Human Endogenous Retrovirus Group E and Its Involvement in Diseases
Abstract
:1. Introduction
2. The HERV-E Family
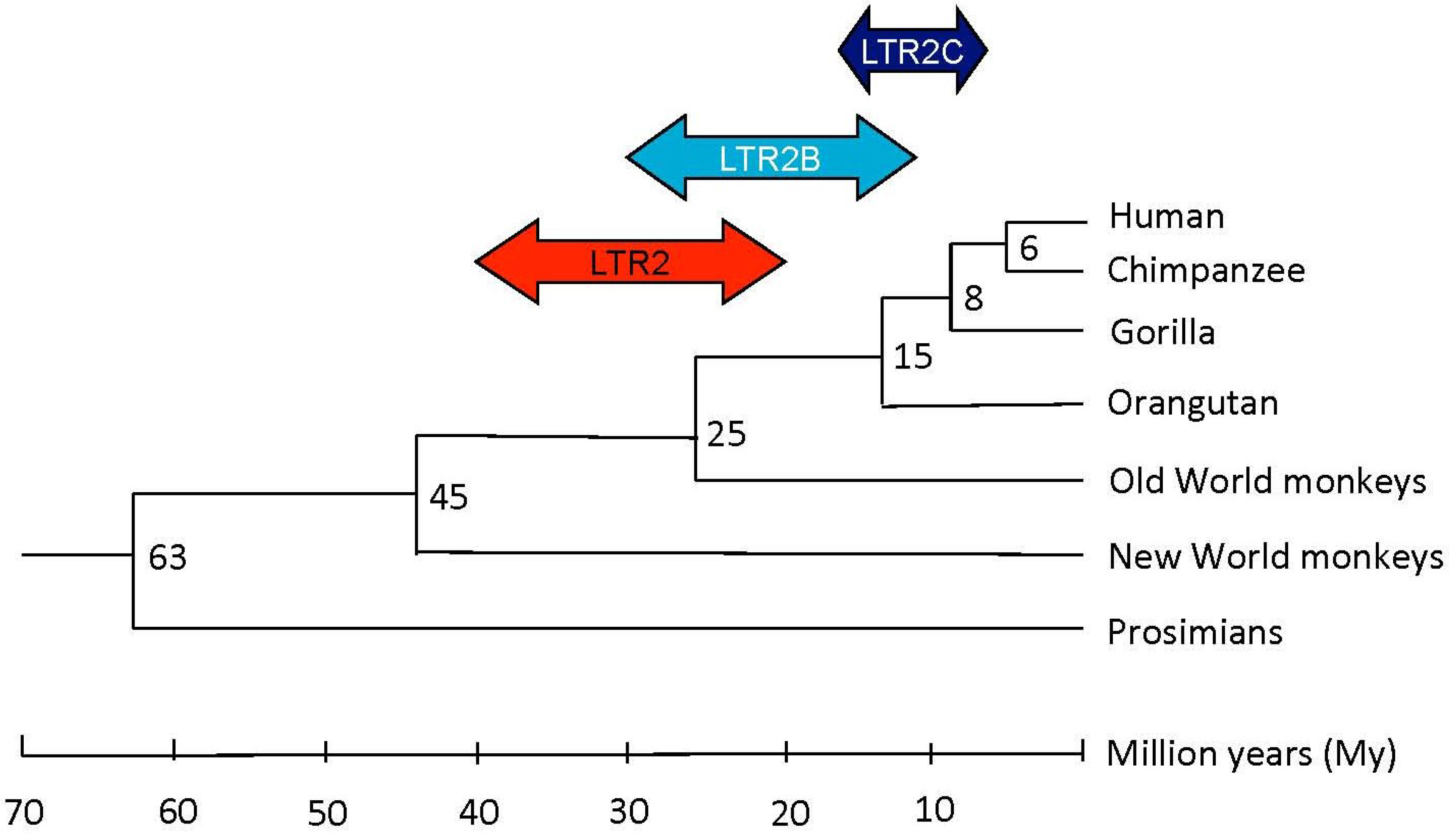
2.1. HERV-E Phylogeny
| Locus | HERV Name | Subgroup | 5’LTR Genomic Location | 3’LTR Genomic Location | Size (bp) | Structure | 5’ and 3’LTR Divergences | Molecular Clock Dating | EPO Alignment Dating | Genomic Amplification |
|---|---|---|---|---|---|---|---|---|---|---|
| 19q13.32 | HERV-E.ApoC1 | LTR2 | 19:44913964-44914947 | - | 987 | solo LTR | - | - | >15 My | >15 My [ 65] |
| 3q22.2 | - | LTR2 | 3:134027135-134027667 | 3:134019829-134020359 | 7839 | gag, pol truncated | 9.5% | 32 My | >15 My | |
| 8q12.1 | - | LTR2 | 8:58690826-58691341 | 8:58698386-58698895 | 8069 | gag, pol truncated | 7.0% | 23 My | >15 My | |
| 13q22.3 | HERV-E.EDNRB | LTR2 | 13:77975613-77976074 | 13:77975589-77976108 | 5777 | gag, pol, env truncated | 49.7% | >50 My | >25 My | >25 My [ 65] |
| 17q21.31 | HERV-E.BRCA1 | LTR2 | 17:43160221-43160752 | 17:43167223-43167735 | 7514 | gag, pol truncated | 9.4% | 31 My | >15 My | |
| 7p22.1 | - | LTR2 | 7:100919594-100920108 | - | 514 | solo LTR | - | - | >15 My | |
| 3p21.33 | LTR2 | 3:43678285-34678772 | - | 487 | solo LTR | - | - | >15 My | ||
| 6q22.31 | HERV-E.FABP7 | LTR2 | 6:122748781-122749289 | 6:122741492-122741993 | 7752 | pol truncated | 9.0% | 30 My | >25 My | |
| 11q12.2 | HERV-E.CD5 | LTR2 | 11:61093564-61094073 | 11:61098368-61098863 | 5254 | pol, env truncated | 7.8% | 26 My | >25 My | > 25 My [ 11] |
| 4q35.1 | - | LTR2 | 4:183377788-183378289 | - | 501 | solo LTR | - | - | >25 My | |
| 2q11.2 | - | LTR2 | 2:101186575-101186124 | - | 451 | solo LTR | - | - | - | |
| 17q24.3 | - | LTR2 | 17:71020856-71021358 | - | 502 | solo LTR | - | - | >15 My | |
| 4q28.2 | - | LTR2 | 4:128703926-128704424 | - | 498 | solo LTR | - | - | >25 My | |
| 1q44 | - | LTR2 | 1:247127397-247127939 | - | 542 | Solo LTR | 12.3% | 41 My | >15 My | |
| 19q13.43 | - | LTR2 | 19:57815303-57815707 | - | 1163 | solo LTR | - | - | >25 My | |
| 12p12.2 | - | LTR2 | 12:20944374-20944873 | - | 499 | solo LTR | - | - | >15 My | |
| 10q23.1 | - | LTR2B | 10:84172465-84171987 | - | 997 | solo LTR | - | - | >15 My | |
| 6q21.33 | LTR2B | 6:31186291-31186769 | - | 478 | solo LTR | - | - | >8 My | ||
| 5q31.2 | HERV-E.UBE2D2 | LTR2B | 5:139525866-139526346 | 5:139531087-139531583 | 5644 | gag, pol truncated | 5.2% | 17 My | >15 My | |
| 1p21 | HERV-E-AMY1B | LTR2B | 1:103696448-103696893 | 1:103703817-103704285 | 7837 | full length | 11.9% | 40 My | >6 My | |
| 2p22.3 | - | LTR2B | 2:34677243-34677662 | - | 888 | solo LTR | - | - | >15 My | |
| 1q24.2 | HERV-E.IQWD1 (DCAF6, PC326) | LTR2B | 1:167863096-167863572 | 1:167869427-167869928 | 6832 | gag, pol truncated | 6.9% | 23 My | >15 My | |
| 11q13.4 | - | LTR2B | 11:73271302-73271710 | - | 408 | solo LTR | - | - | >15 My | |
