European Bats as Carriers of Viruses with Zoonotic Potential
Abstract
:1. Introduction
| Virus Family | Genus | Bat Species | Origin | Detection | Refs. |
|---|---|---|---|---|---|
| Adenoviridae | Mastadenovirus | Pipistrellus nathusii Pipistrellus pipistrellus | Germany | Isolation PCR | [13,14] |
| Nyctalus noctula Rhinolophus ferrumequinum | Hungary | PCR | [15] | ||
| Myotis myotis | Germany | PCR | [16] | ||
| Astroviridae | Mamastrovirus | Myotis myotis | Germany | PCR | [16] |
| Mamastrovirus | Myotis daubentonii Myotis bechsteinii Plecotus auritus | Hungary | PCR | [17] | |
| Borna-viridae | unclassified | Myotis nattereri Pipistrellus pipistrellus | France | Metagenomics | [18] |
| Bunyaviridae | Phlebovirus Toscana virus | Pipistrellus kuhlii | Italy | Isolation | [19] |
| Nairovirus | Myotis mystacinus | France | Metagenomics | [18] | |
| Coronaviridae | Alphacoronavirus | Myotis bechsteinii Myotis dasycneme Myotis daubentonii Pipistrellus nathusii Pipistrellus pygmaeus | Germany | PCR | [20] |
| Myotis blythii Myotis daubentonii Myotis myotis Mineropterus schreibersii Nyctalus lasiopterus Pipistrellus kuhlii Pipistrellus sp. | Spain | PCR | [21] | ||
| Hypsugo savii Nyctalus noctula Pipistrellus kuhlii Pipistrellus spp. Rhinolophus hipposideros | Italy | PCR | [22] | ||
| Miniopterus schreibersii Nyctalus leisleri Rhinolophus euryale Rhinolophus blasii Rhinolophus ferrumequinum Rhinolophus mehelyi | Germany | PCR | [16] | ||
| Myotis daubentonii Myotis nattereri | United Kingdom | PCR | [23] | ||
| Betacoronavirus | Miniopterus schreibersii Nyctalus leisleri Myotis daubentonii Rhinolophus euryale Rhinolophus blasii Rhinolophus ferrumequinumv Rhinolophus mehelyi Rhinolophus hipposideros | Bulgaria Germany | PCR | [24] | |
| Rhinolophus hipposideros | Slovenia | PCR | [25] | ||
| Pipistrellus nathusii | Ukraine | PCR | [26] | ||
| Pipistrellus pipistrellus | Netherlands | PCR | [27] | ||
| Eptesicus isabellinus Hypsugo savii | Spain | PCR | [21] | ||
| Eptesicus serotinus Hypsugo savii Nyctalus noctula Pipistrellus kuhlii Pipistrellus sp. Rhinolophus hipposideros | Italy | PCR | [22,28] | ||
| Filoviridae | Cuevovirus | Miniopterus schreibersii | Spain | PCR | [29] |
| Hepeviruses | Hep-E-related viruses | Eptesicus serotinus Myotis bechsteinii Myotis daubentonii | Germany Bulgaria | PCR | [30] |
| Herpesviridae | Betaherpesvirus Gammaherpesvirus | Myotis myotis Myotis nattereri Nyctalus noctula Pipistrellus pipistrellus Plecotus auritus | Germany | PCR | [31] |
