Recrystallization of Commercial Carbamazepine Samples—A Strategy to Control Dissolution Variability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polymorphic Characterization
2.2. Morphological Characterization
2.3. Water Activity Measurement
2.4. Unidirectional Dissolution Method
2.4.1. Sample Preparation
2.4.2. Dissolution
2.4.3. Evaluation of DIDR Profiles
2.4.4. Analysis of Compact Surface
2.5. Tensile Strength
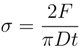
3. Results and Discussion
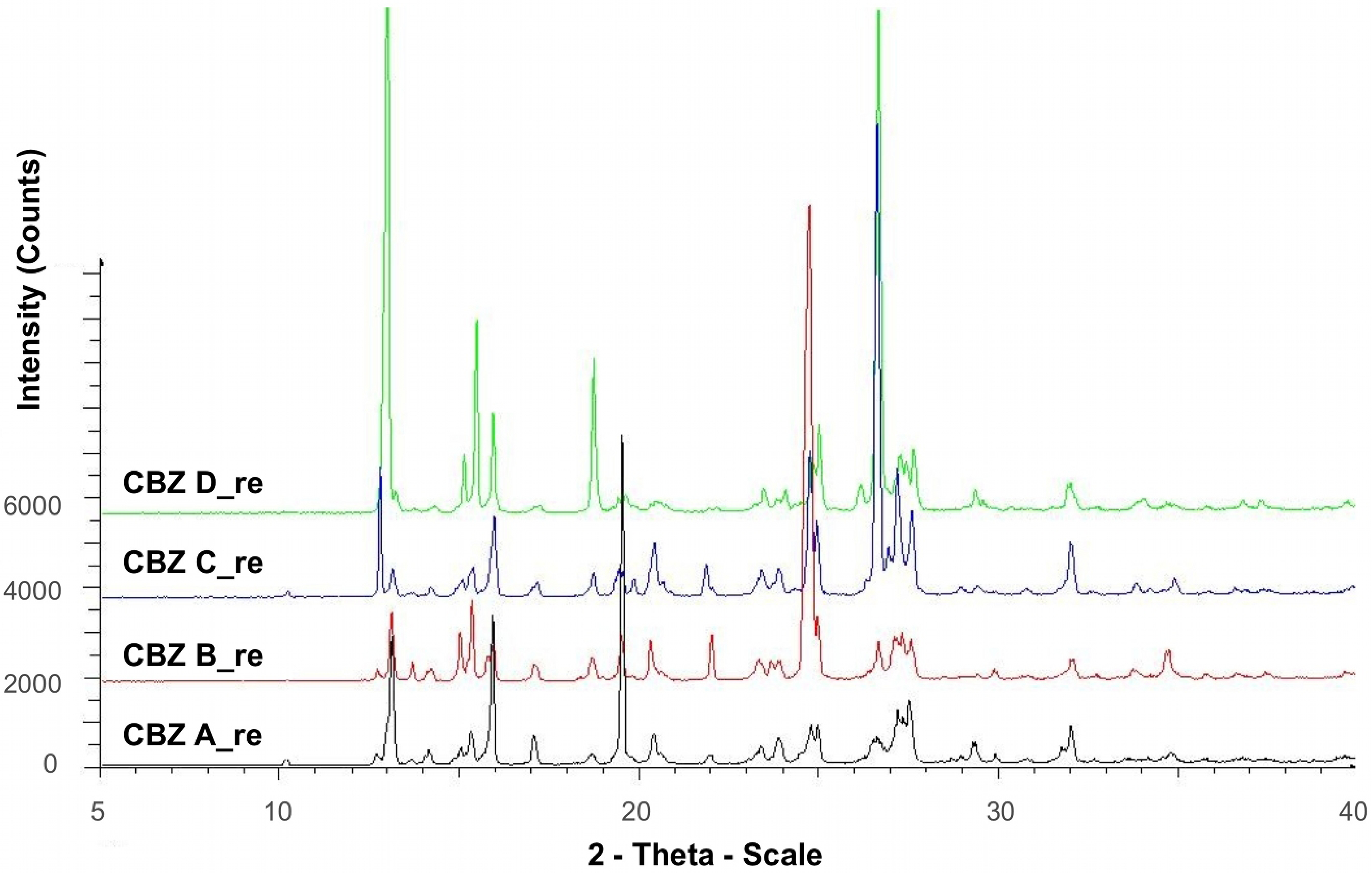
3.1. Polymorphic Characterization

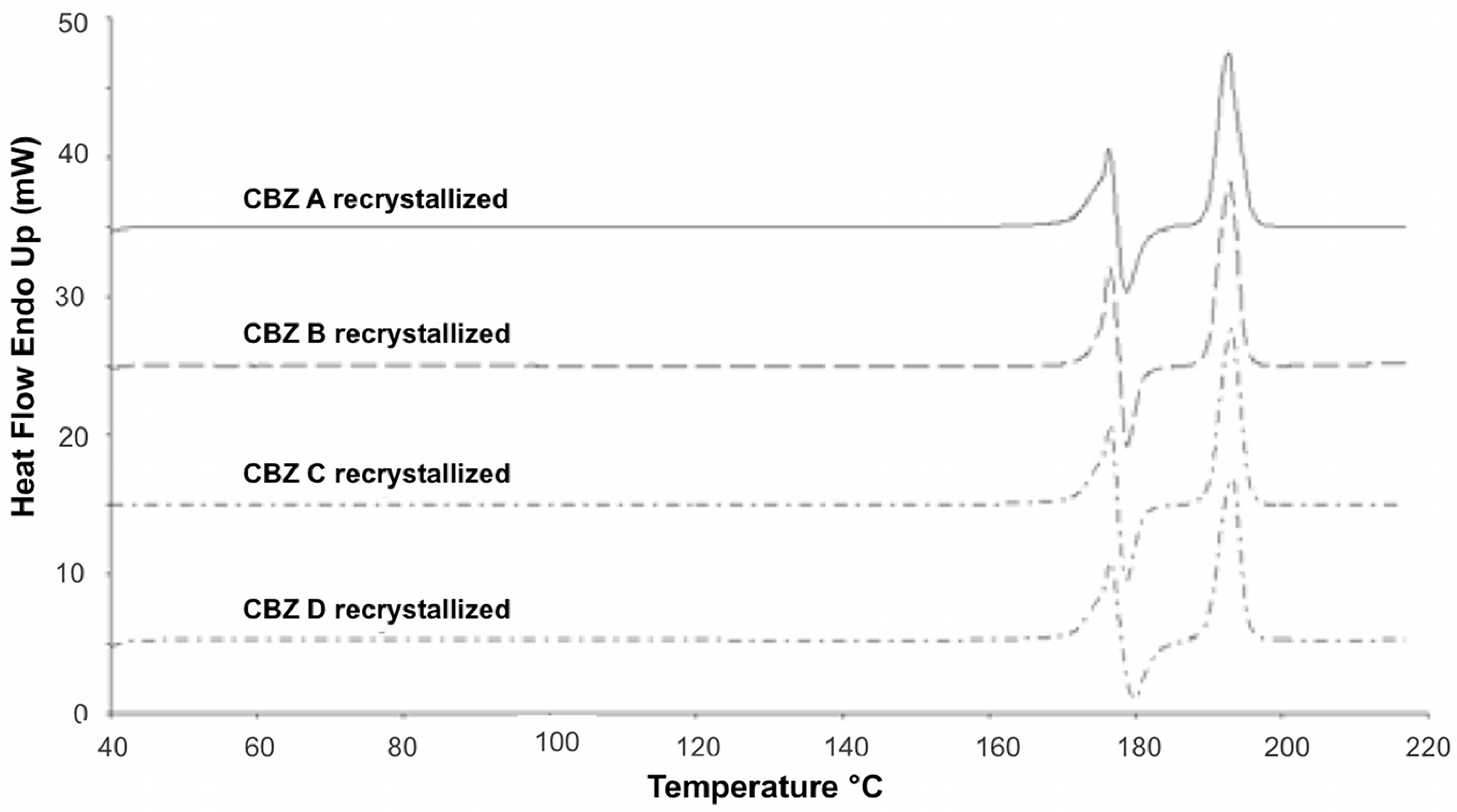
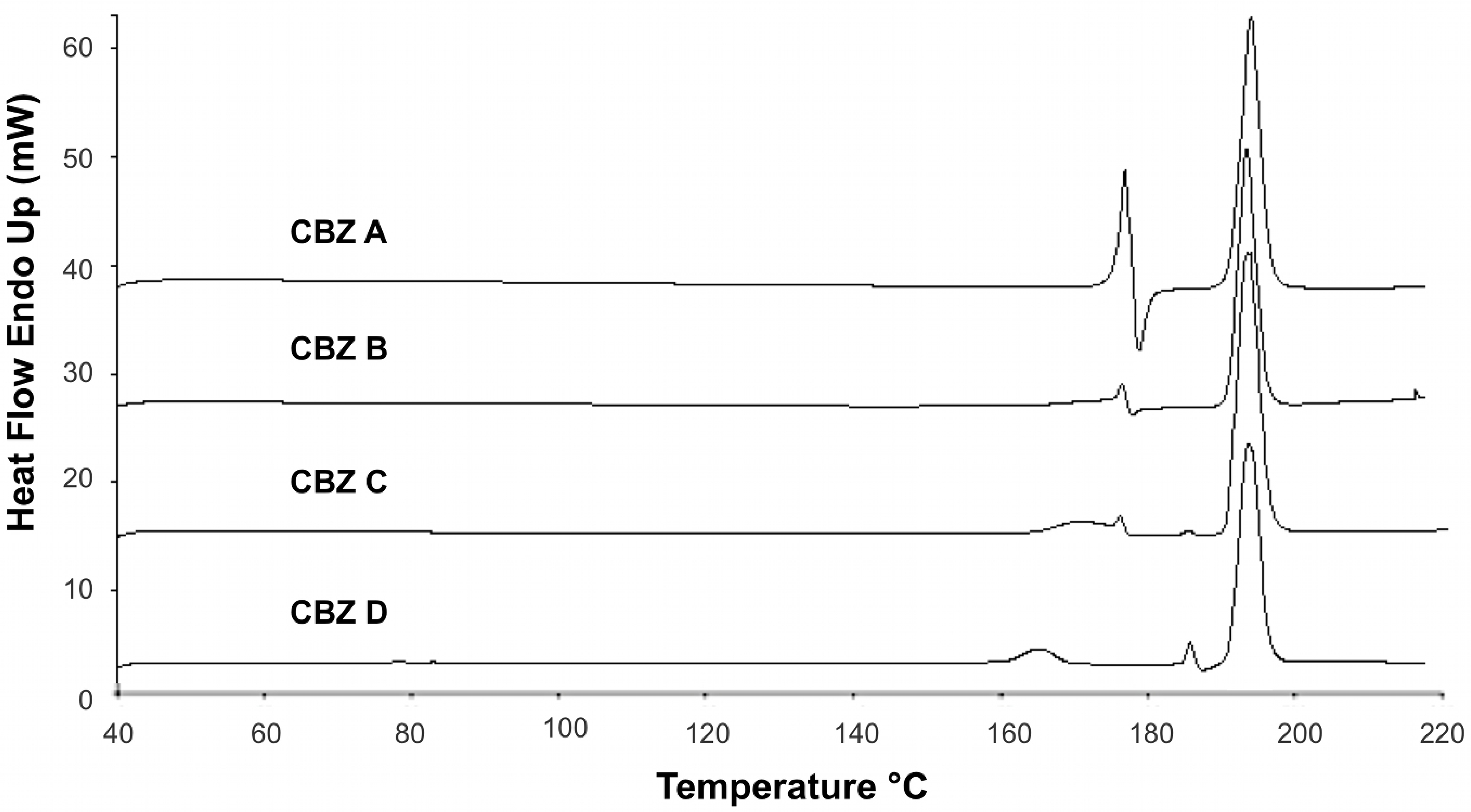
3.2. Morphological Characterization
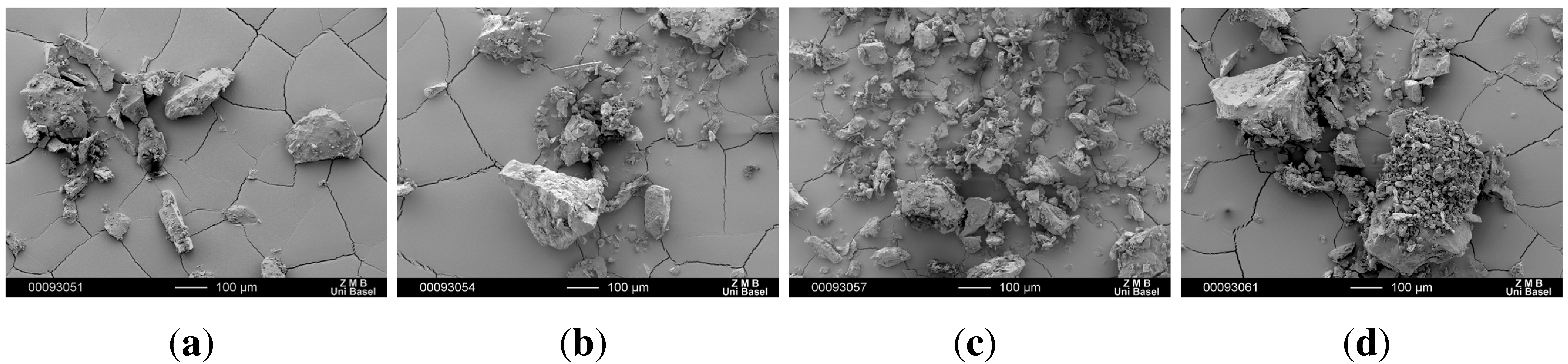
3.3. Water Activity
3.4. DIDR Profiles of Recrystallized CBZ
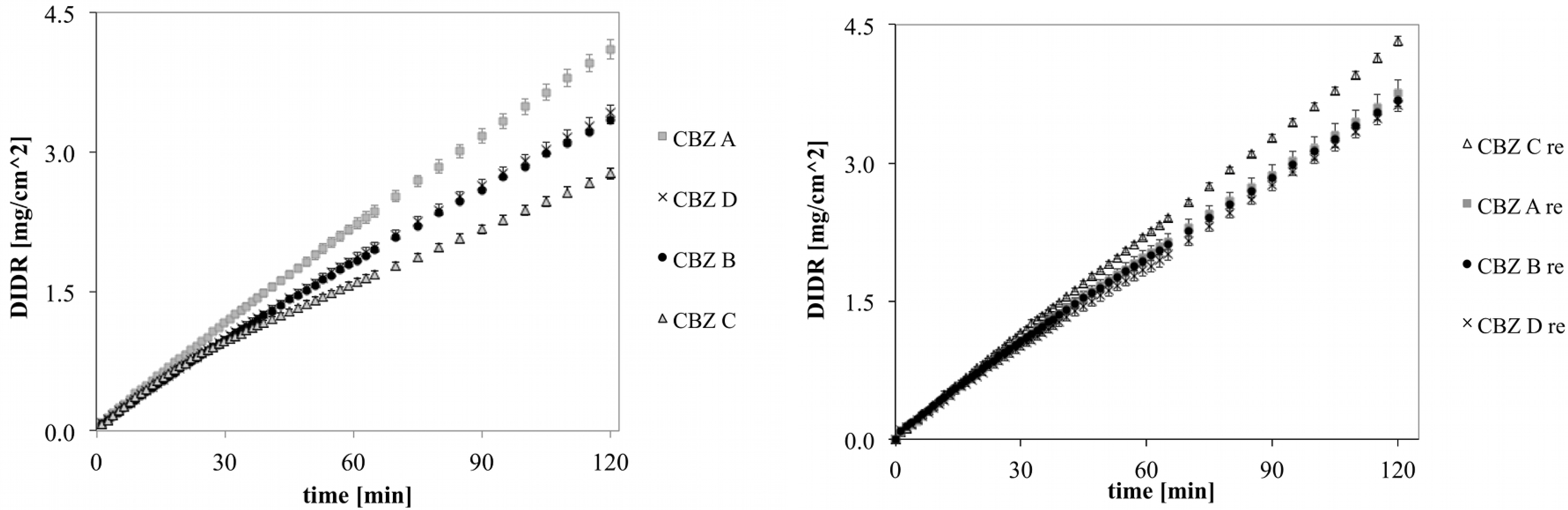
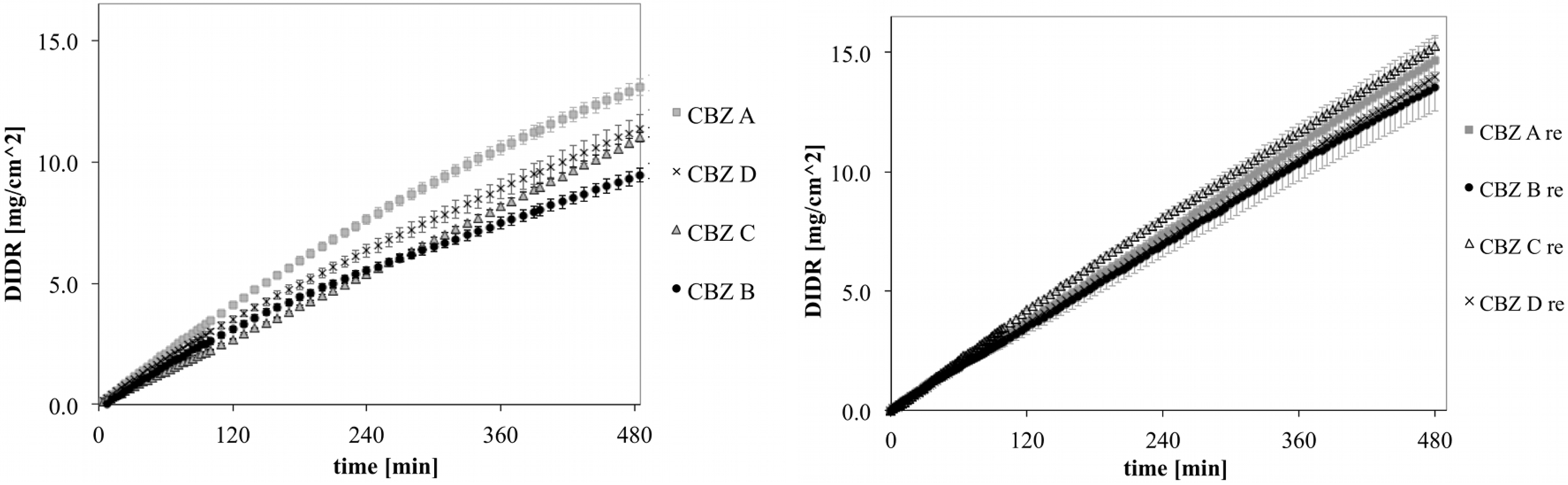
| Sample n = 3 | IP untreated [min] | IP recrystallized [min] |
|---|---|---|
| CBZ A | 35 ± 6 | 31 ± 10 |
| CBZ B | 23 ± 6 | 28 ± 4 |
| CBZ C | 19 ± 2 | 24 ± 6 |
| CBZ D | 26 ± 6 | 21 ± 3 |

3.5. Properties of CBZ Compacts
| n ≥ 5 | untreated CBZ | recrystallized CBZ | ||
|---|---|---|---|---|
| CF [MPa] | TS [MPa] | CF [MPa] | TS [MPa] | |
| CBZ A | 10.5 | 0.355 ± 0.036 | 21.0 | 0.630 ± 0.098 |
| CBZ B | 8.4 | 0.505 ± 0.127 | 21.0 | 0.556 ± 0.262 |
