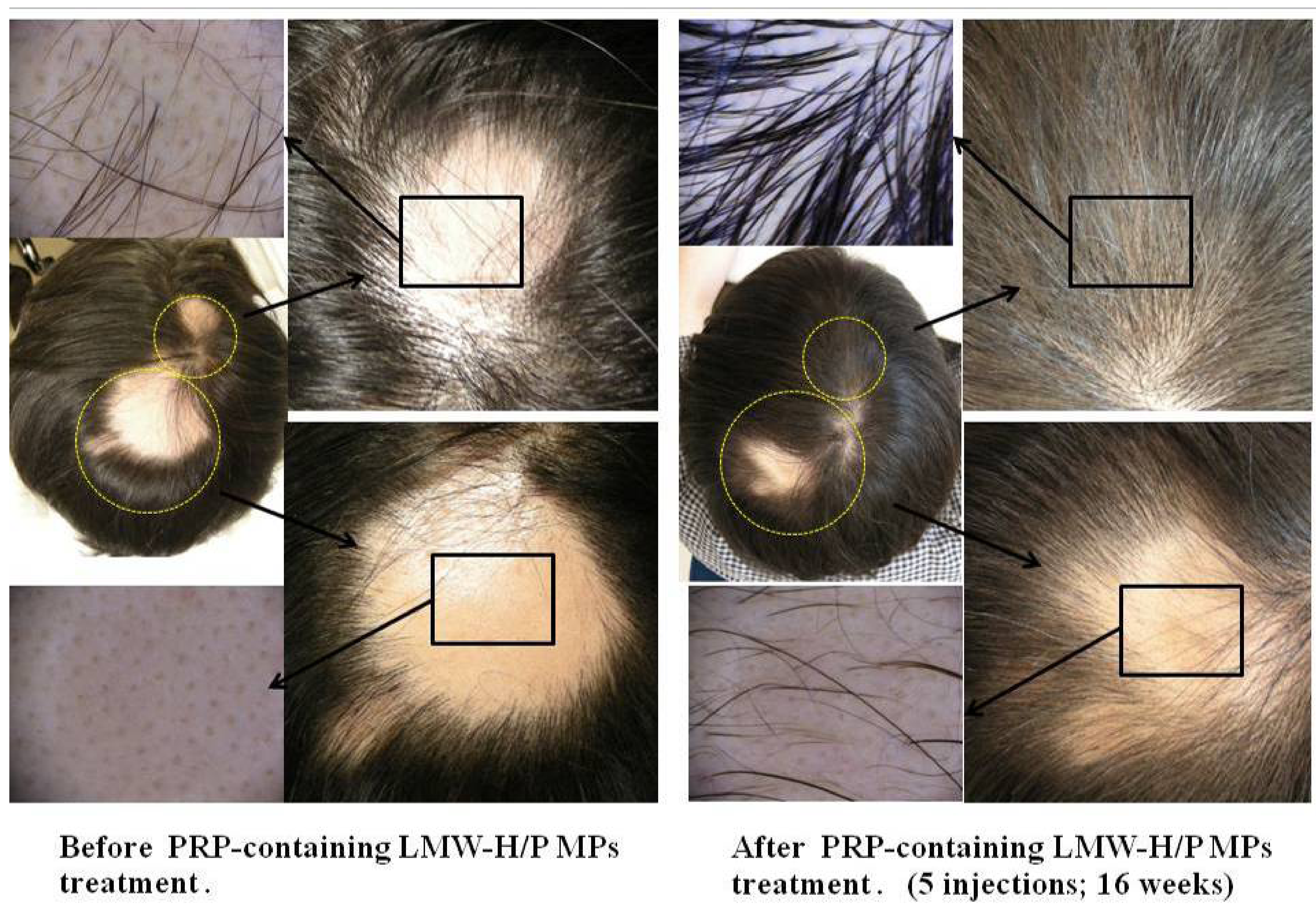
2.2. Preparation of PRP-Containing LMW-H/P MPs and Their Applications
3. Cell-Delivery Microparticles
3.1. LMW-H/P MPs as Stromal Cell Carriers
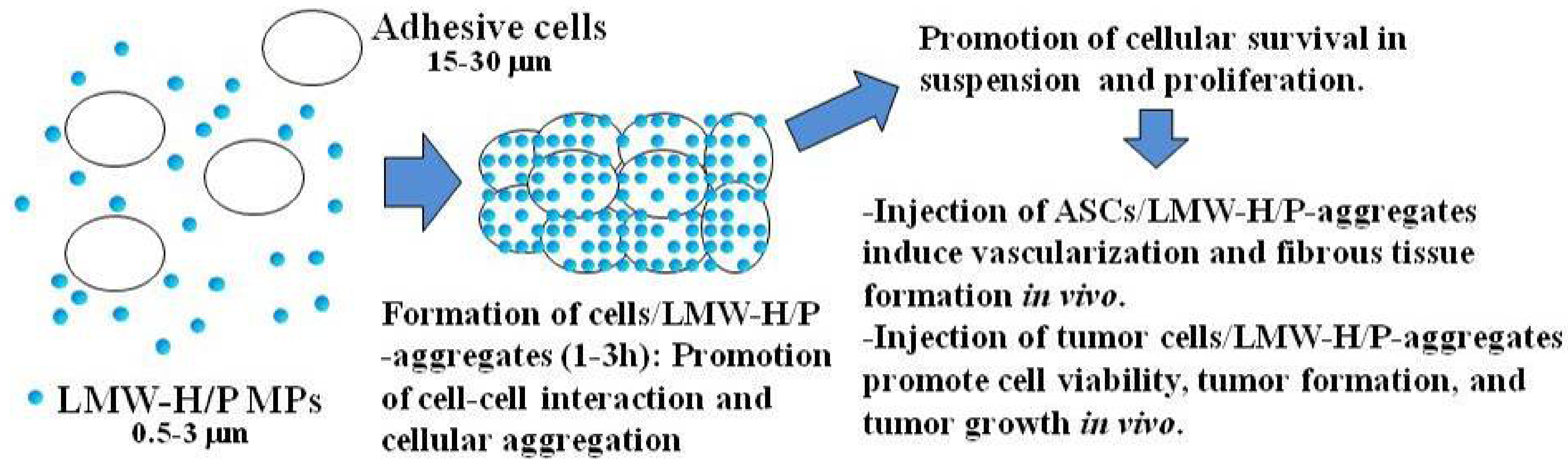
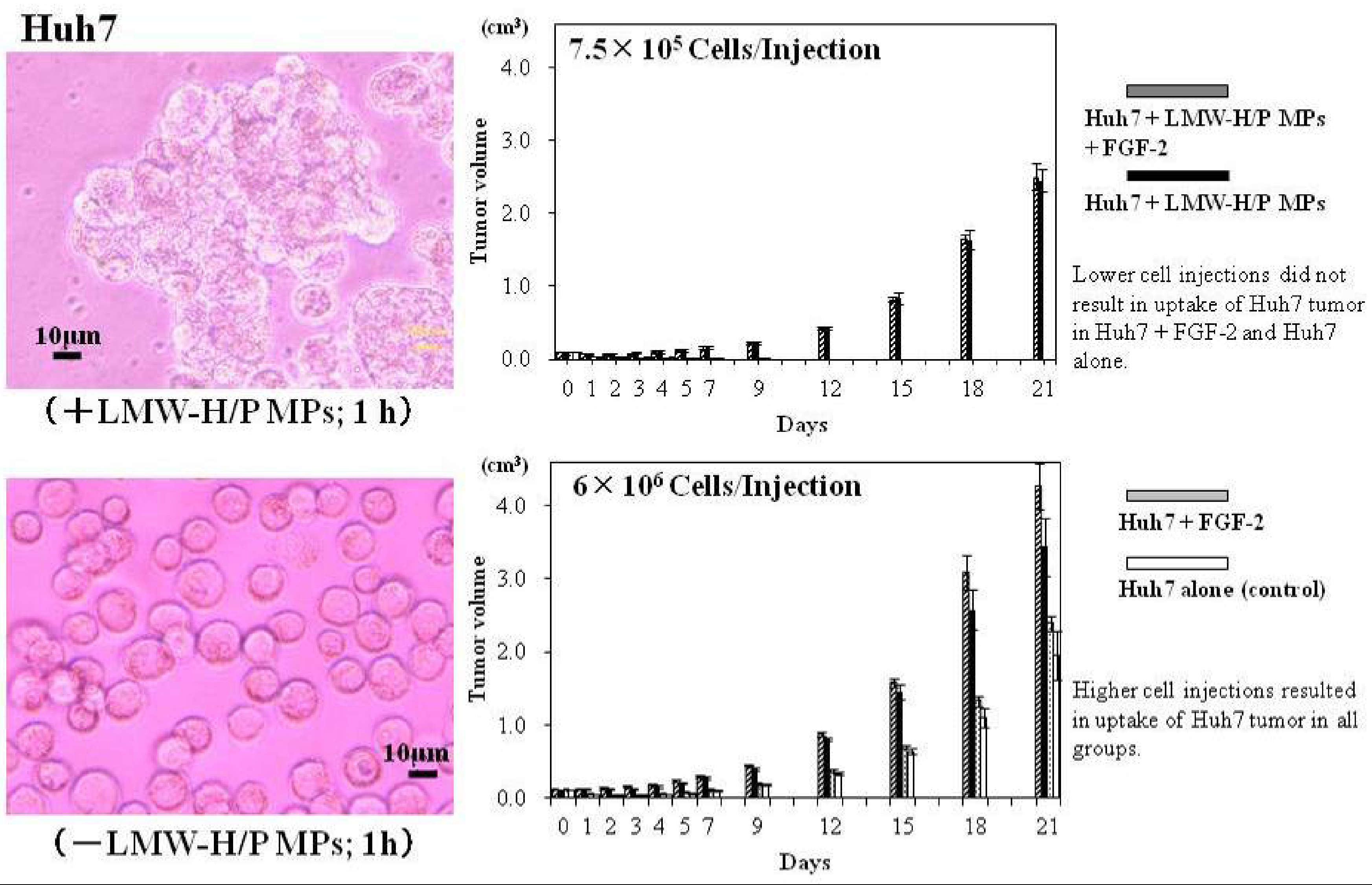
3.2. LMW-H/P MPs as Tumor Cell Carriers
4. A Cell Culture System Using LMW-H/P MPs-Coated Plates
4.1. Various Types of Cell Cultures Using LMW-H/P MPs-Coated Plates

4.2. Culture Expansion of BMSCs and ASCs on LMW-H/P MPs-Coated Plates
4.3. Proliferation of CD34+ Hematopoietic Progenitor Cells (CD34+ HCs) on LMW-H/P MPs-Coated Plates
5. Conclusions
References
- Dautzenberg, H.; Hartmann, J.; Grunewald, S.; Brand, F. Stoichiometry and structure of polyelectrolyte complex particles in diluted solution. Ber. Bunsenges Phys. Chem. 1996, 100, 1024–1032. [Google Scholar]
- Wassmer, K.H.; Schroeder, U.; Horn, D. Characterization and detection of polyanions by direct polyelectrolyte titration. Makromol. Chem. 1991, 192, 553–565. [Google Scholar] [CrossRef]
- Park, J.M.; Muhoberac, B.B.; Dubin, P.L.; Xia, J. Effect of protein charge heterogeneity in protein-polyelectrolyte complexation. Macromolecules 1992, 25, 290–295. [Google Scholar] [CrossRef]
- Mattison, K.W.; Dubin, P.L.; Brittain, I.J. Complex formation between bovine serum albumin and strong polyelectrolytes: Effect of polymer charge density. J. Phys. Chem. B. 1998, 102, 3830–3836. [Google Scholar] [CrossRef]
- Dragan, S.; Cristea, M.; Luca, C.; Simionescu, B.C. Polyelectrolyte complex. I: Synthesis and characterization of some insoluble polyanion-polycation complexes. J. Polym. Sci. A 1996, 34, 3487–3495. [Google Scholar]
- Nordmeier, E.; Beyer, P. Nonstoichiometric polyelectrolyte complexes: A mathematical model and some experimental results. J. Polym. Sci. Pol. Phys. 1999, 37, 335–348. [Google Scholar] [CrossRef]
- Holappa, S.; Andersson, T.; Kantonen, L.; Plattnerm, P.; Tenhun, H. Soluble polyelectrolyte complexes composed of poly (ethylene oxide)-block-poly (sodium methacrylate) and poly (methacryloyloxyethyl trimethylammonium chloride). Polymer 2003, 44, 7907–7916. [Google Scholar] [CrossRef]
