Phocine Distemper Virus: Current Knowledge and Future Directions
Abstract
:1. Introduction
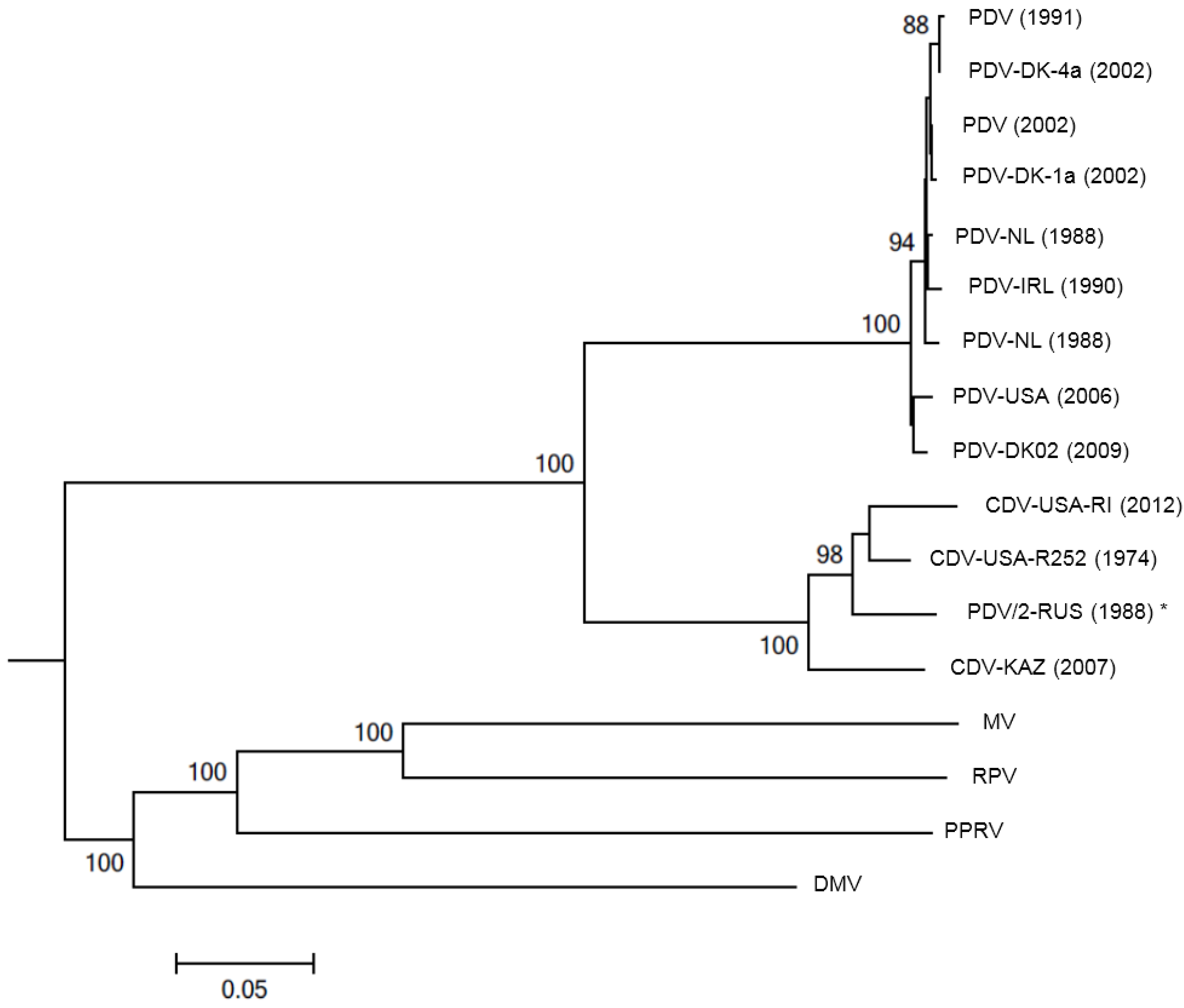
2. Antigenic and Molecular Characteristics of PDV
3. Clinical Signs, Pathogenesis and Pathology
3.1. Clinical Signs of Infection
3.2. Pathogenesis, Cell Receptors and Tissue Tropism
3.3. Gross Pathology
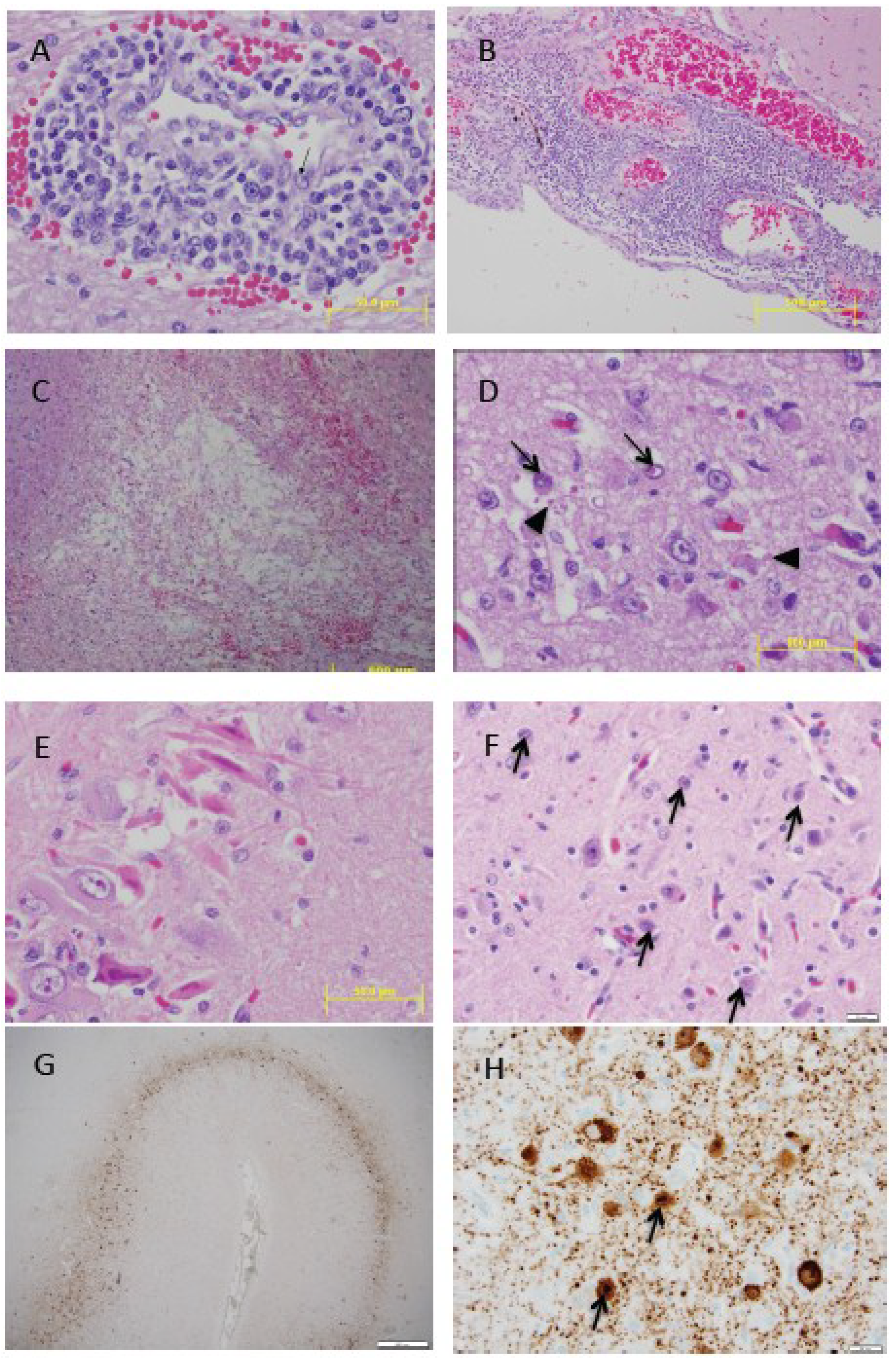
3.4. Histopathology
3.5. Age-Specific Pathology
4. Diagnosis
4.1. Serology
4.2. Histopathology and Immunohistochemistry
4.3. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.4. Virus Isolation
5. Immunology, Species Susceptibility and Vaccination
5.1. Immune Response to PDV
5.2. Vaccines and Vaccination Strategies for Free-Living Pinnipeds: Hawaiian Monk Seal Case Study
6. Epidemiology
6.1. Transmission and Persistence
6.2. Global Distribution of PDV
| Ocean Province | Species | Pathology | Serology | PCR | Reference |
|---|---|---|---|---|---|
| Eastern North Atlantic | Phoca vitulina | Yes | Yes | Yes | [4,5,6,28,29,51,91,92,94,96,174] |
| (Inc. North, Baltic and Irish Seas) | Halichoerus grypus | No | Yes | No | [28,90,92,125,126,142] |
| Arctic | Cystophora cristata | No | Yes | No | [11] |
| (Inc. Greenland, Barents, White and Norwegian Seas) | Pagophilus groenlandicus | No | Yes | No | [10,11,14] |
| Pusa hispida | No | Yes | No | [14] | |
| Western North Atlantic | Phoca vitulina | Yes | Yes | Yes | [49,106,175] |
| (Eastern Canadian Arctic to Caribbean) | Halichoerus grypus | Yes | Yes | Yes | [13,56,106,175] |
| Pagophilus groenlandicus | Yes | Yes | Yes | [11,12,53,55] | |
| Cystophora cristata | Yes | Yes | Yes | [11,12,56] | |
| Pusa hispida | No | Yes | No | [11,12] | |
| Odobenus rosmarus rosmarus | No | Yes | No | [107,176] | |
| Eastern North Pacific and Bering Sea | Phoca vitulina richardsii | No | No | No | [6,106,177,178,179] |
| Pusa hispida | No | No | No | [6] | |
| Phoca largha | No | No | No | [6] | |
| Histriophoca fasciata | No | No | No | [6] | |
| Erignathus barbatus | No | No | No | [6] | |
| Odobenus rosmarus divergens | No | No | No | [6] | |
| Eumetopias jubatus | No | No | No | [6,180] | |
| Callorhinus ursinus | No | No | No | Gulland unpublished | |
| Enhydra lutris kenyoni | No | Yes | Yes | [117,179,181,182] | |
| Enhydra lutris nereis | No | No | No | [179] | |
| Zalophus californianus | No | No | No | Gulland unpublished | |
| Arctocephalus townsendi | No | No | No | Gulland unpublished | |
| Western North Pacific | Phoca vitulina stejnegeri | No | Yes | No | [183,184] |
| (Sea of Okhotsk, Sea of Japan, Yellow Sea) | Eumetopias jubatus | No | Yes | No | [184] |
| Phoca largha | No | Yes | No | [184] | |
| Southern Oceans | |||||
| New Zealand | Phocarctos hookeri | No | Yes | No | [185,186] |
| Arctocephalus forsteri | No | Yes | No | [185,186] | |
| Australia | Arctocephalus pusillus doriferus | No | No * | No | [187] |
| Antarctica | Lobodon carcinophagus | No | No | No | [111,188]# |
| Hydrurga leptonyx | No | No | No | [188] | |
| Leptonychotes weddellii | No | No | No | [111,188] | |
| Ommatophoca rossii | No | No | No | [111] |
6.2.1. Western North Atlantic
6.2.2. Eastern North Atlantic
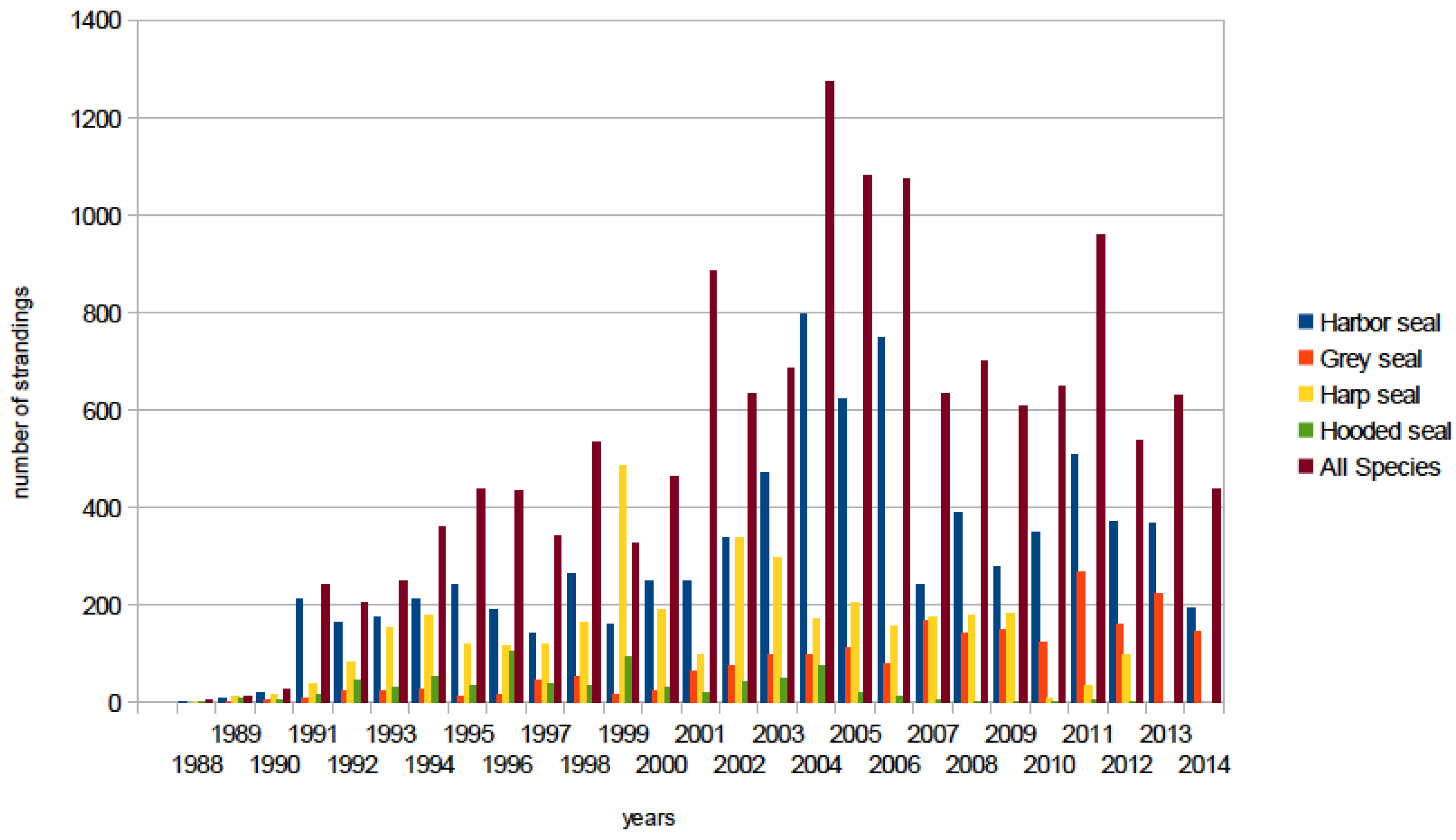

6.2.3. North Pacific
6.2.4. Southern Oceans
7. Conclusions and Future Directions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grachev, M.A.; Kumarev, V.P.; Mamaev, L.V.; Zorin, V.L.; Baranova, L.V.; Denikina, N.N.; Belikov, S.I.; Petrov, E.A.; Kolesnik, V.S.; Kolesnik, R.S.; et al. Distemper virus in Baikal seals. Nature 1989, 338, 209. [Google Scholar] [CrossRef] [PubMed]
- Osterhaus, A.D.M.E.; Groen, J.; Uytdehaag, F.G.C.M.; Visser, I.K.G.; Van De Bildt, M.W.G.; Bergman, A.; Klingeborn, B. Distemper virus in Baikal seals. Nature 1989, 338, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Heide-Jorgensen, M.-P.; Harkonen, T.; Dietz, R.; Thompson, P.M. Retrospective of the 1988 European Seal Epizootic. Dis. Aquat. Org. 1992, 13, 37–62. [Google Scholar] [CrossRef]
- Cosby, S.L.; Mcquaid, S.; Duffy, N.; Lyons, C.; Rima, B.K.; Allan, G.M.; Mccullough, S.J.; Kennedy, S.; Smyth, J.A.; Mcneilly, F.; et al. Characterization of a seal morbillivirus. Nature 1988, 336, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Mahy, B.W.J.; Barrett, T.; Evans, S.; Anderson, E.C.; Bostock, C.J. Characterization of a seal morbillivirus. Nature 1988, 336, 115. [Google Scholar] [CrossRef] [PubMed]
- Osterhaus, A.D.M.E.; Vedder, E.J. Identification of virus causing recent seal deaths. Nature 1988, 335, 20. [Google Scholar] [CrossRef] [PubMed]
- Gorham, J.R. The epizootiology of distemper. J. Am. Vet. Med. Assoc. 1966, 149, 610–622. [Google Scholar] [PubMed]
- Harwood, J. Lessons from the Seal Epidemic. New Sci. 1989, 121, 38–42. [Google Scholar]
- Thompson, P.M.; Cornwell, H.J.; Ross, H.M.; Miller, D. Serologic study of phocine distemper in a population of harbor seals in Scotland. J. Wildl. Dis. 1992, 28, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Markussen, N.H.; Have, P. Phocine distemper virus infection in harp seals (Phoca groenlandica). Mar. Mamm. Sci. 1992, 8, 19–26. [Google Scholar] [CrossRef]
- Stuen, S.; Have, P.; Osterhaus, A.D.; Arnemo, J.M.; Moustgaard, A. Serological investigation of virus infections in harp seals (Phoca groenlandica) and hooded seals (Cystophora cristata). Vet. Rec. 1994, 134, 502–503. [Google Scholar] [CrossRef] [PubMed]
- Duignan, P.J.; Nielsen, O.; House, C.; Kovacs, K.M.; Duffy, N.; Early, G.; Sadove, S.; St Aubin, D.J.; Rima, B.K.; Geraci, J.R. Epizootiology of morbillivirus infection in harp, hooded, and ringed seals from the Canadian Arctic and western Atlantic. J. Wildl. Dis. 1997, 33, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.; Trudgett, A.; Lyons, C.; Ronald, K. Demonstration of antibodies in archival sera from Canadian seals reactive with a European isolate of phocine distemper virus. Sci. Total Environ. 1992, 115, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Dietz, R.; Ansen, C.T.; Have, P.; Heide-Jorgensen, M.P. Clue to seal epizootic? Nature 1989, 338, 627–627. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Blixenkrone-Moller, M.; di Guardo, G.; Domingo, M.; Duignan, P.; Hall, A.; Mamaev, L.; Osterhaus, A.D. Morbilliviruses in aquatic mammals: Report on round table discussion. Vet. Microbiol. 1995, 44, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Osterhaus, A.D.M.E.; de Swart, R.L.; Vos, H.W.; Ross, P.S.; Kenter, M.J.; Barrett, T. Morbillivirus infections of aquatic mammals: Newly identified members of the genus. Vet. Microbiol. 1995, 44, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T. Morbillivirus infections, with special emphasis on morbilliviruses of carnivores. Vet. Microbiol. 1999, 69, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.; Lau, S.K.; Wong, B.H.; Fan, R.Y.; Wong, A.Y.; Zhang, A.J.; Wu, Y.; Choi, G.K.; Li, K.S.; Hui, J.; et al. Feline morbillivirus, a previously undescribed paramyxovirus associated with tubulointerstitial nephritis in domestic cats. Proc. Natl. Acad. Sci. USA 2012, 109, 5435–5440. [Google Scholar] [CrossRef] [PubMed]