| 15q14 | - | LTR2B | 15:34812862-34813351 | - | 489 | solo LTR | - | - | >15 My | |
| 6q23.1 | - | LTR2B | 6:130260127-130260610 | 6:130267508-130267970 | 7843 | gag, pol truncated | 9.7% | 32 My | >15 My | |
| 7p12.3 | - | LTR2B | 7:46031148-46031644 | - | 496 | solo LTR | - | - | >15 My | |
| 6p21.31 | - | LTR2B | 6:35004307-35003824 | - | 483 | solo LTR | - | - | >15 My | |
| Xp22.22 | HERV-E.MID1 | LTR2B | X:10585282-10584793 | X:10590339-10589853 | 5,546 | gag, pol, env truncated | 6.4% | 20 My | >15 My | > 25 My [ 55] |
| 16q24.3 | - | LTR2B | 16:90056524-90057012 | - | 488 | solo LTR | - | - | >15 My | |
| 21q22.3 | - | LTR2B | 21:42807398-42807919 | - | 521 | solo LTR | - | - | >15 My | |
| 3q11.2 | - | LTR2B | 3:97856881-97857316 | - | 435 | solo LTR | - | - | - | |
| 7q33 | HERV-E.PTN | LTR2B | 7:136947291-136947711 | 7:137262486-137263044 | 6,360 | gag, pol, env truncated | 7.9% | 26 My | >15 My | >15 My [ 11,20,40] |
| 18q21.33 | - | LTR2B | 18:61617742-61618212 | - | 470 | solo LTR | - | - | - | |
| 1q24.2 | LTR2B | 1:167869414-167869892 | - | 478 | solo LTR | - | - | >15 My | ||
| 5p13.1 | - | LTR2B | 5:40872315-40872812 | - | 497 | solo LTR | - | - | > 15 My | |
| Yq11.21 | - | LTR2B | Y:12245468-12245973 | Y:12251519-12252038 | 6571 | gag truncated | 14.4% | 48 My | - | |
| 3p25.3 | - | LTR2B | 3:9590155-9590642 | - | 487 | solo LTR | - | - | > 25 My | |
| 6q15 | CT-RCC HERV-E | LTR2C | 6:88662137-88662613 | 6:88670478-88670972 | 8792 | full length | 5.8% | 19 My | > 15 My | |
| 2q37.1 | - | LTR2C | 2:231400694-231401230 | 2:231408328-231408867 | 8173 | full length | 2.9% | 10 My | > 15 My | |
| 2q23.1 | - | LTR2C | 2:149035320-149035863 | - | 543 | solo LTR | - | - | > 8 My | |
| 12p13.31 | - | LTR2C | 12:7703612-7704158 | - | 546 | solo LTR | - | - | > 8 My | |
| 11q13.31 | - | LTR2C | 11:66000626-66001172 | - | 546 | solo LTR | - | - | > 8 My | |
| 2q14.3 | - | LTR2C | 2:127683400-127683948 | - | 548 | solo LTR | - | - | > 8 My | |
| 19p12 | HERV-E clone 4-1 | LTR2C | 19:20755113-20755654 | 19:20746795-20747340 | 8,806 | full length | 4.5% | 15 My | > 8 My | |
| 8p12 | - | LTR2C | 8:30728558-30729101 | - | 5,121 | env, 3’LTR truncated | - | - | > 8 My | |
| 7p14.3 | - | LTR2C | 7:32762447-32762988 | - | 541 | solo LTR | - | - | > 6 My |
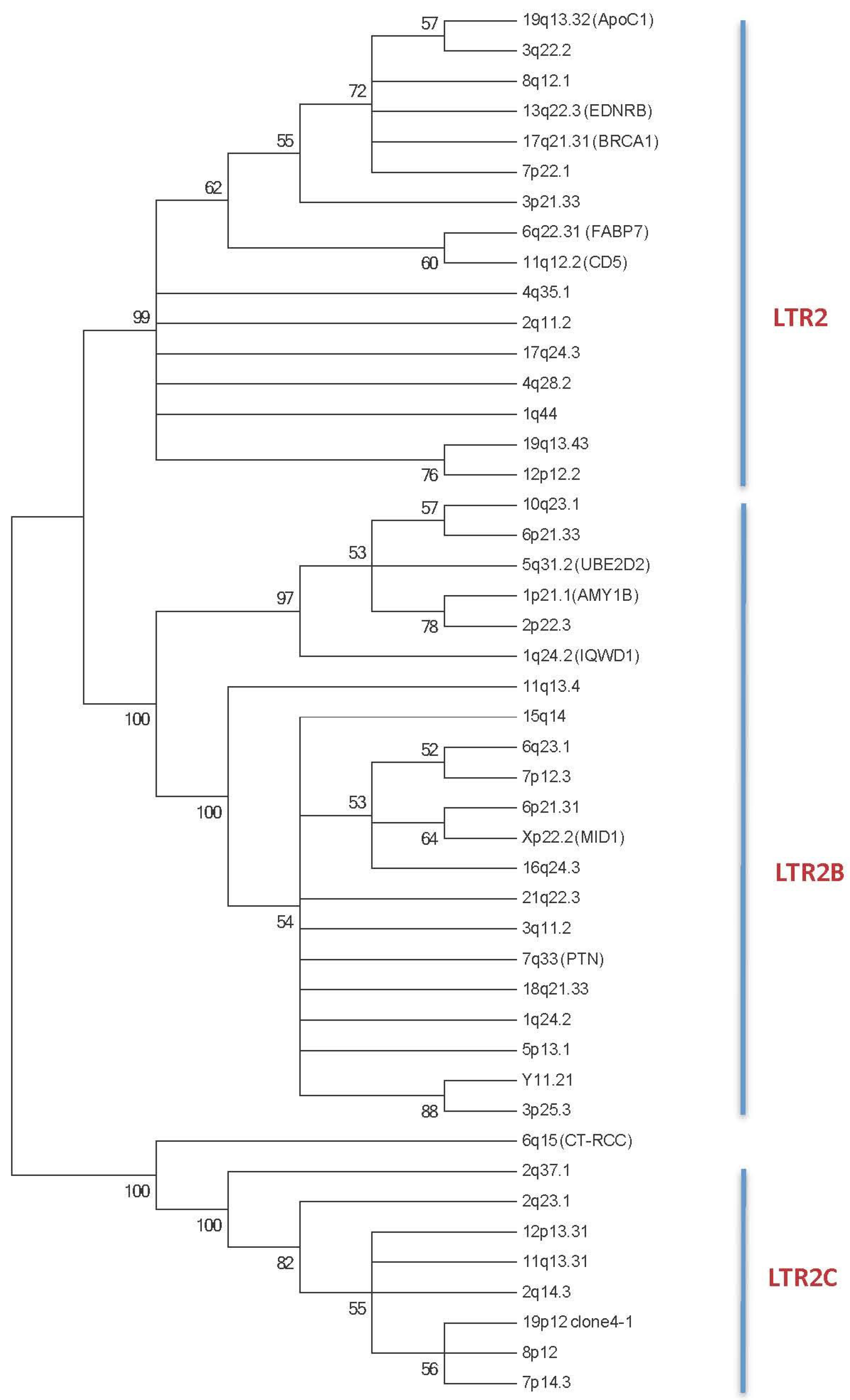
2.1.1. HERV-E Subgroups and Integration Time
2.1.2. HERV-E Subgroups and Structure
2.1.3. HERV-E Subgroups and Promoter Activity
2.1.4. Chromosomal Integration and Consequences

2.2. Examples
2.2.1. Alternative Promoter: LTR2B HERV-E.PTN and LTR2B HERV-E.MID1
| Locus | Gene Name (Function) | Subgroup | Gene Location | Orientation | Transcripts | Chimeric Transcript | Protein Variant | Reference |
|---|---|---|---|---|---|---|---|---|
| 1p21 | AMY1B (salivary amylase) | LTR2 | Upstream | antisense | salivary | No (enhancer) | No | [18,19] |