- Webster, L.; Huglin, M.B.; Robb, I.D. Complex formation between polyelectrolytes in dilute aqueous solution. Polymer 1997, 38, 1373–1380. [Google Scholar] [CrossRef]
- Hashimoto, M.; Koyama, Y.; Sato, T. In vitro gene delivery by pDNA/chitosan complexes coated with anionic PEG derivatives that have a sugar side chain. Chem. Lett. 2008, 37, 266–267. [Google Scholar] [CrossRef]
- Denuziere, A.; Ferrier, D.; Domard, A. Chitosan-chondroitin sulfate and chitosan-hyaluronate polyelectrolyte complexes. Physico-chemical aspects. Carbohydr. Polym. 1996, 29, 317–323. [Google Scholar]
- Kohane, D.S.; Lipp, M.; Kinney, R.C.; Louis, D.N.; Lotan, N. Biocompatibility of lipid-protein-sugar particles containing bupivacaine in the epineurium. J. Biomed. Mater. Res. 2002, 59, 450–409. [Google Scholar] [CrossRef]
- Kohane, D.S.; Tse, J.Y.; Yeo, Y.; Shubina, M.; Langer, R. Biodegradable polymeric microspheres and nanospheres for drug delivery in the peritoneum. J. Biomed. Mater. Res. 2006, 77, 351–361. [Google Scholar]
- Lindahl, U.; Lidholt, K.; Spillmann, D.; Kjellen, L. More to “heparin” than anti-coagulation. Thromb. Res. 1994, 75, 1–32. [Google Scholar] [CrossRef]
- Salmivirta, M.; Lidhold, K.; Lindahl, U. Heparan sulfate: A piece of information. FASEB J. 1996, 10, 1270–1279. [Google Scholar]
- Ishihara, M.; Ono, K. Structure and function of heparin and heparan sulfate: Heparinoid library and modification of FGF-activities. Trends Glycosci. Glycotechnol. 1998, 10, 223–233. [Google Scholar] [CrossRef]
- Hirsh, J.; Warkentin, T.E.; Shaughnessy, S.G.; Anand, S.S.; Halperin, J.L.; Raschke, R.; Granger, C. Heparin and low-molecular heparin, mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest 2001, 119, 64–94. [Google Scholar] [CrossRef]
- Wolzt, M.; Wetermann, A.; Nieszpaur-Los, M.; Schneider, B.; Fassolt, A.; Lechner, K.; Eichler, H.; Kyrle, P.A. Studies on the neutralizing effects of protamine on unfractionated and low molecular weight heparin (Fragmin®) at the site of activation of the coagulation system in man. Thromb. Haemost. 1995, 73, 439–443. [Google Scholar]