| Betaherpesvirus Alphaherpesvirus | Rousettus aegyptiacus | Hungary | PCR | [15] | |
| Gammaherpesvirus | Eptesicus serotinus | Hungary | PCR | [32] | |
| Papillomavirus | Papillomavirus | Eptesicus serotinus Rhinolophus ferrumequinum | Spain | PCR | [33] |
| Paramyxoviridae | Unassigned | Myotis mystacinus Nyctalus noctula Pipistrellus pipistrellus | Germany | PCR | [34] |
| Morbillivirus | Myotis bechsteinii Myotis daubentonii Myotis myotis Myotis mystacinus Myotis alcathoe Myotis capaccinii | Bulgaria Germany Romania | PCR | [35] | |
| Reoviridae | Orthoreovirus | Myotis mystacinus Nyctalus noctula Pipistrellus pipistrellus Pipistrellus nathusii Pipistrellus kuhlii Plecotus auritus | Germany | IsolationPCR | [36] |
| Pipistrellus kuhlii Rhinolophus hipposideros Nyctalus noctula Tadarida teniotis Nyctalus noctula | Italy | Isolation PCR | [37] | ||
| Rotavirus | Myotis mystacinus | France | Metagenomics | [18] | |
| Retrovirus | Gammaretrovirus | Eptesicus serotinus | France | Metagenomics | [18] |
| Rhabdoviridae | Various European bat lyssaviruses | Eptesicus serotinus Eptesicus isabellinus Hypsugo savii Minopterus schreibersii Myotis myotis Myotis daubentonii Myotis dasycneme Myotis nattereri Plectorus auritus Pipistrellus pipistrellus Rhinolophus ferrumequinum Rousettus aegyptiacus Vespertilio murinus unclassified Chiroptera | Denmark
France Finland Germany Hungary Netherlands Poland Slovakia Spain Switzerland Ukraine United Kingdom | Microscopy isolation PCR | [33,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54] |
2. Molecular Detection of European Bat Viruses
2.2. European Bat Filoviruses
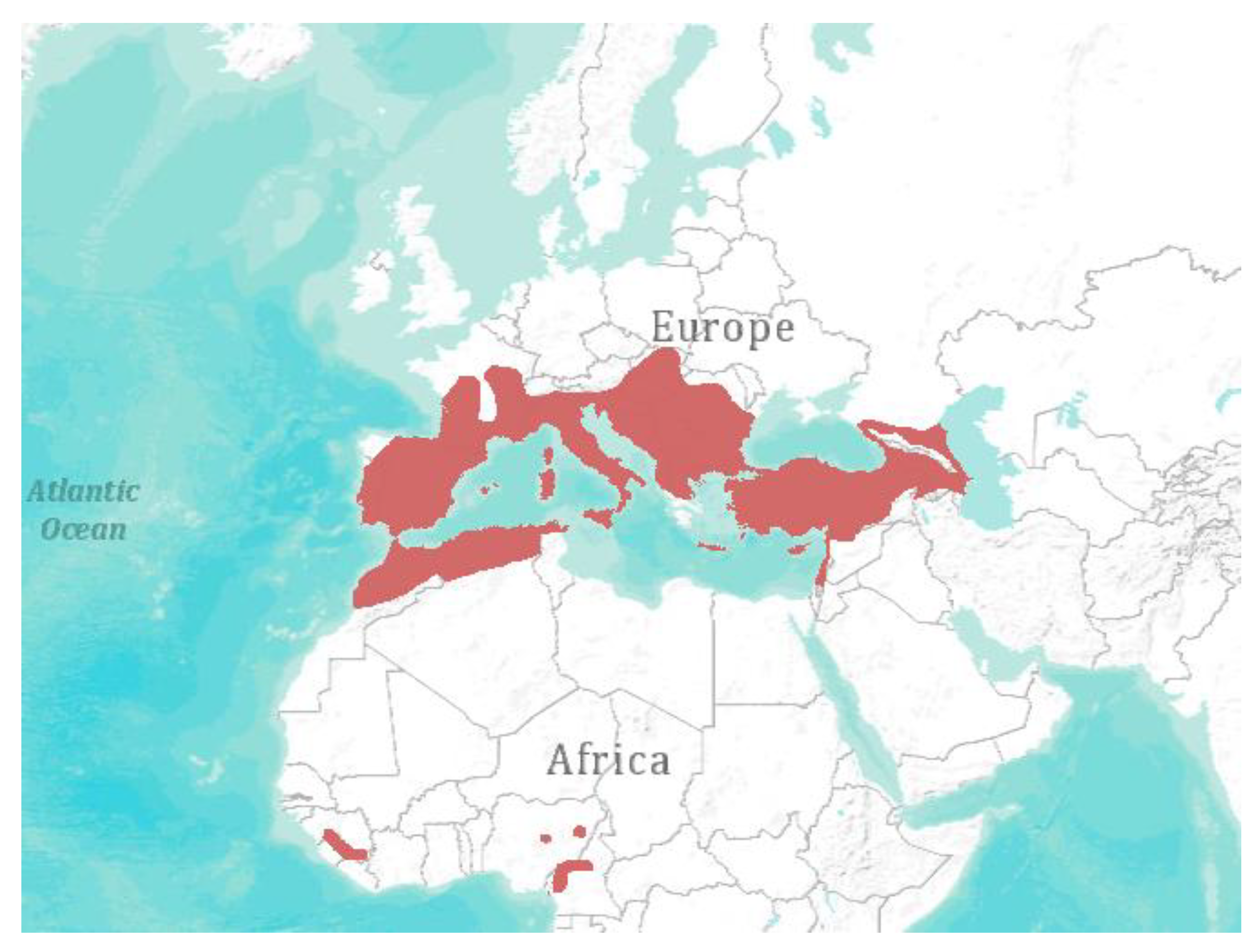
2.3. European Bat Paramyxoviruses
2.4. Other Human-Pathogenic Viruses
3. European Bat Virus Isolates
3.1. European Bat Bunyavirus Isolate
3.2. European Bat Adenovirus Isolate
3.3. European Bat Reovirus Isolates
3.4. European Bat Lyssaviruses
4. Spatial Abundance and Biodiversity
5. Conclusive Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- International Union for Conservation of Nature and Natural Resources (IUCN). 2013 The IUCN Red List of Threatened Species. Version 2013.2. Available online: http://www.iucnredlist.org/ (accessed on 15 April 2014).
- CMS. Convention on the Conservation of Migratory Species of Wild Animals. Available online: http://www.cms.int/ (accessed on 15 April 2014).