| 1q24.2 | IQWD1/DCAF6 (nuclear receptor) | LTR2B | Upstream | sense | placenta | Yes | unknown | [11,40] |
| 7q33 | PTN (secreted heparin-binding cytokine) | LTR2B | Intron 1 | sense | Placenta, trophoblast | Yes | No | [11,20,40] |
| 5q31.2 | UBE2D2 (ubiquitin conjugating enzyme) | LTR2B | Upstream | sense | unknown | Yes | No | [11] |
| 11q12.2 | CD5 (T cell and B cell subset antigen) | LTR2 | Upstream | sense | B cells | Yes | Yes | [11,66] |
| 13q22.3 | EDNRB (G protein-coupled receptor of endothelin) | LTR2 | Upstream | sense | placenta | Yes | No | [11,40,65,67] |
| 19q13.32 | ApoC1 (lipoprotein metabolism) | LTR2 | Upstream | Liver, macrophage | Two variants | No | [65] | |
| 6q22.31 | FABP7 (brain lipid binding protein) | LTR2 | Upstream | antisense | B cell lymphoma | Yes | Yes | [17] |
| Xp22.22 | MID1 (microtubule-associated protein) | LTR2B | Intron 1 | sense | Placenta, fetal kidney | Yes | No | [11,23,40,67] |
2.2.2. Alternative Variants: HERV-E.CD5 and HERV-E.FABP7
2.3. DNA Methylation Controls Transcription
3. HERV-E and Diseases
3.1. Cancer
3.2. Autoimmune Diseases
3.3. Human Placentation
4. Experimental Section
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Belshaw, R.; Pereira, V.; Katzourakis, A.; Talbot, G.; Paces, J.; Burt, A.; Tristem, M. Long-term reinfection of the human genome by endogenous retroviruses. Proc. Natl. Acad. Sci. USA 2004, 101, 4894–4899. [Google Scholar] [CrossRef]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef] [PubMed]
- Jurka, J. Repbase update: a database and an electronic journal of repetitive elements. Trends Genet. 2000, 16, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Jern, P.; Sperber, G.O.; Ahlsen, G.; Blomberg, J. Sequence variability, gene structure, and expression of full-length human endogenous retrovirus H. J. Virol. 2005, 79, 6325–6337. [Google Scholar] [CrossRef] [PubMed]
- Katzourakis, A.; Rambaut, A.; Pybus, O.G. The evolutionary dynamics of endogenous retroviruses. Trends Microbiol 2005, 13, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.; Blomberg, J.; Seal, R.L. A revised nomenclature for transcribed human endogenous retroviral loci. Mob. DNA 2011, 2, e7. [Google Scholar] [CrossRef]
- Wheeler, T.J.; Clements, J.; Eddy, S.R.; Hubley, R.; Jones, T.A.; Jurka, J.; Smit, A.F.; Finn, R.D. Dfam: a database of repetitive DNA based on profile hidden Markov models. Nucl. Acids Res. 2013, 41, D70–D82. [Google Scholar] [CrossRef] [PubMed]
- Belshaw, R.; Watson, J.; Katzourakis, A.; Howe, A.; Woolven-Allen, J.; Burt, A.; Tristem, M. Rate of recombinational deletion among human endogenous retroviruses. J. Virol. 2007, 81, 9437–9442. [Google Scholar] [CrossRef] [PubMed]
- Hayward, A.; Grabherr, M.; Jern, P. Broad-scale phylogenomics provides insights into retrovirus-host evolution. Proc. Natl. Acad. Sci. USA 2013, 110, 20146–20151. [Google Scholar] [CrossRef] [PubMed]