- McCullough, S.J.; McNeilly, F.; Allan, G.M.; Kennedy, S.; Smyth, J.A.; Cosby, S.L.; McQuaid, S.; Rima, B.K. Isolation and characterisation of a porpoise morbillivirus. Arch. Virol. 1991, 118, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Van Bressem, M.F.; Visser, I.K.G.; van de Bildt, M.W.; Teppema, J.S.; Raga, J.A.; Osterhaus, A.D.M.E. Morbillivirus infection in Mediterranean striped dolphins (Stenella coeruleoalba). Vet. Rec. 1991, 129, 471–472. [Google Scholar]
- Visser, I.K.G.; van Bressem, M.F.; de Swart, R.L.; van de Bildt, M.W.; Vos, H.W.; van der Heijden, R.W.; Saliki, J.T.; Orvell, C.; Kitching, P.; Kuiken, T.; et al. Characterization of morbilliviruses isolated from dolphins and porpoises in Europe. J. Gen. Virol. 1993, 74, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, J.K.; Tsai, M.M.; Atkin, T.J.; Fanning, T.G.; Krafft, A.E.; Moeller, R.B.; Kodsi, S.E.; Mense, M.G.; Lipscomb, T.P. Molecular genetic evidence of a novel morbillivirus in a long-finned pilot whale (Globicephalus melas). Emerg. Infect. Dis. 2000, 6, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Belliere, E.N.; Esperon, F.; Fernandez, A.; Arbelo, M.; Munoz, M.J.; Sanchez-Vizcaino, J.M. Phylogenetic analysis of a new Cetacean morbillivirus from a short-finned pilot whale stranded in the Canary Islands. Res. Vet. Sci. 2011, 90, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Stone, B.M.; Blyde, D.J.; Saliki, J.T.; Blas-Machado, U.; Bingham, J.; Hyatt, A.; Wang, J.; Payne, J.; Crameri, S. Fatal cetacean morbillivirus infection in an Australian offshore bottlenose dolphin (Tursiops truncatus). Aust. Vet. J. 2011, 89, 452–457. [Google Scholar] [CrossRef] [PubMed]
- West, K.L.; Sanchez, S.; Rotstein, D.; Robertson, K.M.; Dennison, S.; Levine, G.; Davis, N.; Schofield, D.; Potter, C.W.; Jensen, B. A Longman’s beaked whale (Indopacetus pacificus) strands in Maui, Hawaii, with first case of morbillivirus in the central Pacific. Mar. Mamm. Sci. 2013, 29, 767–776. [Google Scholar]
- Groch, K.R.; Colosio, A.C.; Marcondes, M.C.; Zucca, D.; Diaz-Delgado, J.; Niemeyer, C.; Marigo, J.; Brandao, P.E.; Fernandez, A.; Catão-Dias, J.L. Novel cetacean morbillivirus in Guiana dolphin, Brazil. Emerg. Infect. Dis. 2014, 20, 511–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephens, N.; Duignan, P.J.; Wang, J.; Bingham, J.; Finn, H.; Bejder, L.S.; Patterson, A.P.; Holyoake, C. Cetacean morbillivirus in coastal Indo-Pacific bottlenose dolphins, Western Australia. Emerg. Infect. Dis. 2014, 20, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S. A review of the 1988 European seal morbillivirus epizootic. Vet. Rec. 1990, 127, 563–567. [Google Scholar] [PubMed]
- Bostock, C.J.; Barrett, T.; Crowther, J.R. Characterization of the European seal morbillivirus. Vet. Microbiol. 1990, 23, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Rima, B.K.; Cosby, S.L.; Duffy, N.; Lyons, C.; O’Loan, D.; Kennedy, S.; McCullough, S.J.; Smyth, J.A.; McNeilly, F. Humoral immune responses in seals infected by phocine distemper virus. Res. Vet. Sci. 1990, 49, 114–116. [Google Scholar] [PubMed]
- Rima, B.K.; Curran, M.D.; Kennedy, S. Phocine distemper virus, the agent responsible for the 1988 mass mortality of seals. Sci. Total Environ. 1992, 115, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Curran, M.D.; Lu, Y.J.; Rima, B.K. The fusion protein gene of phocine distemper virus: Nucleotide and deduced amino acid sequences and a comparison of morbillivirus fusion proteins. Arch. Virol. 1992, 126, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Curran, M.D.; O’Loan, D.; Rima, B.K.; Kennedy, S. Nucleotide sequence analysis of phocine distemper virus reveals its distinctness from canine distemper virus. Vet. Rec. 1990, 127, 430–431. [Google Scholar] [PubMed]
- Blixenkrone-Moller, M.; Sharma, B.; Varsanyi, T.M.; Hu, A.; Norrby, E.; Kovamees, J. Sequence analysis of the genes encoding the nucleocapsid protein and phosphoprotein (P) of phocid distemper virus, and editing of the P gene transcript. J. Gen. Virol. 1992, 73, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Kovamees, J.; Blixenkrone-Moller, M.; Sharma, B.; Orvell, C.; Norrby, E. The nucleotide sequence and deduced amino acid composition of the hemagglutinin and fusion proteins of the morbillivirus phocid distemper. J. Gen. Virol. 1991, 72, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.J.; Goodman, S.J. Reassessing conflicting evolutionary histories of the Paramyxoviridae and the origins of respiroviruses with Bayesian multigene phylogenies. Infect. Genet. Evol. 2010, 10, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Drexler, J.F.; Corman, V.M.; Muller, M.A.; Maganga, G.D.; Vallo, P.; Binger, T.; Gloza-Rausch, F.; Cottontail, V.M.; Rasche, A.; Yordanov, S.; et al. Bats host major mammalian paramyxoviruses. Nat. Commun. 2012, 3, e796. [Google Scholar] [CrossRef]
- Wilkinson, D.A.; Melade, J.; Dietrich, M.; Ramasindrazana, B.; Soarimalala, V.; Lagadec, E.; le Minter, G.; Tortosa, P.; Heraud, J.M.; de Lamballerie, X.; et al. Highly diverse morbillivirus-related paramyxoviruses in wild fauna of the southwestern Indian Ocean Islands: Evidence of exchange between introduced and endemic small mammals. J. Virol. 2014, 88, 8268–8277. [Google Scholar] [CrossRef] [PubMed]
- de Vries, R.D.; Verburgh, R.J.; van de Bildt, M.W.; Osterhaus, A.D.M.E.; de Swart, R.L. Complete genome sequence of phocine distemper virus isolated from a harbor seal (Phoca vitulina) during the 1988 North Sea epidemic. Genome Announc. 2013, 1, e00291. [Google Scholar]