- Calisher, C.H.; Childs, J.E.; Field, H.E.; Holmes, K.V.; Schountz, T. Bats: Important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006, 19, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Calisher, C.H.; Holmes, K.V.; Dominguez, S.R.; Schountz, T.; Cryan, P. Bats prove to be rich reservoirs for emerging viruses. Microbe 2008, 3, 521–528. [Google Scholar]
- Kuzmin, I.V.; Bozick, B.; Guagliardo, S.A.; Kunkel, R.; Shak, J.R.; Tong, S.; Rupprecht, C.E. Bats, emerging infectious diseases, and the rabies paradigm revisited. Emerg. Health Threats J. 2011, 4, 7159. [Google Scholar] [PubMed]
- Smith, I.; Wang, L.-F. Bats and their virome: An important source of emerging viruses capable of infecting humans. Curr. Opin. Virol. 2013, 3, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, C.; Wang, L.F.; Real, L.A. Bat zoonoses: The realities. In Food Security in a Global Economy Veterinary Medicine and Public Health; Smith, G., Kelly, A.M., Eds.; University of Pennsylvania Press: Philadelphia, PA, USA, 2008; Chapter 13; p. 145. [Google Scholar]
- Wang, L.-F. Bats and viruses: A brief review. Virol. Sin. 2009, 24, 93–99. [Google Scholar] [CrossRef]
- Wang, L.-F.; Walker, P.J.; Poon, L.L.M. Mass extinctions, biodiversity and mitochondrial function: Are bats “special” as reservoirs for emerging viruses? Curr. Opin. Virol. 2011, 1, 649–657. [Google Scholar]
- Wong, S.; Lau, S.; Woo, P.; Yuen, K.Y. Bats as a continuing source of emerging infections in humans. Rev. Med. Virol. 2007, 17, 67–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, B.; Yang, J.; Jin, Q. DBatVir: The database of bat-associated viruses. Database Oxford 2014, 2014. [Google Scholar] [CrossRef]
- Banyard, A.C.; Evans, J.S.; Luo, T.R.; Fooks, A.R. Lyssaviruses and Bats: Emergence and Zoonotic Threat. Viruses 2014, 6, 2974–2990. [Google Scholar]
- Sonntag, M.; Mühldorfer, K.; Speck, S.; Wibbelt, G.; Kurth, A. New adenovirus in bats, Germany. Emerg. Infect. Dis. 2009, 15, 2052. [Google Scholar] [CrossRef] [PubMed]
- Kohl, C.; Vidovszky, M.Z.; Mühldorfer, K.; Dabrowski, P.W.; Radonić, A.; Nitsche, A.; Wibbelt, G.; Kurth, A.; Harrach, B. Genome analysis of bat adenovirus 2: Indications of interspecies transmission. J. Virol. 2012, 86, 1888–1892. [Google Scholar] [CrossRef] [PubMed]
- Jánoska, M.; Vidovszky, M.; Molnár, V.; Liptovszky, M.; Harrach, B.; Benko, M. Novel adenoviruses and herpesviruses detected in bats. Vet. J. 2011, 189, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Drexler, J.F.; Corman, V.M.; Wegner, T.; Tateno, A.F.; Zerbinati, R.M.; Gloza-Rausch, F.; Seebens, A.; Müller, M.A.; Drosten, C. Amplification of emerging viruses in a bat colony. Emerg. Infect. Dis. 2011, 17, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Kemenesi, G.; Dallos, B.; Görföl, T.; Boldogh, S.; Estók, P.; Kurucz, K.; Oldal, M.; Németh, V.; Madai, M.; Bányai, K.; et al. Novel European lineages of bat astroviruses identified in Hungary. Acta Virol. 2014, 58, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Dacheux, L.; Cervantes-Gonzalez, M.; Guigon, G.; Thiberge, J.-M.; Vandenbogaert, M.; Maufrais, C.; Caro, V.; Bourhy, H. A preliminary study of viral metagenomics of French bat species in contact with humans: Identification of new mammalian viruses. PLoS One 2014, 9, e87194. [Google Scholar] [CrossRef] [PubMed]
- Verani, P.; Ciufolini, M.G.; Caciolli, S.; Renzi, A.; Nicoletti, L.; Sabatinelli, G.; Bartolozzi, D.; Volpi, G.; Amaducci, L.; Coluzzi, M. Ecology of viruses isolated from sand flies in Italy and characterized of a new Phlebovirus (Arabia virus). Am. J. Trop. Med. Hyg. 1988, 38, 433–439. [Google Scholar] [PubMed]
- Gloza-Rausch, F.; Ipsen, A.; Seebens, A.; Göttsche, M.; Panning, M.; Drexler, J.F.; Petersen, N.; Annan, A.; Grywna, K.; Müller, M.; et al. Detection and prevalence patterns of group I coronaviruses in bats, northern Germany. Emerg. Infect. Dis. 2008, 14, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Falcón, A.; Vázquez-Morón, S.; Casas, I.; Aznar, C.; Ruiz, G.; Pozo, F.; Perez-Breña, P.; Juste, J.; Ibáñez, C.; Garin, I.; et al. Detection of alpha and betacoronaviruses in multiple Iberian bat species. Arch. Virol. 2011, 156, 1883–1890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lelli, D.; Papetti, A.; Sabelli, C.; Rosti, E.; Moreno, A.; Boniotti, M.B. Detection of coronaviruses in bats of various species in Italy. Viruses 2013, 5, 2679–2689. [Google Scholar] [CrossRef] [PubMed]