- DFAM. Available online: http://www.dfam.org (accessed on 1 December 2014).
- Steele, P.E.; Rabson, A.B.; Bryan, T.; Martin, M.A. Distinctive termini characterize two families of human endogenous retroviral sequences. Science 1984, 225, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Renaudineau, Y.; Vallet, S.; Le Dantec, C.; Hillion, S.; Saraux, A.; Youinou, P. Characterization of the human CD5 endogenous retrovirus-E in B lymphocytes. Genes Immun. 2005, 6, 663–671. [Google Scholar] [PubMed]
- Yi, J.M.; Kim, H.S. Molecular evolution of the HERV-E family in primates. Arch. Virol. 2006, 151, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Tanimura, M. The molecular clock runs more slowly in man than in apes and monkeys. Nature 1987, 326, 93–96. [Google Scholar] [CrossRef]
- Tristem, M. Identification and characterization of novel human endogenous retrovirus families by phylogenetic screening of the human genome mapping project database. J. Virol. 2000, 74, 3715–3730. [Google Scholar] [CrossRef] [PubMed]
- Prusty, B.K.; zur Hausen, H.; Schmidt, R.; Kimmel, R.; de Villiers, E.M. Transcription of HERV-E and HERV-E-related sequences in malignant and non-malignant human haematopoietic cells. Virology 2008, 382, 37–45. [Google Scholar] [CrossRef] [PubMed]
- van de Lagemaat, L.N.; Medstrand, P.; Mager, D.L. Multiple effects govern endogenous retrovirus survival patterns in human gene introns. Genome Biol. 2006, 7, eR86. [Google Scholar]
- Lock, F.E.; Rebollo, R.; Miceli-Royer, K.; Gagnier, L.; Kuah, S.; Babaian, A.; Sistiaga-Poveda, M.; Lai, C.B.; Nemirovsky, O.; Serrano, I.; et al. Distinct isoform of FABP7 revealed by screening for retroelement-activated genes in diffuse large B-cell lymphoma. Proc. Natl. Acad. Sci. USA 2014, 111, E3534–E3543. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, L.C.; Wiebauer, K.; Snow, C.M.; Meisler, M.H. Retroviral and pseudogene insertion sites reveal the lineage of human salivary and pancreatic amylase genes from a single gene during primate evolution. Mol. Cell. Biol. 1990, 10, 2513–2520. [Google Scholar] [PubMed]
- Ting, C.N.; Rosenberg, M.P.; Snow, C.M.; Samuelson, L.C.; Meisler, M.H. Endogenous retroviral sequences are required for tissue-specific expression of a human salivary amylase gene. Genes Dev 1992, 6, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Schulte, A.M.; Wellstein, A. Structure and phylogenetic analysis of an endogenous retrovirus inserted into the human growth factor gene pleiotrophin. J. Virol. 1998, 72, 6065–6072. [Google Scholar] [PubMed]