- Calain, P.; Roux, L. The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J. Virol. 1993, 67, 4822–4830. [Google Scholar] [PubMed]
- Harkonen, T.; Dietz, R.; Reijnders, P.; Teilmann, J.; Harding, K.; Hall, A.; Brasseur, S.; Siebert, U.; Goodman, S.J.; Jepson, P.D.; et al. The 1988 and 2002 phocine distemper virus epidemics in European harbour seals. Dis. Aquat. Organ. 2006, 68, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Wohlsein, P.; Muller, G.; Haas, L.; Siebert, U.; Harder, T.C.; Baumgartner, W. Antigenic characterization of phocine distemper virus causing mass mortality in 2002 and its relationship to other morbilliviruses. Arch. Virol. 2007, 152, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.; Arctander, P.; Jensen, T.H.; Dietz, H.H.; Hammer, A.S.; Banyard, A.C.; Barrett, T.; Blixenkrone-Moller, M. Genetic diversity and phylogenetic analysis of the attachment glycoprotein of phocine distemper viruses of the 2002 and 1988 epizootics. Virus Res. 2009, 144, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Earle, J.A.P.; Melia, M.M.; Doherty, N.V.; Nielsen, O.; Cosby, S.L. Phocine distemper virus in seals, east coast, United States, 2006. Emerg. Infect. Dis. 2011, 17, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Martella, V.; Elia, G.; Lucente, M.S.; Decaro, N.; Lorusso, E.; Banyai, K.; Blixenkrone-Moller, M.; Lan, N.T.; Yamaguchi, R.; Cirone, F.; et al. Genotyping canine distemper virus (CDV) by a hemi-nested multiplex PCR provides a rapid approach for investigation of CDV outbreaks. Vet. Microbiol. 2007, 122, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Rima, B.K.; Earle, J.A.; Baczko, K.; ter Meulen, V.; Liebert, U.G.; Carstens, C.; Carabana, J.; Caballero, M.; Celma, M.L.; Fernandez-Munoz, R. Sequence divergence of measles virus haemagglutinin during natural evolution and adaptation to cell culture. J. Gen. Virol. 1997, 78, 97–106. [Google Scholar] [PubMed]
- Kennedy, S.; Smyth, J.A.; Cush, P.F.; Duignan, P.; Platten, M.; McCullough, S.J.; Allan, G.M. Histopathologic and immunocytochemical studies of distemper in seals. Vet. Pathol. 1989, 26, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Have, P.; Nielsen, J.; Bottner, A. The seal death in Danish waters 2: Virological studies. Acta Vet. Scand. 1991, 32, 211–219. [Google Scholar] [PubMed]
- Daoust, P.Y.; Haines, D.M.; Thorsen, J.; Duignan, P.J.; Geraci, J.R. Phocine distemper in a harp seal (Phoca groenlandica) from the Gulf of St. Lawrence, Canada. J. Wildl. Dis. 1993, 29, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Duignan, P.J.; Sadove, S.; Saliki, J.T.; Geraci, J.R. Phocine distemper in harbor seals (Phoca vitulina) from Long Island, New York. J. Wildl. Dis. 1993, 29, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, T.P.; Mense, M.G.; Habecker, P.L.; Taubenberger, J.K.; Schoelkopf, R. Morbilliviral dermatitis in seals. Vet. Pathol. 2001, 38, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Prendiville, S. Death and diagnosis of two grey seals (Halichoerus grypus) in the Northeast U.S. In Proceedings of the 17th Biennial Conference on the Biology of Marine Mammals, Cape Town, South Africa, 29 November–3 December 2007.
- Siebert, U.; Gulland, F.; Harder, T.; Janiaux, T.; Seibel, H.; Wohlsein, P.; Baumgartner, W. Epizootics in Harbour Seals (Phoca vitulina): Clinical Aspects. NAMMCO Sci. Publ. 2010, 8, 265–274. [Google Scholar]
- Wild, T.F.; Naniche, D.; Rabourdin-Combe, C.; Gerlier, D.; Malvoisin, E.; Lecouturier, V.; Buckland, R. Mode of entry of morbilliviruses. Vet. Microbiol. 1995, 44, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Melia, M.M.; Earle, J.P.; Abdullah, H.; Reaney, K.; Tangy, F.; Cosby, S.L. Use of SLAM and PVRL4 and identification of pro-HB-EGF as cell entry receptors for wild type phocine distemper virus. PLoS One 2014, 9, e106281. [Google Scholar] [CrossRef] [PubMed]
- Dunster, L.M.; Schneider-Schaulies, J.; Loffler, S.; Lankes, W.; Schwartz-Albiez, R.; Lottspeich, F.; ter Meulen, V. Moesin: A cell membrane protein linked with susceptibility to measles virus infection. Virology 1994, 198, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Tatsuo, H.; Ono, N.; Tanaka, K.; Yanagi, Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature 2000, 406, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Tatsuo, H.; Ono, N.; Yanagi, Y. Morbilliviruses use signaling lymphocyte activation molecules (CD150) as cellular receptors. J. Virol. 2001, 75, 5842–5850. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, K.; Suzuki, R.; Maeda, T.; Tsuda, M.; Abe, E.; Yoshida, T.; Endo, Y.; Okamura, M.; Nagamine, T.; Yamamoto, H.; et al. Recent host range expansion of canine distemper virus and variation in its receptor, the signaling lymphocyte activation molecule, in carnivores. J. Wildl. Dis. 2014, 50, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, K.; Ando, A.; Suzuki, R.; Takishita, K.; Kawato, M.; Katsumata, E.; Ohtsu, D.; Okutsu, K.; Tokutake, K.; Miyahara, H.; et al. Host-virus specificity of morbilliviruses predicted by structural modeling of the marine mammal SLAM, a receptor. Comp. Immunol. Microbiol. Infect. Dis. 2010, 33, 227–241. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.J.; Shaw, M.A.; Jepson, P.D.; Brasseur, S.M.; Reijnders, P.J.; Goodman, S.J. Variation in European harbour seal immune response genes and susceptibility to phocine distemper virus (PDV). Infect. Genet. Evol. 2011, 11, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Cocks, B.G.; Chang, C.C.; Carballido, J.M.; Yssel, H.; de Vries, J.E.; Aversa, G. A novel receptor involved in T-cell activation. Nature 1995, 376, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, H.; Tanaka, K.; Ono, N.; Tatsuo, H.; Yanagi, Y. Induction of the measles virus receptor SLAM (CD150) on monocytes. J. Gen. Virol. 2001, 82, 2913–2917. [Google Scholar] [PubMed]
- McQuaid, S.; Cosby, S.L. An immunohistochemical study of the distribution of the measles virus receptors, CD46 and SLAM, in normal human tissues and subacute sclerosing panencephalitis. Lab. Investig. 2002, 82, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, K.; Suzuki, R.; Maruyama, T. Host-Virus Specificity of the Morbillivirus Receptor, SLAM, in Marine Mammals: Risk Assessment of Infection Based on Three-Dimensional Models. In New Approaches to the Study of Marine Mammals; Romero, A., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Seki, F.; Ono, N.; Yamaguchi, R.; Yanagi, Y. Efficient isolation of wild strains of canine distemper virus in Vero cells expressing canine SLAM (CD150) and their adaptability to marmoset B95a cells. J. Virol. 2003, 77, 9943–9950. [Google Scholar] [CrossRef] [PubMed]