- August, T.A.; Mathews, F.; Nunn, M.A. Alphacoronavirus detected in bats in the United Kingdom. Vector Borne Zoonotic Dis. 2012, 12, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Drexler, J.F.; Gloza-Rausch, F.; Glende, J.; Corman, V.M.; Muth, D.; Goettsche, M.; Seebens, A.; Niedrig, M.; Pfefferle, S.; Yordanov, S.; et al. Genomic characterization of severe acute respiratory syndrome-related coronavirus in European bats and classification of coronaviruses based on partial RNA-dependent RNA polymerase gene sequences. J. Virol. 2010, 84, 11336–11349. [Google Scholar]
- Rihtaric, D.; Hostnik, P.; Steyer, A.; Grom, J.; Toplak, I. Identification of SARS-like coronaviruses in horseshoe bats (Rhinolophus hipposideros) in Slovenia. Arch. Virol. 2010, 155, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Annan, A.; Baldwin, H.J.; Corman, V.M.; Klose, S.M.; Owusu, M.; Nkrumah, E.E.; Badu, E.K.; Anti, P.; Agbenyega, O.; Meyer, B.; et al. Human betacoronavirus 2c EMC/2012-related viruses in bats, Ghana and Europe. Emerg. Infect. Dis. 2013, 19, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Reusken, C.B.E.M.; Lina, P.H.C.; Pielaat, A.; de Vries, A.; Dam-Deisz, C.; Adema, J.; Drexler, J.F.; Drosten, C.; Kooi, E.A. Circulation of group 2 coronaviruses in a bat species common to urban areas in Western Europe. Vector Borne Zoonotic Dis. 2010, 10, 785–791. [Google Scholar] [CrossRef] [PubMed]
- De Benedictis, P.; Marciano, S.; Scaravelli, D.; Priori, P.; Zecchin, B.; Capua, I.; Monne, I.; Cattoli, G. Alpha and lineage C betaCoV infections in Italian bats. Virus Genes 2014, 48, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Negredo, A.; Palacios, G.; Vázquez-Morón, S.; González, F.; Dopazo, H.; Molero, F.; Juste, J.; Quetglas, J.; Savji, N.; de la Cruz Martínez, M.; et al. Discovery of an Ebolavirus-like filovirus in Europe. PLoS Pathog. 2011, 7, e1002304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drexler, J.F.; Seelen, A.; Corman, V.M.; Fumie Tateno, A.; Cottontail, V.; Melim Zerbinati, R.; Gloza-Rausch, F.; Klose, S.M.; Adu-Sarkodie, Y.; Oppong, S.K.; et al. Bats worldwide carry hepatitis E-related viruses that form a putative novel genus within the family Hepeviridae. J. Virol. 2012, 86, 9134–9147. [Google Scholar] [CrossRef] [PubMed]
- Wibbelt, G.; Kurth, A.; Yasmum, N.; Bannert, M.; Nagel, S.; Nitsche, A.; Ehlers, B. Discovery of herpesviruses in bats. J. Gen. Virol. 2007, 88, 2651–2655. [Google Scholar] [CrossRef] [PubMed]
- Molnár, V.; Jánoska, M.; Harrach, B.; Glávits, R.; Pálmai, N.; Rigó, D.; Sós, E.; Liptovszky, M. Detection of a novel bat gammaherpesvirus in Hungary. Acta Vet. Hung. 2008, 56, 529–538. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, R.; Ibáñez, C.; Godínez, J.M.; Aréchiga, N.; Garin, I.; Pérez-Suárez, G.; de Paz, O.; Juste, J.; Echevarría, J.E.; Bravo, I.G. Novel papillomaviruses in free-ranging Iberian bats: No virus-host co-evolution, no strict host specificity, and hints for recombination. Genome Biol. Evol. 2014, 6, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Kurth, A.; Kohl, C.; Brinkmann, A.; Ebinger, A.; Harper, J.A.; Wang, L.-F.; Mühldorfer, K.; Wibbelt, G. Novel paramyxoviruses in free-ranging European bats. PLoS One 2012, 7, e38688. [Google Scholar] [CrossRef] [PubMed]
- Drexler, J.F.; Corman, V.M.; Müller, M.A.; Maganga, G.D.; Vallo, P.; Binger, T.; Gloza-Rausch, F.; Rasche, A.; Yordanov, S.; Seebens, A.; et al. Bats host major mammalian paramyxoviruses. Nat. Commun. 2012, 3, 796. [Google Scholar] [CrossRef] [PubMed]
- Kohl, C.; Lesnik, R.; Brinkmann, A.; Ebinger, A.; Radonić, A.; Nitsche, A.; Mühldorfer, K.; Wibbelt, G.; Kurth, A. Isolation and characterization of three mammalian orthoreoviruses from European bats. PLoS One 2012, 7, e43106. [Google Scholar] [CrossRef] [PubMed]
- Lelli, D.; Moreno, A.; Lavazza, A.; Bresaola, M.; Canelli, E.; Boniotti, M.B.; Cordioli, P. Identification of Mammalian Orthoreovirus Type 3 in Italian Bats. Zoonoses Public Health 2012, 60, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Bourhy, H.; Kissi, B.; Lafon, M.; Sacramento, D.; Tordo, N. Antigenic and molecular characterization of bat rabies virus in Europe. J. Clin. Microbiol. 1992, 30, 2419–2426. [Google Scholar] [PubMed]