- Schulte, A.M.; Malerczyk, C.; Cabal-Manzano, R.; Gajarsa, J.J.; List, H.J.; Riegel, A.T.; Wellstein, A. Influence of the human endogenous retrovirus-like element HERV-E.PTN on the expression of growth factor pleiotrophin: a critical role of a retroviral Sp1-binding site. Oncogene 2000, 19, 3988–3998. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wu, K.; Didoronkute, A.; Levy, M.V.; Todi, N.; Shchelokova, A.; Massiah, M.A. MID1 catalyzes the ubiquitination of protein phosphatase 2A and mutations within its Bbox1 domain disrupt polyubiquitination of alpha4 but not of PP2Ac. PLoS One 2014, 9, e107428. [Google Scholar] [CrossRef] [PubMed]
- Landry, J.R.; Rouhi, A.; Medstrand, P.; Mager, D.L. The Opitz syndrome gene Mid1 is transcribed from a human endogenous retroviral promoter. Mol. Biol. Evol. 2002, 19, 1934–1942. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Ramirez, R.; Loisel, S.; Buors, C.; Pers, J.O.; Montero, E.; Youinou, P.; Renaudineau, Y. Rationale for Targeting CD6 as a Treatment for Autoimmune Diseases. Arthritis 2010, 2010, 130646. [Google Scholar] [CrossRef] [PubMed]
- Renaudineau, Y.; Hillion, S.; Saraux, A.; Mageed, R.A.; Youinou, P. An alternative exon 1 of the CD5 gene regulates CD5 expression in human B lymphocytes. Blood 2005, 106, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Garaud, S.; Le Dantec, C.; Berthou, C.; Lydyard, P.M.; Youinou, P.; Renaudineau, Y. Selection of the alternative exon 1 from the cd5 gene down-regulates membrane level of the protein in B lymphocytes. J. Immunol. 2008, 181, 2010–2018. [Google Scholar] [CrossRef] [PubMed]
- Renaudineau, Y.; Bariller, E.; Pers, J.Q. B1 and CD5-positive B cells. eLS 2014. [Google Scholar] [CrossRef]
- Martin, M.A.; Bryan, T.; Rasheed, S.; Khan, A.S. Identification and cloning of endogenous retroviral sequences present in human DNA. Proc. Natl. Acad. Sci. USA 1981, 78, 4892–4896. [Google Scholar] [CrossRef] [PubMed]
- Repaske, R.; Steele, P.E.; O'Neill, R.R.; Rabson, A.B.; Martin, M.A. Nucleotide sequence of a full-length human endogenous retroviral segment. J. Virol. 1985, 54, 764–772. [Google Scholar] [PubMed]
- Wang-Johanning, F.; Frost, A.R.; Jian, B.; Azerou, R.; Lu, D.W.; Chen, D.T.; Johanning, G.L. Detecting the expression of human endogenous retrovirus E envelope transcripts in human prostate adenocarcinoma. Cancer 2003, 98, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Deplus, R.; Blanchon, L.; Rajavelu, A.; Boukaba, A.; Defrance, M.; Luciani, J.; Rothe, F.; Dedeurwaerder, S.; Denis, H.; Brinkman, A.B.; et al. Regulation of DNA methylation patterns by CK2-mediated phosphorylation of Dnmt3a. Cell. Rep. 2014, 8, 743–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, H.M.; Trono, D. Dynamic control of endogenous retroviruses during development. Virology 2011, 411, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Macfarlan, T.S.; Gifford, W.D.; Driscoll, S.; Lettieri, K.; Rowe, H.M.; Bonanomi, D.; Firth, A.; Singer, O.; Trono, D.; Pfaff, S.L. Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature 2012, 487, 57–63. [Google Scholar] [PubMed]
- Brooks, W.H.; Le Dantec, C.; Pers, J.O.; Youinou, P.; Renaudineau, Y. Epigenetics and autoimmunity. J. Autoimmun. 2010, 34, J207–J219. [Google Scholar] [CrossRef] [PubMed]