- Cosby, S.L. Morbillivirus cross species infection: Is there a risk for humans? Future Virol. 2012, 7, 1103–1113. [Google Scholar] [CrossRef]
- Vongpunsawad, S.; Oezgun, N.; Braun, W.; Cattaneo, R. Selectively receptor-blind measles viruses: Identification of residues necessary for SLAM- or CD46-induced fusion and their localization on a new hemagglutinin structural model. J. Virol. 2004, 78, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Von Messling, V.; Oezguen, N.; Zheng, Q.; Vongpunsawad, S.; Braun, W.; Cattaneo, R. Nearby clusters of hemagglutinin residues sustain SLAM-dependent canine distemper virus entry in peripheral blood mononuclear cells. J. Virol. 2005, 79, 5857–5862. [Google Scholar]
- McCarthy, A.J.; Shaw, M.A.; Goodman, S.J. Pathogen evolution and disease emergence in carnivores. Proc. Biol. Sci. 2007, 274, 3165–3174. [Google Scholar] [CrossRef] [PubMed]
- Muhlebach, M.D.; Mateo, M.; Sinn, P.L.; Prufer, S.; Uhlig, K.M.; Leonard, V.H.; Navaratnarajah, C.K.; Frenzke, M.; Wong, X.X.; Sawatsky, B.; et al. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 2011, 480, 530–533. [Google Scholar] [PubMed]
- Noyce, R.S.; Bondre, D.G.; Ha, M.N.; Lin, L.T.; Sisson, G.; Tsao, M.S.; Richardson, C.D. Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 2011, 7, e1002240. [Google Scholar] [CrossRef] [PubMed]
- Noyce, R.S.; Richardson, C.D. Nectin 4 is the epithelial cell receptor for measles virus. Trends Microbiol. 2012, 20, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Delpeut, S.; Noyce, R.S.; Richardson, C.D. The tumor-associated marker, PVRL4 (nectin-4), is the epithelial receptor for morbilliviruses. Viruses 2014, 6, 2268–2286. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, H.; Brankin, B.; Brady, C.; Cosby, S.L. Wild-type measles virus infection upregulates poliovirus receptor-related 4 and causes apoptosis in brain endothelial cells by induction of tumor necrosis factor-related apoptosis-inducing ligand. J. Neuropathol. Exp. Neurol. 2013, 72, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Yoneda, M.; Honda, T.; Kai, C. Morbillivirus receptors and tropism: Multiple pathways for infection. Front. Microbiol. 2012, 3, e75. [Google Scholar]
- De Vries, R.D.; Mesman, A.W.; Geijtenbeek, T.B.; Duprex, W.P.; de Swart, R.L. The pathogenesis of measles. Curr. Opin. Virol. 2012, 2, 248–255. [Google Scholar]
- Sawatsky, B.; Wong, X.X.; Hinkelmann, S.; Cattaneo, R.; von Messling, V. Canine distemper virus epithelial cell infection is required for clinical disease but not for immunosuppression. J. Virol. 2012, 86, 3658–3666. [Google Scholar] [CrossRef] [PubMed]
- De Swart, R.L.; Ludlow, M.; de Witte, L.; Yanagi, Y.; van Amerongen, G.; McQuaid, S.; Yuksel, S.; Geijtenbeek, T.B.H.; Duprex, W.P.; Osterhaus, A.D.M.E. Predominant infection of CD150+ lymphocytes and dendritic cells during measles virus infection of macaques. PLoS Pathog. 2007, 3, e178. [Google Scholar] [CrossRef] [PubMed]
- De Swart, R.L. The pathogenesis of measles revisited. Pediatr. Infect. Dis. J. 2008, 27, S84–S88. [Google Scholar]
- Ludlow, M.; McQuaid, S.; Milner, D.; Swart, R.L.; Duprex, W.P. Pathological consequences of systemic measles virus infection. J. Pathol. 2014. [Google Scholar] [CrossRef]
- Bogomolni, A. Assessing Differences in Species Susceptibility to Phocine Distemper Virus (PDV) in Harp (Phoca groenlandica), Harbor (Phoca vitulina concolor) and Gray Seals (Halichoerus grypus) of the Northwest Atlantic. Ph.D. Thesis, University of Connecticut, Storrs, CT, USA, 2014. [Google Scholar]
- Appel, M.J. Pathogenesis of canine distemper. Am. J. Vet. Res. 1969, 30, 1167–1182. [Google Scholar] [PubMed]
- Harder, T.C.; Willhaus, T.; Leibold, W.; Liess, B. Investigations on course and outcome of phocine distemper virus infection in harbour seals (Phoca vitulina) exposed to polychlorinated biphenyls. Virological and serological investigations. Zbl. Vet. Med. B 1992, 39, 19–31. [Google Scholar] [CrossRef]
- Krakowka, S.; Higgins, R.J.; Koestner, A. Canine distemper virus: Review of structural and functional modulations in lymphoid tissues. Am. J. Vet. Res. 1980, 41, 284–292. [Google Scholar] [PubMed]
- Bergman, A.; Jarplid, B.; Svensson, B.M. Pathological findings indicative of distemper in European seals. Vet. Microbiol. 1990, 23, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Munro, R.; Ross, H.; Cornwell, C.; Gilmour, J. Disease conditions affecting common seals (Phoca vitulina) around the Scottish mainland, September-November 1988. Sci. Total Environ. 1992, 115, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S. Morbillivirus infections in Aquatic Mammals. In Infectious Diseases of Wild Mammals, 3rd ed.; Williams, E.S., Barker, I.K., Eds.; Iowa State Press: Ames, IA, USA, 2001; pp. 64–76. [Google Scholar]
- Duignan, P.J. Morbillivirus infections of marine mammals. In Zoo and Wild Animal Medicine: Current Therapy 4; Fowler, M.E., Miller, R.E., Eds.; W. B. Saunders: Philadelphia, USA, 1999; pp. 497–500. [Google Scholar]
- Rijks, J.M.; Read, F.L.; van de Bildt, M.W.; van Bolhuis, H.G.; Martina, B.E.; Wagenaar, J.A.; van der Meulen, K.; Osterhaus, A.D.; Kuiken, T. Quantitative analysis of the 2002 phocine distemper epidemic in the Netherlands. Vet. Pathol. 2008, 45, 516–530. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.S. Canine distemper. In Infectious Diseases of Wild Mammals, 3rd ed.; Williams, E.S., Barker, I.K., Eds.; Iowa State Press: Ames, IA, USA, 2001; pp. 50–59. [Google Scholar]