- Pan, M.; Lezo, J.S.; Medina, A.; Romero, M.; Hernandez, E.; Segura, J.; Melian, F.; Wanguemert, F.; Landin, M.; Benitez, F.; et al. In-laboratory removal of femoral sheath following protamine administration in patients having intracoronary stent implantation. Am. J. Cardiol. 1997, 80, 1336–1338. [Google Scholar]
- Rosenstock, J.; Fonseca, V.; McGill, J.B.; Riddle, M.; Halle, J.P.; Hramiak, I.; Johnston, P.; David, M. Similar risk of malignancy with insulin glargine and neutral protamine Hagerdorn (NPH) in patients with type 2 diabetes: Finding from a 5-year randomized, open-label study. Diabetologia 2009. [Google Scholar] [CrossRef]
- Fujita, M.; Ishihara, M.; Shimizu, M.; Obara, K.; Ishizuka, T.; Saito, Y.; Yura, H.; Morimoto, Y.; Takase, B.; Matsui, T.; et al. Vascularization in vivo caused by the controlled release of fibroblast growth factor-2 from an injectable chitosan/non-anticoagulant heparin hydrogel. Biomaterials 2004, 25, 699–706. [Google Scholar]
- Nakamura, S.; Nambu, M.; Kishimoto, S.; Ishizuka, T.; Hattori, H.; Kanatani, Y.; Takase, B.; Aoki, H.; Kiyosawa, T.; Maehara, T.; et al. Effect of controlled release of fibroblast growth factor-2 from chitosan/fucoidan micro complex hydrogel on in vitro and in vivo vascularization. J. Biomed. Mater. Res. A 2008, 85, 619–627. [Google Scholar]
- Nakamura, S.; Kanatani, Y.; Kishimoto, S.; Nambu, M.; Ohno, C.; Hattori, H.; Takase, B.; Tanaka, Y.; Yura, H.; Kiyosawa, T.; et al. Controlled release of FGF-2 using fragmin/protamine microparticles and effect on neovascularization. J. Biomed. Mater. Res. A 2009, 91, 814–823. [Google Scholar]
- Takikawa, M.; Nakamura, S.-I.; Nakamura, S.; Nambu, M.; Ishihara, M.; Fujita, M.; Kishimoto, S.; Doumoto, T.; Yanagibayashi, S.; Azuma, R.; et al. Enhancement of vascularization and granulation tissue formation by growth factors in human platelet-rich plasma-containing fragmin/protamine microparticles. J. Biomed. Mater. Res. B 2011, 97, 373–380. [Google Scholar]