- Renaudineau, Y.; Youinou, P. Epigenetics and autoimmunity, with special emphasis on methylation. Keio J. Med. 2011, 60, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Garaud, S.; Le Dantec, C.; Jousse-Joulin, S.; Hanrotel-Saliou, C.; Saraux, A.; Mageed, R.A.; Youinou, P.; Renaudineau, Y. IL-6 modulates CD5 expression in B cells from patients with lupus by regulating DNA methylation. J. Immunol. 2009, 182, 5623–5632. [Google Scholar] [CrossRef] [PubMed]
- Garaud, S.; Le Dantec, C.; de Mendoza, A.R.; Mageed, R.A.; Youinou, P.; Renaudineau, Y. IL-10 production by B cells expressing CD5 with the alternative exon 1B. Ann. N.Y. Acad. Sci. 2009, 1173, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Garaud, S.; Morva, A.; Lemoine, S.; Hillion, S.; Bordron, A.; Pers, J.O.; Berthou, C.; Mageed, R.A.; Renaudineau, Y.; Youinou, P. CD5 promotes IL-10 production in chronic lymphocytic leukemia B cells through STAT3 and NFAT2 activation. J. Immunol. 2011, 186, 4835–4844. [Google Scholar] [CrossRef]
- Mageed, R.A.; Garaud, S.; Taher, T.E.; Parikh, K.; Pers, J.O.; Jamin, C.; Renaudineau, Y.; Youinou, P. CD5 expression promotes multiple intracellular signaling pathways in B lymphocyte. Autoimmun Rev 2012, 11, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Reiss, D.; Zhang, Y.; Mager, D.L. Widely variable endogenous retroviral methylation levels in human placenta. Nucl. Acids Res. 2007, 35, 4743–4754. [Google Scholar] [CrossRef] [PubMed]
- Sukapan, P.; Promnarate, P.; Avihingsanon, Y.; Mutirangura, A.; Hirankarn, N. Types of DNA methylation status of the interspersed repetitive sequences for LINE-1, Alu, HERV-E and HERV-K in the neutrophils from systemic lupus erythematosus patients and healthy controls. J. Hum. Genet. 2014, 59, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Nakkuntod, J.; Sukkapan, P.; Avihingsanon, Y.; Mutirangura, A.; Hirankarn, N. DNA methylation of human endogenous retrovirus in systemic lupus erythematosus. J. Hum. Genet. 2013, 58, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Garaud, S.; Youinou, P.; Renaudineau, Y. DNA methylation and B-cell autoreactivity. Adv Exp Med Biol 2011, 711, 50–60. [Google Scholar] [PubMed]
- Frank, O.; Verbeke, C.; Schwarz, N.; Mayer, J.; Fabarius, A.; Hehlmann, R.; Leib-Mosch, C.; Seifarth, W. Variable transcriptional activity of endogenous retroviruses in human breast cancer. J Virol 2008, 82, 1808–1818. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, J.; Montgiraud, C.; Pichon, J.P.; Bonnaud, B.; Arsac, M.; Ruel, K.; Bouton, O.; Mallet, F. Custom human endogenous retroviruses dedicated microarray identifies self-induced HERV-W family elements reactivated in testicular cancer upon methylation control. Nucl. Acids Res. 2010, 38, 2229–2246. [Google Scholar] [CrossRef] [PubMed]