| CBZ C | 6.3 | 0.294 ± 0.017 | 21.0 | 0.436 ± 0.252 |
| CBZ D | 8.4 | 0.651 ± 0.095 | 21.0 | 0.498 ± 0.180 |
| x ± SD | 8.4 | 0.451 ± 0.160 | 21.0 | 0.530 ± 0.083 |
3.6. Recrystallized CBZ in Binary Mixtures

4. Conclusions
Declaration of Interest
Acknowledgments
References
- Šehić, S.; Betz, G.; Hadžidedić, Š.; El-Arini, S.K.; Leuenberger, H. Investigation of intrinsic dissolution behavior of different carbamazepine samples. Int. J. Pharm. 2010, 386, 77–90. [Google Scholar] [CrossRef]
- Flicker, F.; Betz, G. Effect of crospovidone and hydroxypropyl cellulose on carbamazepine in high-dose tablet formulation. Drug Dev. Ind. Pharm. 2011. [Google Scholar] [CrossRef]
- Lindenberg, M.; Kopp, S.; Dressman, J.B. Classification of orally administered drugs on the World Health Organization Model list of Essential Medicines according to the biopharmaceutics classification system. Eur. J. Pharm. Biopharm. 2004, 58, 265–278. [Google Scholar] [CrossRef]
- Wang, J.; Shiu, G.; Ting, O.; Viswanathan, C.; Skelly, J. Effects of humidity and temperature on in-vitro dissolution of carbamazepine tablets. J. Pharm. Sci. 1993, 82, 1002–1005. [Google Scholar] [CrossRef]
- Davidson, A. A multinational survey of the quatity of carbamazepine tablets. Drug Dev. Ind. Pharm. 1995, 21, 2167–2186. [Google Scholar] [CrossRef]