- Mori, Y.; Nakamura, S.; Kishimoto, S.; Kawakami, M.; Suzuki, S.; Matsui, T.; Ishihara, M. Preparation and characterization of low-molecular-weight heparin/protamine nanoparticles (LMW-H/P NPs) as FGF-2 carrier. Int. J. Nanomed. 2010, 5, 147–155. [Google Scholar]
- Nakamura, S.; Kishimoto, S.; Nakamura, S.-I.; Nambu, M.; Fujita, M.; Tanaka, Y.; Mori, Y.; Tagawa, M.; Maehara, T.; Ishihara, M. Fragmin/protamine microparticles as cell carriers to enhance viability of adipose-derived stromal cells and their subsequent effect on in vivo neovascularization. J. Biomed. Mater. Res. A 2010, 92, 1614–1622. [Google Scholar]
- Kumano, I.; Kishimoto, S.; Nakamura, S.; Hattori, H.; Tanaka, Y.; Nakata, M.; Sato, T.; Fujita, M.; Maehara, T.; Ishihara, M. Fragmin/protamine microparticles (F/P MPs) as cell carriers enhance the formation and growth of tumors in vivo. Cell. Mol.Bioeng. 2011, 4, 476–483. [Google Scholar] [CrossRef]
- Kishimoto, S.; Nakamura, S.; Nakamura, S.-I.; Kanatani, Y.; Hattori, H.; Tanaka, Y.; Harada, Y.; Tagawa, M.; Mori, Y.; Maehara, T.; et al. Fragmin/protamine microparticle-coated matrix immobilized cytokines to stimulate various cell proliferations with low serum media. Artif. Org. 2009, 33, 431–438. [Google Scholar] [CrossRef]
- Kishimoto, S.; Hattori, H.; Nakamura, S.; Amano, Y.; Kanatani, Y.; Tanaka, Y.; Mori, Y.; Harada, Y.; Tagawa, M.; Ishihara, M. Expansion and characterization of human bone marrow-derived mesenchymal stem cells cultured on fragmin/protamine microparticle-coated matrix with fibroblast growth factor-2 in low serum medium. Tissue Eng. Part C 2009, 15, 523–527. [Google Scholar] [CrossRef]
- Kishimoto, S.; Nakamura, S.; Nakamura, S.-I.; Hattori, H.; Oomuma, F.; Kanatani, Y.; Tanaka, Y.; Harada, Y.; Tagawa, M.; Maehara, T.; Ishihara, M. Cytokine-immobilized microparticle-coated plates for culturing hematopoietic progenitor cells. J. Control.Release 2009, 133, 185–190. [Google Scholar] [CrossRef]
- Gospodarowicz, D.; Cheng, J. Heparin protects basic and acidic FGF from inactivation. J. Cell Physiol. 1986, 128, 475–484. [Google Scholar] [CrossRef]