- Fooks, A.R.; Brookes, S.M.; Johnson, N.; McElhinney, L.M.; Hutson, A.M. European bat lyssaviruses: An emerging zoonosis. Epidemiol. Infect. 2003, 131, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Schatz, J.; Fooks, A.R.; McElhinney, L.; Horton, D.; Echevarria, J.; Vázquez-Moron, S.; Kooi, E.A.; Rasmussen, T.B.; Müller, T.; Freuling, C.M. Bat rabies surveillance in Europe. Zoonoses Public Health 2013, 60, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Van Der Poel, W.H.M.; Van Der Heide, R.; Verstraten, E.R.A.M.; Takumi, K.; Lina, P.H.C.; Kramps, J.A. European bat lyssaviruses, The Netherlands. Emerg. Infect. Dis. 2005, 11, 1854–1859. [Google Scholar] [CrossRef]
- Delmas, O.; Holmes, E.C.; Talbi, C.; Larrous, F.; Dacheux, L.; Bouchier, C.; Bourhy, H. Genomic diversity and evolution of the lyssaviruses. PLoS One 2008, 3, e2057. [Google Scholar] [CrossRef] [PubMed]
- Dacheux, L.; Berthet, N.; Dissard, G.; Holmes, E.C.; Delmas, O.; Larrous, F.; Guigon, G.; Dickinson, P.; Faye, O.; Sall, A.A.; et al. Application of broad-spectrum resequencing microarray for genotyping rhabdoviruses. J. Virol. 2010, 84, 9557–9574. [Google Scholar] [CrossRef] [PubMed]
- Badrane, H.; Bahloul, C.; Perrin, P.; Tordo, N. Evidence of two Lyssavirus phylogroups with distinct pathogenicity and immunogenicity. J. Virol. 2001, 75, 3268–3276. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Johnson, N.; Freuling, C.M.; Fooks, A.R.; Selhorst, T.; Vos, A. Epidemiology of bat rabies in Germany. Arch. Virol. 2007, 152, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Picard-Meyer, E.; Barrat, J.; Tissot, E.; Barrat, M.J.; Bruyère, V.; Cliquet, F. Genetic analysis of European bat lyssavirus type 1 isolates from France. Vet. Rec. 2004, 154, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Amengual, B.; Whitby, J.; King, A.; Cobo, J.; Bourhy, H. Evolution of European bat lyssaviruses. J. Gen. Virol. 1997, 78, 2319–2328. [Google Scholar] [PubMed]
- Freuling, C.M.; Beer, M.; Conraths, F.J.; Finke, S.; Hoffmann, B.; Keller, B.; Kliemt, J.; Mettenleiter, T.C.; Mühlbach, E.; Teifke, J.P.; et al. Novel lyssavirus in Natterer’s bat, Germany. Emerg. Infect. Dis. 2011, 17, 1519–1522. [Google Scholar] [PubMed]
- Jakava-Viljanen, M.; Lilley, T.; Kyheröinen, E.-M.; Huovilainen, A. First encounter of European bat lyssavirus type 2 (EBLV-2) in a bat in Finland. Epidemiol. Infect. 2010, 138, 1581–1585. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Moron, S.; Juste, J.; Ibáñez, C.; Berciano, J.M.; Echevarria, J.E. Phylogeny of European bat Lyssavirus 1 in Eptesicus isabellinus bats, Spain. Emerg. Infect. Dis. 2011, 17, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.; Selden, D.; Parsons, G.; Healy, D.; Brookes, S.M.; McElhinney, L.M.; Hutson, A.M.; Fooks, A.R. Isolation of a European bat lyssavirus type 2 from a Daubenton’s bat in the United Kingdom. Vet. Rec. 2003, 152, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Picard-Meyer, E.; Servat, A.; Robardet, E.; Moinet, M.; Borel, C.; Cliquet, F. Isolation of Bokeloh bat lyssavirus in Myotis nattereri in France. Arch. Virol. 2013, 158, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Freuling, C.M.; Abendroth, B.; Beer, M.; Fischer, M.; Hanke, D.; Hoffmann, B.; Höper, D.; Just, F.; Mettenleiter, T.C.; Schatz, J.; et al. Molecular diagnostics for the detection of Bokeloh bat lyssavirus in a bat from Bavaria, Germany. Virus Res. 2013, 177, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Aznar-Lopez, C.; Vazquez-Moron, S.; Marston, D.A.; Juste, J.; Ibáñez, C.; Berciano, J.M.; Salsamendi, E.; Aihartza, J.; Banyard, A.C.; McElhinney, L.; et al. Detection of rhabdovirus viral RNA in oropharyngeal swabs and ectoparasites of Spanish bats. J. Gen. Virol. 2013, 94, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, R. Die aetiologie der tuberkulose. Klin. Wochenschr. 1932, 11, 490–492. [Google Scholar] [CrossRef]
- International Committee on Taxonomy of Viruses (ICTV) online 2013. Available online: http://www.ictvonline.org/virusTaxonomy.asp?version=2013 (accessed on 15 April 2014).