- Schumacher, U.; Horny, H.P.; Heidemann, G.; Schultz, W.; Welsch, U. Histopathological findings in harbour seals (Phoca vitulina) found dead on the German North sea coast. J. Comp. Pathol. 1990, 102, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, W.; Orvell, C.; Reinacher, M. Naturally occurring canine distemper virus encephalitis: Distribution and expression of viral polypeptides in nervous tissues. Acta Neuropathol. 1989, 78, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Vandevelde, M.; Zurbriggen, A. The neurobiology of canine distemper virus infection. Vet. Microbiol. 1995, 44, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Vandevelde, M.; Kristensen, F.; Kristensen, B.; Steck, A.J.; Kihm, U. Immunological and pathological findings in demyelinating encephalitis associated with canine distemper virus infection. Acta Neuropathol. 1982, 56, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Alldinger, S.; Baumgartner, W.; Orvell, C. Restricted expression of viral surface proteins in canine distemper encephalitis. Acta Neuropathol. 1993, 85, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Headley, S.A.; Amude, A.M.; Alfieri, A.F.; Bracarense, A.P.; Alfieri, A.A.; Summers, B.A. Molecular detection of Canine distemper virus and the immunohistochemical characterization of the neurologic lesions in naturally occurring old dog encephalitis. J. Vet. Diagn. Invest. 2009, 21, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Geraci, J.R.; St Aubin, D.J.; Barker, I.K.; Webster, R.G.; Hinshaw, V.S.; Bean, W.J.; Ruhnke, H.L.; Prescott, J.H.; Early, G.; Baker, A.S.; et al. Mass mortality of harbor seals: Pneumonia associated with influenza A virus. Science 1982, 215, 1129–1131. [Google Scholar] [CrossRef] [PubMed]
- Callan, R.J.; Early, G.; Kida, H.; Hinshaw, V.S. The appearance of H3 influenza viruses in seals. J. Gen. Virol. 1995, 76, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Scholin, C.A.; Gulland, F.; Doucette, G.J.; Benson, S.; Busman, M.; Chavez, F.P.; Cordaro, J.; DeLong, R.; de Vogelaere, A.; Harvey, J.; et al. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature 2000, 403, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Visser, I.K.G.; van Bressem, M.F.; Barrett, T.; Osterhaus, A.D.M.E. Morbillivirus infections in aquatic mammals. Vet. Res. 1993, 24, 169–178. [Google Scholar] [PubMed]
- Duignan, P.J.; Saliki, J.T.; St Aubin, D.J.; Early, G.; Sadove, S.; House, J.A.; Kovacs, K.; Geraci, J.R. Epizootiology of morbillivirus infection in North American harbor seals (Phoca vitulina) and gray seals (Halichoerus grypus). J. Wildl. Dis. 1995, 31, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, O.; Stewart, R.E.; Measures, L.; Duignan, P.; House, C. A morbillivirus antibody survey of Atlantic walrus, narwhal and beluga in Canada. J. Wildl. Dis. 2000, 36, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Saliki, J.T.; Lehenbauer, T.W. Monoclonal antibody-based competitive enzyme-linked immunosorbent assay for detection of morbillivirus antibody in marine mammal sera. J. Clin. Microbiol. 2001, 39, 1877–1881. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, O.; Smith, G.; Weingartl, H.; Lair, S.; Measures, L. Use of a SLAM transfected Vero cell line to isolate and characterize marine mammal morbilliviruses using an experimental ferret model. J. Wildl. Dis. 2008, 44, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Van Bressem, M.F.; Waerebeek, K.V.; Jepson, P.D.; Raga, J.A.; Duignan, P.J.; Nielsen, O.; Beneditto, A.P.D.; Siciliano, S.; Ramos, R.; Kant, W.; et al. An insight into the epidemiology of dolphin morbillivirus worldwide. Vet. Microbiol. 2001, 81, 287–304. [Google Scholar]
- Tryland, M.; Nymo, I.H.; Nielsen, O.; Nordoy, E.S.; Kovacs, K.M.; Krafft, B.A.; Thoresen, S.I.; Asbakk, K.; Osterrieder, K.; Roth, S.J.; et al. Serum chemistry and antibodies against pathogens in antarctic fur seals, Weddell seals, crabeater seals, and Ross seals. J. Wildl. Dis. 2012, 48, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, W.; Boyce, R.W.; Weisbrode, S.E.; Aldinger, S.; Axthelm, M.K.; Krakowka, S. Histologic and immunocytochemical characterization of canine distemper-associated metaphyseal bone lesions in young dogs following experimental infection. Vet. Pathol. 1995, 32, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Stanton, J.B.; Brown, C.C.; Poet, S.; Lipscomb, T.P.; Saliki, J.; Frasca, S., Jr. Retrospective differentiation of canine distemper virus and phocine distemper virus in phocids. J. Wildl. Dis. 2004, 40, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Visser, I.K.G.; Mamaev, L.; Goatley, L.; van Bressem, M.F.; Osterhaus, A.D.M.E. Dolphin and porpoise morbilliviruses are genetically distinct from phocine distemper virus. Virology 1993, 193, 1010–1012. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Sahoo, P.; Jepson, P.D. Seal distemper outbreak 2002. Microbiol. Today 2003, 30, 162–164. [Google Scholar]
- Jensen, T.; Van De Bildt, M.W.G.; Dietz, H.H.; Andersen, T.H.; Hammer, A.S.; Kuiken, T.; Osterhaus, A. Another phocine distemper outbreak in Europe. Science 2002, 297, 209. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, T.; Mazet, J.A.; Gill, V.A.; Doroff, A.M.; Burek, K.A.; Hammond, J.A. Phocine distemper virus in northern sea otters in the Pacific Ocean, Alaska, USA. Emerg. Infect. Dis. 2009, 15, 925–927. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.A.; Pomeroy, P.P.; Hall, A.J.; Smith, V.J. Identification and real-time PCR quantification of Phocine distemper virus from two colonies of Scottish grey seals in 2002. J. Gen. Virol. 2005, 86, 2563–2567. [Google Scholar] [CrossRef] [PubMed]
- Bogomolni, A.L.; Frasca, J.S.; Matassa, K.A.; Nielsen, O.; Rogers, K.; De Guise, S. Development of a one-step duplex RT-qPCR for the quantification of phocine distemper virus. J. Wildl. Dis. 2014, in press. [Google Scholar]
- Sierra, E.; Sanchez, S.; Saliki, J.T.; Blas-Machado, U.; Arbelo, M.; Zucca, D.; Fernandez, A. Retrospective study of etiologic agents associated with nonsuppurative meningoencephalitis in stranded cetaceans in the Canary Islands. J. Clin. Microbiol. 2014, 52, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Appel, M.J.; Jones, O.R. Use of alveolar macrophages for cultivation of canine distemper virus. Proc. Soc. Exp. Biol. Med. 1967, 126, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.; Smyth, J.A.; Cush, P.F.; McCullough, S.J.; Allan, G.M.; McQuaid, S. Viral distemper now found in porpoises. Nature 1988, 336, 21. [Google Scholar] [CrossRef] [PubMed]