- Nakamura, S.-I.; Ishihara, M.; Takikawa, M.; Murakami, K.; Kishimoto, S.; Nakamura, S.; Yanagibayashi, S.; Mori, Y.; Fujita, M.; Kubo, S.; et al. Increased survival of free fat grafts and vascularization in rats with local delivery of fragmin/protamine microparticles containing FGF-2 (F/P MP-F). J. Biomed. Mater. Res. B 2011, 96, 234–241. [Google Scholar]
- Horio, T.; Fujita, M.; Tanaka, Y.; Ishihara, M.; Kishimoto, S.; Nakamura, S.; Shimizu, M.; Nogami, Y.; Hattori, H.; Hase, K.; et al. Efficacy of fragmin/protamine microparticles containing fibroblast growth factor-2 (F/P MP/FGF-2) in a rabbit model of hindlimb ischemia. J. Vasc. Surg. 2011, 54, 791–798. [Google Scholar]
- Marx, R.E. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant. Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef]
- Bhanot, S.; Alex, J.C. Current applications of platelet gels in facial plastic surgery. Facial. Plast. Surg. 2002, 18, 27–33. [Google Scholar] [CrossRef]
- Takikawa, M.; Sumi, Y.; Ishihara, M.; Kishimoto, S.; Nakamura, S.; Yanagibayashi, S.; Hattori, H.; Azuma, R.; Yamamoto, N.; Kiyosawa, T. PRP&F/P MPs improved survival of dorsal paired pedicle skin flaps in rats. J. Surg. Res. 2011, 170, e189–e196. [Google Scholar] [CrossRef]
- Takikawa, M.; Nakamura, S.-I.; Nakamura, S.; Nambu, M.; Ishihara, M.; Murakami, K.; Kishimoto, S.; Sasaki, K.; Yanagishita, S.; Azuma, R.; et al. Enhanced Effect of Platelet-Rich Plasma containing a new carrier on hair growth. Dermatol. Surg. 2011, 37, 1721–1729. [Google Scholar] [CrossRef]
- Hattori, H.; Masuoka, K.; Sato, M.; Ishihara, M.; Asazuma, T.; Takase, B.; Kikuchi, M.; Nemoto, K.; Ishihara, M. Bone formation using human adipose tissue-derived stromal cells and a biodegradable scaffold. J. Biomed. Mater. Res. B 2006, 76, 230–239. [Google Scholar]
- Wang, H.J.; Pieper, J.; Schotel, R.; van Blitterswijk, C.A.; Lamme, E.N. Stimulation of skin repair is dependent on fibroblast source and presence of extracellular matrix. Tissue Eng. 2004, 10, 1054–1064. [Google Scholar]
- Nakagami, H.; Morishita, R.; Maeda, K.; Kikuchi, Y.; Ogihara, T.; Kaneda, Y. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J. Atheroscler. Thromb. 2006, 13, 77–81. [Google Scholar]