- Drosten, C.; Günther, S.; Preiser, W.; van der Werf, S.; Brodt, H.-R.; Becker, S.; Rabenau, H.; Panning, M.; Kolesnikova, L.; Fouchier, R.A.M.; et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Fouchier, R.A.M.; Kuiken, T.; Schutten, M.; van Amerongen, G.; van Doornum, G.J.J.; van den Hoogen, B.G.; Peiris, M.; Lim, W.; Stöhr, K.; Osterhaus, A.D.M.E. Aetiology: Koch’s postulates fulfilled for SARS virus. Nature 2003, 423, 240. [Google Scholar] [CrossRef] [PubMed]
- Kuiken, T.; Fouchier, R.A.M.; Schutten, M.; Rimmelzwaan, G.F.; van Amerongen, G.; van Riel, D.; Laman, J.D.; de Jong, T.; van Doornum, G.; Lim, W.; et al. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet 2003, 362, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Peiris, J.S.M.; Guan, Y.; Yuen, K.Y. Severe acute respiratory syndrome. Nat. Med. 2004, 10, S88–S97. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-F.; Shi, Z.; Zhang, S.; Field, H.; Daszak, P.; Eaton, B.T. Review of bats and SARS. Emerg. Infect. Dis. 2006, 12, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- WHO. Available online: http://www.who.int/csr/don/2014_06_26_mers/en (accessed on 26 June 2014).
- The WHO MERS-CoV Research Group. State of knowledge and data gaps of Middle East respiratory syndrome coronavirus (MERS-CoV) in humans. PLOS Curr. 2013, 5. doi:10.1371/currents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8. [Google Scholar]
- Briese, T.; Mishra, N.; Jain, K.; Zalmout, I.S.; Jabado, O.J.; Karesh, W.B.; Daszak, P.; Mohammed, O.B.; Alagaili, A.N.; Lipkin, W.I. Middle East respiratory syndrome coronavirus quasispecies that include homologues of human isolates revealed through whole-genome analysis and virus cultured from dromedary camels in Saudi Arabia. MBio 2014, 5, e01146–14. [Google Scholar] [PubMed]
- Ithete, N.L.; Stoffberg, S.; Corman, V.M.; Cottontail, V.M.; Richards, L.R.; Schoeman, M.C.; Drosten, C.; Drexler, J.F.; Preiser, W. Close relative of human Middle East respiratory syndrome coronavirus in bat, South Africa. Emerg. Infect. Dis. 2013, 19, 1697–1699. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wong, S.-K.; Li, F.; Kuhn, J.H.; Huang, I.-C.; Choe, H.; Farzan, M. Animal origins of the severe acute respiratory syndrome coronavirus: Insight from ACE2-S-protein interactions. J. Virol. 2006, 80, 4211–4219. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.-Y.; Li, J.-L.; Yang, X.-L.; Chmura, A.A.; Zhu, G.; Epstein, J.H.; Mazet, J.K.; Hu, B.; Zhang, W.; Peng, C.; et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 2013, 503, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Memish, Z.A.; Mishra, N.; Olival, K.J.; Fagbo, S.F.; Kapoor, V. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg. Infect. Dis. 2013, 19, 7–13. [Google Scholar] [CrossRef]
- Mole, B. Deadly coronavirus found in bats. Nat. News 2013. [Google Scholar] [CrossRef]
- Van der Putten, W.H.; Macel, M.; Visser, M.E. Predicting species distribution and abundance responses to climate change: Why it is essential to include biotic interactions across trophic levels. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 2025–2034. [Google Scholar] [CrossRef] [PubMed]
- ArcGIS Explorer; ESRI Inc: ESRI Inc, 1999.
- Appleton, B.R.; McKenzie, J.A.; Christidis, L. Molecular systematics and biogeography of the bent-wing bat complex Miniopterus schreibersii (Kuhl, 1817) (Chiroptera: Vespertilionidae). Mol. Phylogenet. Evol. 2004, 31, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Mühldorfer, K.; Speck, S.; Kurth, A.; Lesnik, R.; Freuling, C.; Müller, T.; Kramer-Schadt, S.; Wibbelt, G. Diseases and causes of death in European bats: Dynamics in disease susceptibility and infection rates. PLoS One 2011, 6, e29773. [Google Scholar] [CrossRef] [PubMed]
- Fereidouni, S.; Kwasnitschka, L.; Balkema Buschmann, A.; Müller, T.; Freuling, C.; Schatz, J.; Pikula, J.; Bandouchova, H.; Hoffmann, R.; Ohlendorf, B.; et al. No Virological Evidence for an Influenza A—Like Virus in European Bats. Zoonoses Public Health 2014. [Google Scholar] [CrossRef]
- Drexler, J.F.; Geipel, A.; König, A.; Corman, V.M.; van Riel, D.; Leijten, L.M.; Bremer, C.M.; Rasche, A.; Cottontail, V.M.; Maganga, G.D.; et al. Bats carry pathogenic hepadnaviruses antigenically related to hepatitis B virus and capable of infecting human hepatocytes. Proc. Natl. Acad. Sci. USA 2013, 110, 16151–16156. [Google Scholar] [CrossRef]
- Kohl, C.; Kurth, A. Robert Koch Institute: Berlin, Germany, Unpublished work. 2013.