- Visser, I.K.G.; Kumarev, V.P.; Orvell, C.; de Vries, P.; Broeders, H.W.; van de Bildt, M.W.; Groen, J.; Teppema, J.S.; Burger, M.C.; Uytdehaag, F.G.C.M.; et al. Comparison of two morbilliviruses isolated from seals during outbreaks of distemper in north west Europe and Siberia. Arch. Virol. 1990, 111, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Harder, T.; Willhaus, T.; Frey, H.R.; Liess, B. Morbillivirus infections of seals during the 1988 epidemic in the Bay of Heligoland: III. Transmission studies of cell culture-propagated phocine distemper virus in harbour seals (Phoca vitulina) and a grey seal (Halichoerus grypus): Clinical, virological and serological results. Zbl. Vet. Med. B 1990, 37, 641–650. [Google Scholar] [CrossRef]
- Carter, S.D.; Hughes, D.E.; Taylor, V.J.; Bell, S.C. Immune responses in common and grey seals during the seal epizootic. Sci. Total Environ. 1992, 115, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Harwood, J.; Carter, S.D.; Hughes, D.E.; Bell, S.C.; Baker, J.R.; Cornwell, H.J. Seal disease predictions. Nature 1989, 339, 670. [Google Scholar] [CrossRef] [PubMed]
- Krakowka, S.; Cockerell, G.; Koestner, A. Effects of canine distemper virus infection on lymphoid function in vitro and in vivo. Infect. Immun. 1975, 11, 1069–1078. [Google Scholar] [PubMed]
- Miele, J.A.; Krakowka, S. Antibody responses to virion polypeptides in gnotobiotic dogs infected with canine distemper virus. Infect. Immun. 1983, 41, 869–871. [Google Scholar] [PubMed]
- Duignan, P.J.; Duffy, N.; Rima, B.K.; Geraci, J.R. Comparative antibody response in harbour and grey seals naturally infected by a morbillivirus. Vet. Immunol. Immunopathol. 1997, 55, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.J. Patterns of extensive genetic differentiation and variation among European harbor seals (Phoca vitulina vitulina) revealed using microsatellite DNA polymorphisms. Mol. Biol. Evol. 1998, 15, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.A.; Guethlein, L.A.; Norman, P.J.; Parham, P. Natural selection on marine carnivores elaborated a diverse family of classical MHC class I genes exhibiting haplotypic gene content variation and allelic polymorphism. Immunogenetics 2012, 64, 915–933. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.I.; Simpson, F.; David, P.; Rijks, J.M.; Kuiken, T.; Thorne, M.A.; Lacy, R.C.; Dasmahapatra, K.K. High-throughput sequencing reveals inbreeding depression in a natural population. Proc. Natl. Acad. Sci. USA 2014, 111, 3775–3780. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.T.; Andersen, L.W.; Dietz, R.; Teilmann, J.; Harkonen, T.; Siegismund, H.R. Integrating genetic data and population viability analyses for the identification of harbour seal (Phoca vitulina) populations and management units. Mol. Ecol. 2014, 23, 815–831. [Google Scholar] [CrossRef] [PubMed]
- Rijks, J.M.; Hoffman, J.I.; Kuiken, T.; Osterhaus, A.D.M.E.; Amos, W. Heterozygosity and lungworm burden in harbour seals (Phoca vitulina). Heredity (Edinb) 2008, 100, 587–593. [Google Scholar] [CrossRef]
- Goodman, S.J. Phocine Distemper Virus. In Mononegaviruses of Veterinary Importance; Munir, M., Ed.; CABI: Wallingford, UK, 2013; Volume 1, pp. 261–268. [Google Scholar]
- Dinarello, C.A. Interleukin-18, a proinflammatory cytokine. Eur. Cytokine Netw. 2000, 11, 483–486. [Google Scholar] [PubMed]
- Kiecolt-Glaser, J.K.; Preacher, K.J.; MacCallum, R.C.; Atkinson, C.; Malarkey, W.B.; Glaser, R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc. Natl. Acad. Sci. USA 2003, 100, 9090–9095. [Google Scholar] [CrossRef] [PubMed]
- Frisk, A.L.; Baumgartner, W.; Grone, A. Dominating interleukin-10 mRNA expression induction in cerebrospinal fluid cells of dogs with natural canine distemper virus induced demyelinating and non-demyelinating CNS lesions. J. Neuroimmunol. 1999, 97, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Beineke, A.; Puff, C.; Seehusen, F.; Baumgartner, W. Pathogenesis and immunopathology of systemic and nervous canine distemper. Vet. Immunol. Immunopathol. 2009, 127, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Moss, W.J.; Ryon, J.J.; Monze, M.; Griffin, D.E. Differential regulation of interleukin (IL)-4, IL-5, and IL-10 during measles in Zambian children. J. Infect. Dis. 2002, 186, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Seibel, H.; Siebert, U.; Rosenberger, T.; Baumgartner, W. Variable transcription of pro- and anti-inflammatory cytokines in phocine lymphocytes following canine distemper virus infection. Vet. Immuno. Immupathol. 2014, 161, 170–183. [Google Scholar] [CrossRef]
- Hughes, D.E.; Carter, S.D.; Robinson, I.; Clarke, D.D.; Clarke, C.J. Anti-canine distemper virus antibodies in common and grey seals. Vet. Rec. 1992, 130, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Visser, I.K.G.; vd Bildt, M.W.G.; Brugge, H.N.; Reijnders, P.J.H.; Vedder, E.J.; Kuiper, J.; de Vries, P.; Groen, J.; Walvoort, H.C.; UytdeHaag, F.G.C.M.; et al. Vaccination of harbour seals (Phoca vitulina) against phocid distemper with two different inactivated canine distemper virus (CDV) vaccines. Vaccine 1989, 7, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.W.; Appel, M.J.; Erickson, R.C.; Novilla, M.N. Fatal vaccine-induced canine distemper virus infection in black-footed ferrets. J. Am. Vet. Med. Assoc. 1976, 169, 961–964. [Google Scholar] [PubMed]
- Itakura, C.; Nakamura, K.; Nakatsuka, J.; Goto, M. Distemper infection in lesser pandas due to administration of a canine distemper live vaccine. Nihon Juigaku Zasshi 1979, 41, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Visser, I.K.G.; Vedder, E.J.; van de Bildt, M.W.; Orvell, C.; Barrett, T.; Osterhaus, A.D.M.E. Canine distemper virus ISCOMs induce protection in harbour seals (Phoca vitulina) against phocid distemper but still allow subsequent infection with phocid distemper virus-1. Vaccine 1992, 10, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Osterhaus, A.; van de Bildt, M.; Vedder, L.; Martina, B.; Niesters, H.; Vos, J.; van Egmond, H.; Liem, D.; Baumann, R.; Androukaki, E.; et al. Monk seal mortality: Virus or toxin? Vaccine 1998, 16, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Carretta, J.V.; Oleson, E.; Weller, D.W.; Lang, A.R.; Forney, K.A.; Baker, J.; Hanson, B.; Martien, K.; Muto, M.M.; Orr, A.J.; et al. U.S. Pacific Marine Mammal Stock Assessments, 2013; U.S. Department of Commerce, National Oceanic and Atmospheric Administration - NOAA Technical Memorandum NMFS (National Marine Fisheries Service): Silver Spring, USA, 2014; p. 406, NOAA-TM-NMFS-SWFSC-532.