- Mittapalli, P.K.; Suresh, B.; Hussaini, S.S.; Rao, Y.M.; Apte, S. Comparative in vitro study of six carbamazepine products. AAPS PharmSciTech 2008, 9, 357–365. [Google Scholar] [CrossRef]
- Jung, H.; Milán, R.; Girard, M.; León, F.; Montoya, M. Bioequivalence study of carbamazepine tablets: In vitro/in vivo correlation. Int. J. Pharm. 1997, 152, 37–44. [Google Scholar] [CrossRef]
- Meyer, M.C.; Staughn, A.B.; Mhatre, R.M.; Shah, V.P.; Williams, R.L.; Lesko, L.J. The relative bioavailability and in vivo—In vitro correlations for four marketed carbamazepine tablets. Pharm. Res. 1998, 15, 1787–1791. [Google Scholar] [CrossRef]
- Lake, O.A.; Olling, M.; Barends, D.M. In vitro/in vivo correlations of dissolution data of carbamazepine immediate release tablets with pharmacokinetic data obtained in healthy volunteers. Eur. J. Pharm. Biopharm. 1999, 48, 13–19. [Google Scholar] [CrossRef]
- Meyer, M.C.; Straughn, A.B.; Jarvi, E.J.; Wood, G.C.; Pelsor, F.R.; Shah, V.P. The bioinequivalence of carbamazepine tablets with a history of clinical failures. Pharm. Res. 1992, 9, 1612–1616. [Google Scholar] [CrossRef]
- Rodríguez-Sponga, B.; Priceb, C.P.; Jayasankara, A.; Matzger, A.J.; Rodríguez-Hornedo, N. General principles of pharmaceutical solid polymorphism: A supramolecular perspective. Adv. Drug Deliv. Rev. 2004, 56, 241–274. [Google Scholar] [CrossRef]