- Turbeville, M.A.; Rhodes, J.C.; Hyams, D.M.; Distler, C.M.; Steele, P.E. Expression of a putative immunosuppressive protein in human tumors and tissues. Pathobiology 1996, 64, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Han, J.; Liu, J.; Zheng, J.; Zhong, D.; Liu, R. ISDTool 2.0: a computational model for predicting immunosuppressive domain of retroviruses. J. Theor. Biol. 2014, 360, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Harashima, N.; Kajigaya, S.; Yokoyama, H.; Cherkasova, E.; McCoy, J.P.; Hanada, K.; Mena, O.; Kurlander, R.; Tawab, A.; et al. Regression of human kidney cancer following allogeneic stem cell transplantation is associated with recognition of an HERV-E antigen by T cells. J. Clin. Invest. 2008, 118, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Cherkasova, E.; Malinzak, E.; Rao, S.; Takahashi, Y.; Senchenko, V.N.; Kudryavtseva, A.V.; Nickerson, M.L.; Merino, M.; Hong, J.A.; Schrump, D.S.; et al. Inactivation of the von Hippel-Lindau tumor suppressor leads to selective expression of a human endogenous retrovirus in kidney cancer. Oncogene 2011, 30, 4697–4706. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, H.; Okada, M.; Kaneko, H.; Hishikawa, T.; Sekigawa, I.; Iida, N.; Maruyama, N.; Yamamoto, N.; Hashimoto, H. Quantitative comparison of human endogenous retrovirus mRNA between SLE and rheumatoid arthritis. Lupus 2001, 10, 517–518. [Google Scholar] [CrossRef] [PubMed]
- Le Dantec, C.; Varin, M.M.; Brooks, W.H.; Pers, J.O.; Youinou, P.; Renaudineau, Y. Epigenetics and Sjogren's syndrome. Cur. Pharm. Biotechnol. 2012, 13, 2046–2053. [Google Scholar]
- Konsta, O.D.; Thabet, Y.; Le Dantec, C.; Brooks, W.H.; Tzioufas, A.G.; Pers, J.O.; Renaudineau, Y. The contribution of epigenetics in Sjogren's Syndrome. Front. Genet. 2014, 5, e71. [Google Scholar] [CrossRef] [Green Version]
- Ogasawara, H.; Naito, T.; Kaneko, H.; Hishikawa, T.; Sekigawa, I.; Hashimoto, H.; Kaneko, Y.; Yamamoto, N.; Maruyama, N. Quantitative analyses of messenger RNA of human endogenous retrovirus in patients with systemic lupus erythematosus. J. Rheumatol. 2001, 28, 533–538. [Google Scholar] [PubMed]
- Bessis, D.; Moles, J.P.; Basset-Seguin, N.; Tesniere, A.; Arpin, C.; Guilhou, J.J. Differential expression of a human endogenous retrovirus E transmembrane envelope glycoprotein in normal, psoriatic and atopic dermatitis human skin. Br. J. Dermatol. 2004, 151, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Hishikawa, T.; Ogasawara, H.; Kaneko, H.; Shirasawa, T.; Matsuura, Y.; Sekigawa, I.; Takasaki, Y.; Hashimoto, H.; Hirose, S.; Handa, S.; et al. Detection of antibodies to a recombinant gag protein derived from human endogenous retrovirus clone 4-1 in autoimmune diseases. Viral. Immunol. 1997, 10, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, P.C.; Duriagin, S.; Jagodzinski, P.P. Expression of human endogenous retrovirus clone 4-1 may correlate with blood plasma concentration of anti-U1 RNP and anti-Sm nuclear antibodies. Clin. Rheumatol. 