- Jung, Y.T.; Kim, G.R. Genomic characterization of M and S RNA segments of hantaviruses isolated from bats. Acta Virol. 1995, 39, 231–233. [Google Scholar] [PubMed]
- Kohl, C.; Vidovszky, M.Z.; Kurth, A. Create Two Species, Bat Adenovirus B and Murine Adenovirus B, in the Genus Mastadenovirus, Family Adenoviridae; In the report of the international committee on taxonomy of viruses. Elsevier Academic Press: San Diego, CA, USA; ICTV: 2011 ICTV: 2011, ICTV 2011.024aV. Available online: http://talk.ictvonline.org/ (accessed on 15 April 2014).
- Buonavoglia, C.; Martella, V. Canine respiratory viruses. Vet. Res. 2007, 38, 355–373. [Google Scholar] [PubMed]
- Davis, P.L.; Bourhy, H.; Holmes, E.C. The evolutionary history and dynamics of bat rabies virus. Infect. Genet. Evol. 2006, 6, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Campolo, M.; Desario, C.; Ricci, D.; Camero, M.; Lorusso, E.; Elia, G.; Lavazza, A.; Martella, V.; Buonavoglia, C. Virological and molecular characterization of a mammalian orthoreovirus type 3 strain isolated from a dog in Italy. Vet. Microbiol. 2005, 109, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Tyler, K.L.; Barton, E.S.; Ibach, M.L.; Robinson, C.; Campbell, J.A.; O’Donnell, S.M.; Valyi-Nagy, T.; Clarke, P.; Wetzel, J.D.; Dermody, T.S. Isolation and molecular characterization of a novel type 3 reovirus from a child with meningitis. J. Infect. Dis. 2004, 189, 1664–1675. [Google Scholar] [CrossRef] [PubMed]
- Steyer, A.; Gutiérrez-Aguire, I.; Kolenc, M.; Koren, S.; Kutnjak, D.; Pokorn, M.; Poljšak-Prijatelj, M.; Racki, N.; Ravnikar, M.; Sagadin, M.; et al. High similarity of novel orthoreovirus detected in a child hospitalized with acute gastroenteritis to mammalian orthoreoviruses found in bats in Europe. J. Clin. Microbiol. 2013, 51, 3818–3825. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.B.; Crameri, G.; Hyatt, A.; Yu, M.; Tompang, M.R.; Rosli, J.; McEachern, J.; Crameri, S.; Kumarasamy, V.; Eaton, B.T.; et al. A previously unknown reovirus of bat origin is associated with an acute respiratory disease in humans. Proc. Natl. Acad. Sci. USA 2007, 104, 11424–11429. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.B.; Voon, K.; Crameri, G.; Tan, H.S.; Rosli, J.; McEachern, J.A.; Suluraju, S.; Yu, M.; Wang, L.-F. Identification and characterization of a new orthoreovirus from patients with acute respiratory infections. PLoS One 2008, 3, e3803. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.; Vos, A.; Freuling, C.; Tordo, N.; Fooks, A.R.; Müller, T. Human rabies due to lyssavirus infection of bat origin. Vet. Microbiol. 2010, 142, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Racey, P.A.; Hutson, A.M.; Lina, P.H.C. Bat rabies, public health and European bat conservation. Zoonoses Public Health 2013, 60, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Lumio, J.; Hillbom, M.; Roine, R.; Ketonen, L.; Haltia, M.; Valle, M.; Neuvonen, E.; Lahdevirta, J. Human rabies of bat origin in Europe. Lancet 1986, 1, 378. [Google Scholar] [CrossRef]
- Fooks, A.R.; Mcelhinney, L.M.; Pounder, D.J.; Finnegan, C.J.; Mansfield, K.; Johnson, N.; Brookes, S.M.; Parson, G.; White, K.; Mcintyre, P.G.; et al. Case report: Isolation of a European bat lyssavirus type 2a from a fatal human case of rabies encephalitis. J. Med. Virol. 2003, 71, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Dietz, C.; von Helversen, O.; Nill, D. Handbuch der Fledermäuse Europas und Nordwestafrikas; (in German). Kosmos: Stuttgart, Germany, 2007. [Google Scholar]
- Serra-Cobo, J.; Amengual, B.; Abellán, C.; Bourhy, H. European bat lyssavirus infection in Spanish bat populations. Emerg. Infect. Dis. 2002, 8, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Cox, J.; Peter, W.; Schafer, R.; Johnson, N.; McElhinney, L.M.; Geue, J.L.; Tjornehoj, K.; Fooks, A.R. Spill-over of European bat lyssavirus type 1 into a stone marten (Martes foina) in Germany. J. Vet. Med. B 2004, 51, 49–54. [Google Scholar] [CrossRef]
- Tjornehoj, K.; Fooks, A.R.; Agerholm, J.S.; Ronsholt, L. Natural and experimental infection of sheep with European bat lyssavirus type-1 of Danish bat origin. J. Comp. Pathol. 2006, 134, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Dacheux, L.; Larrous, F.; Mailles, A.; Boisseleau, D.; Delmas, O.; Biron, C.; Bouchier, C.; Capek, I.; Muller, M.; Ilari, F.; et al. European bat Lyssavirus transmission among cats, Europe. Emerg. Infect. Dis. 2009, 15, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Streicker, D.G.; Recuenco, S.; Valderrama, W.; Gomez Benavides, J.; Vargas, I.; Pacheco, V.; Condori Condori, R.E.; Montgomery, J.; Rupprecht, C.E.; Rohani, P.; et al. Ecological and anthropogenic drivers of rabies exposure in vampire bats: Implications for transmission and control. Proc. Biol. Sci. 2012, 279, 3384–3392. [Google Scholar] [CrossRef] [PubMed]