- Bolourtchian, N.; Nokhodchi, A.; Dinarvand, R. The effect of solvent and crystallization conditions on habit modification of carbamazepine. Daru 2001, 9, 12–22. [Google Scholar]
- McMahon, L.E.; Timmins, P.; Williams, A.C.; York, P. Characterization of dihydrates prepared from carbamazepine polymorphs. J. Pharm. Sci. 1996, 85, 1064–1069. [Google Scholar] [CrossRef]
- Li, Y.; Han, J.; Zhang, G.G.Z.; Grant, D.J.W.; Suryanarayanan, R. In situ dehydration of carbamazepine dihydrate: A novel technique to prepare amorphous anhydrous carbamazepine. Pharm. Dev. Technol 2000, 5, 257–266. [Google Scholar] [CrossRef]
- Mahalaxmi, R.; Ravikumar; Pandey, S.; Shirwaikar, A.; Shirwaikar, A. Effect of recrystallization on size, shape, polymorph and dissolution of carbamazepine. Int. J. Pharm. Tech. Res. 2009, 1, 725–732. [Google Scholar]
- Nokhodchi, A.; Bolourtchian, N.; Dinarvand, R. Dissolution and mechanical behaviors of recrystallized carbamazepine from alcohol solution in the presence of additives. J. Cryst. Growth 2005, 274, 573–584. [Google Scholar] [CrossRef]
- Gift, A.D.; Luner, P.E.; Luedeman, L.; Taylor, L.S. Influence of polymeric excipients on crystal hydrate formation kinetics in aqueous slurries. J. Pharm. Sci. 2008, 97, 5198–211. [Google Scholar] [CrossRef]