2005, 24, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Naito, T.; Ogasawara, H.; Kaneko, H.; Hishikawa, T.; Sekigawa, I.; Hashimoto, H.; Maruyama, N. Immune abnormalities induced by human endogenous retroviral peptides: with reference to the pathogenesis of systemic lupus erythematosus. J. Clin. Immunol. 2003, 23, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Pullmann, R., Jr.; Bonilla, E.; Phillips, P.E.; Middleton, F.A.; Perl, A. Haplotypes of the HRES-1 endogenous retrovirus are associated with development and disease manifestations of systemic lupus erythematosus. Arthritis Rheum. 2008, 58, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Fali, T.; Le Dantec, C.; Thabet, Y.; Jousse, S.; Hanrotel, C.; Youinou, P.; Brooks, W.H.; Perl, A.; Renaudineau, Y. DNA methylation modulates HRES1/p28 expression in B cells from patients with Lupus. Autoimmunity 2014, 47, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.; Colombo, E.; Dai, H.; Agarwal, R.; Mark, K.A.; Banki, K.; Poiesz, B.J.; Phillips, P.E.; Hoch, S.O.; Reveille, J.D.; et al. Antibody reactivity to the HRES-1 endogenous retroviral element identifies a subset of patients with systemic lupus erythematosus and overlap syndromes. Correlation with antinuclear antibodies and HLA class II alleles. Arthritis Rheum. 1995, 38, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Lokossou, A.G.; Toudic, C.; Barbeau, B. Implication of human endogenous retrovirus envelope proteins in placental functions. Viruses 2014, 6, 4609–4627. [Google Scholar] [CrossRef] [PubMed]
- Landry, J.R.; Mager, D.L. Functional analysis of the endogenous retroviral promoter of the human endothelin B receptor gene. J Virol 2003, 77, 7459–7466. [Google Scholar] [CrossRef] [PubMed]
- E!Ensembl. Available online: http://www.ensembl.org (accessed on 1 December 2014).
- NCBI DCODE Tool zPicture. Available online: http://zpicture.dcode.org (accessed on 1 December 2014).
- ClustalW2. Available online: http://www.ebi.ac.uk/Tools/msa/clustalw2 (accessed on December 2014).
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000; p. 333. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Dantec, C.; Vallet, S.; Brooks, W.H.; Renaudineau, Y. Human Endogenous Retrovirus Group E and Its Involvement in Diseases. Viruses 2015, 7, 1238-1257. https://doi.org/10.3390/v7031238
Le Dantec C, Vallet S, Brooks WH, Renaudineau Y. Human Endogenous Retrovirus Group E and Its Involvement in Diseases. Viruses. 2015; 7(3):1238-1257. https://doi.org/10.3390/v7031238
Chicago/Turabian StyleLe Dantec, Christelle, Sophie Vallet, Wesley H. Brooks, and Yves Renaudineau. 2015. "Human Endogenous Retrovirus Group E and Its Involvement in Diseases" Viruses 7, no. 3: 1238-1257. https://doi.org/10.3390/v7031238