- von Humboldt, A. Ansichten der Natur, mit wissenschaftlichen Erläuterungen(in German), 2nd ed.; Cottasche, J.G., Ed.; Buchhandlung: Stuttgart/Tübingen, Germany, 1826. [Google Scholar]
- Buckley, L.B.; Davies, T.J.; Ackerly, D.D.; Kraft, N.J.B.; Harrison, S.P.; Anacker, B.L.; Cornell, H.V.; Damschen, E.I.; Grytnes, J.-A.; Hawkins, B.A.; et al. Phylogeny, niche conservatism and the latitudinal diversity gradient in mammals. Proc. Biol. Sci. 2010, 277, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Simmons, N.B. Taxonomy of chiroptera. In Mammal Species of the World: A Taxonomic and Geographic Reference, 3rd ed.; Wilson, D.E., Reeder, D.M., Eds.; Johns Hopkins University: Baltimore, MD, USA, 2005. [Google Scholar]
- Teeling, E.C.; Springer, M.S.; Madsen, O.; Bates, P.; O’Brien, S.J.; Murphy, W.J. A molecular phylogeny for bats illuminates biogeography and the fossil record. Science 2005, 307, 580–584. [Google Scholar] [PubMed]
- Willig, M.; Selcer, K. Bat species density gradients in the New World: A statistical assessment. J. Biogeogr. 1989, 16, 189–195. [Google Scholar] [CrossRef]
- Buckley, H.L.; Miller, T.E.; Ellison, A.M.; Gotelli, N.J. Reverse latitudinal trends in species richness of pitcher-plant food webs. Ecol. Lett. 2003, 6, 825–829. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, H.; Azovsky, A.I. Body size determines the strength of the latitudinal diversity gradient. Ecography 2001, 24, 251–256. [Google Scholar] [CrossRef]
- Martiny, J.B.H.; Bohannan, B.J.M.; Brown, J.H.; Colwell, R.K.; Fuhrman, J.A.; Green, J.L.; Horner-Devine, M.C.; Kane, M.; Krumins, J.A.; Kuske, C.R.; et al. Microbial biogeography: Putting microorganisms on the map. Nat. Rev. Microbiol. 2006, 4, 102–112. [Google Scholar]
- Peterson, A.T. Biogeography of diseases: A framework for analysis. Naturwissenschaften 2008, 95, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, M.; Rohwer, F. Here a virus, there a virus, everywhere the same virus? Trends Microbiol. 2005, 13, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Dolan, J.R. Microbial biogeography? J. Biogeogr. 2006, 33, 199–200. [Google Scholar] [CrossRef]
- Keesing, F.; Belden, L.K.; Daszak, P.; Dobson, A.; Harvell, C.D.; Holt, R.D.; Hudson, P.; Jolles, A.; Jones, K.E.; Mitchell, C.E.; et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 2010, 468, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.N. Biodiversity loss and emerging infectious disease: An example from the rodent-borne hemorrhagic fevers. Biodiversity 2006, 7, 9–17. [Google Scholar] [CrossRef]
- Ostfeld, R.S. Biodiversity loss and the rise of zoonotic pathogens. Clin. Microbiol. Infect. 2009, 15, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Randolph, S.E.; Dobson, A.D.M. Pangloss revisited: A critique of the dilution effect and the biodiversity-buffers-disease paradigm. Parasitology 2012, 139, 847–863. [Google Scholar] [CrossRef] [PubMed]
- Salkeld, D.J.; Padgett, K.A.; Jones, J.H. A meta-analysis suggesting that the relationship between biodiversity and risk of zoonotic pathogen transmission is idiosyncratic. Ecol. Lett. 2013, 16, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Woolhouse, M.E.J.; Gowtage-Sequeria, S. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 2005, 11, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Woolhouse, M.E.J.; Taylor, L.H.; Haydon, D.T. Population biology of multihost pathogens. Science 2001, 292, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- Baas Becking, L.G.M. Geobiologie of Inleiding tot de Milieukunde; (in Dutch). W.P. Van Stockum & Zoon: Gravenhage, The Netherlands, 1934. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kohl, C.; Kurth, A. European Bats as Carriers of Viruses with Zoonotic Potential. Viruses 2014, 6, 3110-3128. https://doi.org/10.3390/v6083110
Kohl C, Kurth A. European Bats as Carriers of Viruses with Zoonotic Potential. Viruses. 2014; 6(8):3110-3128. https://doi.org/10.3390/v6083110
Chicago/Turabian StyleKohl, Claudia, and Andreas Kurth. 2014. "European Bats as Carriers of Viruses with Zoonotic Potential" Viruses 6, no. 8: 3110-3128. https://doi.org/10.3390/v6083110