- Tian, F.; Sandler, N.; Gordon, K.C.; McGoverin, C.M.; Reay, A.; Strachan, C.J.; Saville, D.J.; Rades, T. Visualizing the conversion of carbamazepine in aqueous suspension with and without the presence of excipients: A single crystal study using SEM and Raman microscopy. Eur. J. Pharm. Biopharm. 2006, 64, 326–335. [Google Scholar] [CrossRef]
- Otsuka, M.; Ohfusa, T.; Matsuda, Y. Effect of binders on polymorphic transformation kinetics of carbamazepine in aqueous solution. Colloid Surface 2000, 17, 145–152. [Google Scholar] [CrossRef]
- Flicker, F.; Eberle, V.A.; Betz, G. Variability in commercial carbamazepine samples—Impact on drug release. Int. J. Pharm. 2011, 410, 99–106. [Google Scholar] [CrossRef]
- Jetzer, W.E. Measurement of hardness and strength of tablets and their relation to compaction performance of powders. J. Pharm. Pharmacol. 1986, 38, 254–258. [Google Scholar] [CrossRef]
- Rustichelli, C.; Gamberini, G.; Ferioli, V.; Gamberini, M.; Ficarra, R.; Tommasini, S. Solid-state study of polymorphic drugs: Carbamazepine. J. Pharm. Biomed. Anal. 2000, 23, 41–54. [Google Scholar] [CrossRef]
- Grzesiak, A.L.; Lang, M.; Kim, K.; Matzger, A.J. Comparison of the four anhydrous polymorphs of carbamazepine and the crystal structure of form 1. J. Pharm. Sci. 2003, 92, 2260–2271. [Google Scholar] [CrossRef]