- Volpe, J.P.G.; Milas, L. Influence of tumor transplantation methods on tumor growth rate and metastatic potential of solitary tumors derived metastasis. Clin. Expl. Metasitasis 1990, 8, 381–389. [Google Scholar] [CrossRef]
- Ishihara, M.; Ono, K. Structure and function of heparin and heparin sulfate; heparinoid library and modification of FGF-activities. Trends Glycosci. Glycotechnol. 1998, 10, 223–233. [Google Scholar] [CrossRef]
- Salmivirta, M.; Lidhold, K.; Lindahl, U. Heparan sulfate: A piece of information. FASEB. J. 1996, 10, 1270–1279. [Google Scholar]
- Lindahl, U.; Lidholt, K.; Spillmann, D.; Kjellen, L. More to “heparin” than anti-coagulation. Thromb. Res. 1994, 75, 1–32. [Google Scholar] [CrossRef]
- Prockop, D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997, 276, 71–74. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar]
- Ferrari, G.; Cusella-DeAngelis, G.; Coletta, M.; Paolucci, E.; Stornaiuolo, A.; Cossu, G.; Mavilio, F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science 1998, 279, 1528–1530. [Google Scholar]
- Woodbury, D.; Reynoldsk, K.; Black, I.B. Adult bone marrow stromal stem cells express germline, ectodermal, endodermal, and mesodermal genes prior to neurogenesis. J.Neurosci.Res. 2002, 69, 908–917. [Google Scholar] [CrossRef]
- Spees, J.L.; Gregory, C.A.; Singh, H.; Tucker, H.A.; Peister, A.; Lynch, P.J.; Hsu, S.C.; Smith, J.; Prockop, D.J. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol. Ther. 2004, 9, 747–756. [Google Scholar]
- Martin, M.J.; Muotri, A.; Gage, F.; Varki, A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat. Med. 2005, 11, 228–232. [Google Scholar]
- Gupta, P.; Oegema, T.R.; Brazil, J.J.; Dudek, A.Z.; Slungaard, A.; Verfaillie, C.M. Structurally specific heparan sulfates support primitive human hematopoiesis by formation of a multimolecular stem cell niche. Blood 1998, 92, 4641–4651. [Google Scholar]
- Alvarez-Silva, M.; Borojevic, R. GM-CSF and IL-3 activities in schistosomal liver granulomas are controlled by stroma-associated heparan sulfate proteoglycans. J. Leukoc. Biol. 1996, 59, 435–441. [Google Scholar]