- Aguirre, A.A.; Keefe, T.J.; Reif, J.S.; Kashinsky, L.; Yochem, P.K.; Saliki, J.T.; Stott, J.L.; Goldstein, T.; Dubey, J.P.; Braun, R.; et al. Infectious disease monitoring of the endangered Hawaiian monk seal. J. Wildl. Dis. 2007, 43, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.K.; Baker, J.D.; Toonen, R.J.; Bowen, B.W. Extremely low genetic diversity in the endangered Hawaiian monk seal (Monachus schauinslandi). J. Hered. 2009, 100, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Bronson, E.; Deem, S.L.; Sanchez, C.; Murray, S. Serologic response to a canarypox-vectored canine distemper virus vaccine in the giant panda (Ailuropoda melanoleuca). J. Zoo. Wildl. Med. 2007, 38, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Quinley, N.; Mazet, J.A.; Rivera, R.; Schmitt, T.L.; Dold, C.; McBain, J.; Fritsch, V.; Yochem, P.K. Serologic response of harbor seals (Phoca vitulina) to vaccination with a recombinant canine distemper vaccine. J. Wildl. Dis. 2013, 49, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Jessup, D.A.; Murray, M.J.; Casper, D.R.; Brownstein, D.; Kreuder-Johnson, C. Canine distemper vaccination is a safe and useful preventive procedure for southern sea otters (Enhydra lutra nereis). J. Zoo Wildl. Med. 2009, 40, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Ragen, T.J.; Lavigne, D.M. The Hawaiian monk seal: Biology of an endangered species. In Conservation and Management of Marine Mammals; Twiss, J., Reeves, R., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1999. [Google Scholar]
- Baker, J.D.; Harting, A.L.; Wurth, T.A.; Johanos, T.C. Dramatic shifts in hawaiian monk seal distribution predicted from divergent regional trends. Mar. Mamm. Sci. 2011, 27, 78–93. [Google Scholar] [CrossRef]
- Appel, M.J.G.; Gibbs, E.P.J.; Martin, S.J.; ter Meulen, V.; Rima, B.K.; Stephenson, J.R.; Taylor, W.P. Morbillivirus diseases of animals and man. In Comparative Diagnosis of Viral Diseases; Kurstak, E., Kurstak, C., Eds.; Academic Press: New York, NY, USA, 1981; Volume 4, pp. 235–297. [Google Scholar]
- Harwood, J.; Hall, A. Mass mortality in marine mammals: Its implications for population dynamics and genetics. Trends Ecol. Evol. 1990, 5, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Dietz, R.; Heide-Jorgensen, M.-P.; Harkonen, T. Mass deaths of Harbor seals (Phoca vitulina) in Europe. Ambio 1989, 18, 258–264. [Google Scholar]
- Krakowka, S.; Hoover, E.A.; Koestner, A.; Ketring, K. Experimental and naturally occurring transplacental transmission of canine distemper virus. Am. J. Vet. Res. 1977, 38, 919–922. [Google Scholar] [PubMed]
- Fernández, A.; Esperón, F.; Herraéz, P.; de Los Monteros, A.E.; Clavel, C.; Bernabé, A.; Sanchez-Vizcaino, J.M.; Verborgh, P.; DeStephanis, R.; Toledano, F.; et al. Morbillivirus and pilot whale deaths, Mediterranean Sea. Emerg. Infect. Dis. 2008, 14, 792–794. [Google Scholar] [CrossRef] [PubMed]
- Di Guardo, G.; Cocumelli, C.; Scholl, F.; di Francesco, C.E.; Speranza, R.; Pennelli, M.; Eleni, C. Morbilliviral encephalitis in a striped dolphin Stenella coeruleoalba calf from Italy. Dis. Aquat. Organ. 2011, 95, 247–251. [Google Scholar]
- Warren, K.S.; Anderson, R.M.; Capasso, V.; Cliff, A.D.; Dietz, K.; Fenner, F.; Finnes, R.N.; Grossman, Z.; Knolle, H.; Mann, P.G.; et al. Transmission patterns and dynamics of infectious diseases. In Population Biology of Infectious Diseases; Anderson, R.M., May, R.M., Eds.; Springer Verlag: Berlin, Germany, 1982; pp. 67–85. [Google Scholar]
- Anderson, R.M.; May, R.M. The invasion, persistence and spread of infectious diseases within animal and plant communities. Philos. Trans. Royal Soc. Lond B 1986, 314, 533–570. [Google Scholar] [CrossRef]
- Bartlett, M.S. The critical community size for measles in the United States. J. Roy. Stat. Soc. A 1960, 123, 37–44. [Google Scholar] [CrossRef]
- Black, F.L. Infectious diseases in primitive societies. Science 1975, 187, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Keeling, M.J.; Grenfell, B.T. Disease extinction and community size: Modeling the persistence of measles. Science 1997, 275, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Swinton, J.; Harwood, J.; Grenfell, B.T.; Gilligan, C.A. Persistence thresholds for phocine distemper virus infection in harbour seal Phoca vitulina metapopulations. J. Anim. Ecol. 1998, 67, 54–68. [Google Scholar] [CrossRef]
- Almberg, E.S.; Cross, P.C.; Smith, D.W. Persistence of canine distemper virus in the Greater Yellowstone ecosystem’s carnivore community. Ecol. Appl. 2010, 20, 2058–2074. [Google Scholar] [CrossRef] [PubMed]
- Almberg, E.S.; Mech, L.D.; Smith, D.W.; Sheldon, J.W.; Crabtree, R.L. A serological survey of infectious disease in Yellowstone National Park’s canid community. PLoS One 2009, 4, e7042. [Google Scholar] [CrossRef] [PubMed]
- Duignan, P.J. Studies on the Epizootiology and Immunology of Morbillivirus Infection in Marine Mammals of the Western Atlantic. Ph.D. Thesis, University of Guelph, Guelph, Canada, 1996. [Google Scholar]
- Holmes, J.C. The impact of infectious disease agents on the population growth and geographical distribution of animals. In Population Biology of Infectious Diseases; Anderson, R.M., May, R.M., Eds.; Springer-Verlag: Berlin, Germany, 1982; pp. 37–51. [Google Scholar]
- Anderson, R.M. Directly transmitted viral and bacterial infections of man. In The Population Dynamics of Infectious Diseases: Theory and Applications; Anderson, R.M., Ed.; Springer: New York, NY, USA, 1982; pp. 1–37. [Google Scholar]
- Plowright, W. The effects of rinderpest and rinderpest control on wildlife in Africa. Symp. Zool. Soc. Lond. 1982, 50, 1–28. [Google Scholar]
- Muller, G.; Wohlsein, P.; Beineke, A.; Haas, L.; Greiser-Wilke, I.; Siebert, U.; Fonfara, S.; Harder, T.; Stede, M.; Gruber, A.D.; et al. Phocine distemper in German seals, 2002. Emerg. Infect. Dis. 2004, 10, 723–725. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.S.; Visser, I.K.; Broeders, H.W.; van de Bildt, M.W.; Bowen, W.D.; Osterhaus, A.D. Antibodies to phocine distemper virus in Canadian seals. Vet. Rec. 1992, 130, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Duignan, P.J.; Saliki, J.T.; St. Aubin, D.J.; House, J.A.; Geraci, J.R. Neutralizing antibodies to phocine distemper virus in Atlantic walruses (Odobenus rosmarus rosmarus) from Arctic Canada. J. Wildl. Dis. 1994, 30, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Zarnke, R.L.; Saliki, J.T.; Macmillan, A.P.; Brew, S.D.; Dawson, C.E.; Ver Hoef, J.M.; Frost, K.J.; Small, R.J. Serologic survey for Brucella spp., phocid herpesvirus-1, phocid herpesvirus-2, and phocine distemper virus in harbor seals from Alaska, 1976–1999. J. Wildl. Dis. 2006, 42, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Hanni, K.D.; Mazet, J.A.; Gulland, F.M.; Estes, J.; Staedler, M.; Murray, M.J.; Miller, M.; Jessup, D.A. Clinical pathology and assessment of pathogen exposure in southern and Alaskan sea otters. J. Wildl. Dis. 2003, 39, 837–850. [Google Scholar] [CrossRef] [PubMed]