- Krahn, F.U.; Mielck, J.B. Effect of type and extent of crystalline order on chemical and physical stability of carbamazepine. Int. J. Pharm. 1989, 53, 25–34. [Google Scholar] [CrossRef]
- Tian, F.; Zeitler, J.; Strachan, C.; Saville, D.; Gordon, K.; Rades, T. Characterizing the conversion kinetics of carbamazepine polymorphs to the dihydrate in aqueous suspension using Raman spectroscopy. J. Pharm. Biomed. Anal. 2006, 40, 271–280. [Google Scholar] [CrossRef]
- Murphy, D.; Rodríguez-Cintrón, F.; Langevin, B.; Kelly, R.C.; Rodríguez-Hornedo, N. Solution-mediated phase transformation of anhydrous to dihydrate carbamazepine and the effect of lattice disorder. Int. J. Pharm. 2002, 246, 121–134. [Google Scholar] [CrossRef]
- Mosharraf, M.; Sebhatu, T.; Nyström, C. The effects of disordered structure on the solubility and dissolution rates of some hydrophilic, sparingly soluble drugs. Int. J. Pharm. 1999, 177, 29–51. [Google Scholar] [CrossRef]
- Lefebvre, C.; Guyot-Hermann, A.M.; Draguet-Brughmans, M.; Bouché, R.; Guyot, J.C. Polymorphic transitions of carbamazepine during grinding and compression. Drug Dev. Ind. Pharm. 1986, 12, 1913–1927. [Google Scholar] [CrossRef]
- Qu, H.; Louhi-Kultanen, M.; Kallas, J. Solubility and stability of anhydrate/hydrate in solvent mixtures. Int. J. Pharm. 2006, 321, 101–107. [Google Scholar]