- Roberts, R.; Gallagher, J.; Spooncer, E.; Allen, T.D.; Bloomfield, F.; Dexter, T.M. Heparan sulfate bound growth factors: A mechanism for stromal cell mediated haemopoiesis. Nature 1988, 332, 376–378. [Google Scholar]
- Gordon, M.Y.; Riley, G.P.; Watt, S.M.; Greaves, M.F. Compartmentalization of a haematopoietic growth factor (GM-CSF) by glycosaminoglycans in the bone marrow microenvironment. Nature 1987, 326, 403–405. [Google Scholar]
- Drayer, A.L.; Olthof, S.G.; Vellenga, E. Mammalian target of rapamycin is required for thrombopoietin-induced proliferation of megakaryocyte progenitors. Stem Cells 2006, 24, 105–114. [Google Scholar] [CrossRef]
- Schepers, H.; Wierenga, A.T.; Gasliga, D.V.; Eggen, B.J.; Vellenga, E.; Schuringa, J.J. Reintroduction of C/EBPalpha in leukemic CD34+ stem/progenitor cells impairs self-renewal and partially restores myelopoiesis. Blood 2007, 110, 1317–1325. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kishimoto, S.; Ishihara, M.; Takikawa, M.; Mori, Y.; Hattori, H.; Fujita, M.; Nakamura, S. Novel Experimental and Clinical Therapeutic Uses of Low-Molecular-Weight Heparin/Protamine Microparticles. Pharmaceutics 2012, 4, 42-57. https://doi.org/10.3390/pharmaceutics4010042
Kishimoto S, Ishihara M, Takikawa M, Mori Y, Hattori H, Fujita M, Nakamura S. Novel Experimental and Clinical Therapeutic Uses of Low-Molecular-Weight Heparin/Protamine Microparticles. Pharmaceutics. 2012; 4(1):42-57. https://doi.org/10.3390/pharmaceutics4010042
Chicago/Turabian StyleKishimoto, Satoko, Masayuki Ishihara, Megumi Takikawa, Yasutaka Mori, Hidemi Hattori, Masanori Fujita, and Shingo Nakamura. 2012. "Novel Experimental and Clinical Therapeutic Uses of Low-Molecular-Weight Heparin/Protamine Microparticles" Pharmaceutics 4, no. 1: 42-57. https://doi.org/10.3390/pharmaceutics4010042
APA StyleKishimoto, S., Ishihara, M., Takikawa, M., Mori, Y., Hattori, H., Fujita, M., & Nakamura, S. (2012). Novel Experimental and Clinical Therapeutic Uses of Low-Molecular-Weight Heparin/Protamine Microparticles. Pharmaceutics, 4(1), 42-57. https://doi.org/10.3390/pharmaceutics4010042