- Li, Y.; Chow, P.S.; Tan, R.B.H.; Black, S.N. Effect of water activity on the transformation between hydrate and anhydrate of carbamazepine. Org. Process Res. Dev. 2008, 12, 264–270. [Google Scholar] [CrossRef]
- Salameh, A.K.; Taylor, L.S. Physical stability of crystal hydrates and their anhydrates in the presence of excipients. J. Pharm. Sci. 2006, 95, 446–461. [Google Scholar] [CrossRef]
- Wen, H.; Morris, K.R.; Park, K. Study on the Interactions between Polyvinylpyrrolidone (PVP) and acetaminophen crystals: Partial dissolution pattern change. J. Pharm. Sci. 2005, 94, 2166–2174. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Flicker, F.; Eberle, V.A.; Betz, G. Recrystallization of Commercial Carbamazepine Samples—A Strategy to Control Dissolution Variability. Pharmaceutics 2012, 4, 58-70. https://doi.org/10.3390/pharmaceutics4010058
Flicker F, Eberle VA, Betz G. Recrystallization of Commercial Carbamazepine Samples—A Strategy to Control Dissolution Variability. Pharmaceutics. 2012; 4(1):58-70. https://doi.org/10.3390/pharmaceutics4010058
Chicago/Turabian StyleFlicker, Felicia, Veronika A. Eberle, and Gabriele Betz. 2012. "Recrystallization of Commercial Carbamazepine Samples—A Strategy to Control Dissolution Variability" Pharmaceutics 4, no. 1: 58-70. https://doi.org/10.3390/pharmaceutics4010058