- Ham-Lammé, K.D.; King, D.P.; Taylor, B.C.; House, C.; Jessup, D.A.; Jeffries, S.; Yochem, P.K.; Gulland, F.M.D.; Ferrick, D.A.; Stott, J.L. The application of immuno-assays for serological detection of morbillivirus exposure in free ranging harbor seals (Phoca vitulina) and sea otters (Enhydra lutris) from the western coast of the United States. Mar. Mamm. Sci. 1999, 15, 601–608. [Google Scholar] [CrossRef]
- Burek, K.A.; Gulland, F.M.; Sheffield, G.; Beckmen, K.B.; Keyes, E.; Spraker, T.R.; Smith, A.W.; Skilling, D.E.; Evermann, J.F.; Stott, J.L.; et al. Infectious disease and the decline of Steller sea lions (Eumetopias jubatus) in Alaska, USA: Insights from serologic data. J. Wildl. Dis. 2005, 41, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, T.; Gill, V.A.; Tuomi, P.; Monson, D.; Burdin, A.; Conrad, P.A.; Dunn, J.L.; Field, C.; Johnson, C.; Jessup, D.A.; et al. Assessment of clinical pathology and pathogen exposure in sea otters (Enhydra lutris) bordering the threatened population in Alaska. J. Wildl. Dis. 2011, 47, 579–592. [Google Scholar] [CrossRef] [PubMed]
- White, C.L.; Schuler, K.L.; Thomas, N.J.; Webb, J.L.; Saliki, J.T.; Ip, H.S.; Dubey, J.P.; Frame, E.R. Pathogen exposure and blood chemistry in the Washington, USA population of northern sea otters (Enhydra lutris kenyoni). J. Wildl. Dis. 2013, 49, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Sato, H.; Kakumoto, C.; Kobayashi, M.; Saito, S.; Kariya, T.; Watanabe, Y.; Sakoda, Y.; Kai, C.; Kida, H.; et al. Seroepidemiological survey of morbillivirus infection in Kuril harbor seals (Phoca vitulina stejnegeri) of Hokkaido, Japan. Jpn. J. Vet. Res. 2006, 54, 109–117. [Google Scholar] [PubMed]
- Ohashi, K.; Kai, C. Morbillivirus Infections in wildlife of Japan. J. Vet. Med. 2000, 53, 834–838. (In Japanese) [Google Scholar]
- Duignan, P.J. Diseases of cetaceans and pinnipeds. In Marine Wildlife, Proceedings 335; Post Graduate Foundation in Veterinary Science, University of Sydney: Sydney, Australia, 2000; pp. 419–462. [Google Scholar]
- Duignan, P.J. Gross pathology, histopathology, virology, serology and parasitology. In Unusual Mortality of the New Zealand Sea Lion, Phocarctos Hookeri, Auckland Islands, January–February 1998: A Report of a Workshop Held 8–9 June 1998, Wellington, and a Contingency Plan for Future Events; Baker, A., Ed.; The Department of Conservation, Te Papa Atawhai: Wellington, New Zealand, 1999; pp. 29–33. [Google Scholar]
- Lynch, M.; Nielsen, O.; Duignan, P.J.; Kirkwood, R.; Hoskins, A.; Arnould, J.P. Serologic survey for potential pathogens and assessment of disease risk in Australian fur seals. J. Wildl. Dis. 2011, 47, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Bengtson, J.L.; Boveng, P.; Franzen, U.; Have, P.; Heide-Jorgensen, M.P.; Harkonen, T.J. Antibodies to canine distemper virus in Antarctic seals. Mar. Mamm. Sci. 1991, 7, 85–87. [Google Scholar] [CrossRef]
- Cattet, M.R.; Duignan, P.J.; House, C.A.; Aubin, D.J. Antibodies to canine distemper and phocine distemper viruses in polar bears from the Canadian arctic. J. Wildl. Dis. 2004, 40, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, D.E. Harp Seals, Man and Ice. Can. Spec. Publ. Fish. Aquat. Sci. 1991, 114, 153. [Google Scholar]
- Stenson, G.B.; Hammill, M. Current Status of Northwest Atlantic Harp Seals, (Pagophilus groenlandicus). DFO Can. Sci. Advis. Sec., Sci. Advis. Rep. 2012, 2011/070, 15. [Google Scholar]
- Boness, D.J.; James, H. Reproductive behaviour of the grey seal (Halichoerus grypus) on Sable Island, Nova Scotia. J. Zool. 1979, 188, 477–500. [Google Scholar] [CrossRef]
- Kenney, M.K.; Gilbert, J.R. Increase in Harbor Seal and Gray Seal Populations in Maine; National Marine Fisheries Service, Northeast Fisheries Science Center: Woods Hole, MA, USA, 1994; p. 19, Contract Number 50-ENAF-2-00064.
- Lavigueur, L.; Hammill, M.O. Distribution and seasonal movements of grey seals, Halichoerus grypus, born in the Gulf of St. Lawrence and eastern Nova Scotia shore. Can. Field Nat. 1993, 107, 329–340. [Google Scholar]
- Stobo, W.; Beck, B.; Horne, J.K. Seasonal movements of grey seals (Halichoerus grypus) in the northwest Atlantic. In Population Biology of Seal Worm (Pseudoterranova decipiens) in Relation to its Intermediate and Seal Hosts; Bowen, W.D., Ed.; Canadian Bulletin of Fisheries and Aquatic Sciences Number 222, Canadian Government Publishing Centre, Supply and Services Canada: Ottawa, ON, Canada, 1990; pp. 199–213. [Google Scholar]
- Thomas, L.; Hammill, M.O.; Bowen, W.D. Estimated Size of the Northwest Atlantic Grey Seal Population 1977–2010. DFO Can. Sci. Advis. Sec. Res. Doc. 2011, 2011/017, iv, 23. [Google Scholar]
- Wood, S.A.; Frasier, T.R.; McLeod, B.A.; Gilbert, J.R.; White, B.N.; Bowen, W.D.; Hammill, M.O.; Waring, G.T.; Brault, S. The genetics of recolonization: An analysis of the stock structure of grey seals (Halichoerus grypus) in the northwest Atlantic. Can. J. Zool. 2011, 89, 490–497. [Google Scholar] [CrossRef]
- Bodewes, R.; Morick, D.; van de Bildt, M.W.G.; Osinga, N.; Rubio Garcia, A.; Sanchez Contreras, G.J.; Smits, S.L.; Reperant, L.A.P.; Kuiken, T.; Osterhaus, A.D.M.E. Prevalence of phocine distemper virus specific antibodies: Bracing for the next seal epizootic in north-western Europe. Emerg. Microbes. Infect. 2013, 2, e3. [Google Scholar] [CrossRef]
- Thompson, P.M.; Hall, A.J. Seals and epizootics—What factors migh affect the severity of mass mortalities? Mammal. Rev. 1993, 23, 149–154. [Google Scholar] [CrossRef]
- Hall, A.J.; Jepson, P.D.; Goodman, S.J.; Harkonen, T. Phocine distemper virus in the North and European Seas – Data and models, nature and nurture. Biol. Conserv. 2006, 131, 221–229. [Google Scholar] [CrossRef]
- Seibel, H.; Baumgartner, W.; Muller, G.; Wohlsein, P.; Siebert, U. Retrospective analysis of two phocine distemper epidemics in the North and Baltic Seas in 1988 and 2002. Dtsch. Tierarztl. Woch. 2007, 114, 284–293. [Google Scholar]
- Lonergan, M.; Hall, A.; Thompson, H.; Thompson, P.M.; Pomeroy, P.; Harwood, J. Comparison of the 1988 and 2002 phocine distemper epizootics in British harbour seal Phoca vitulina populations. Dis. Aquat. Org. 2010, 88, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Rijks, J.M.; van de Bildt, M.W.; Jensen, T.; Philippa, J.D.; Osterhaus, A.D.M.E.; Kuiken, T. Phocine distemper outbreak, The Netherlands, 2002. Emerg. Infect. Dis. 2005, 11, 1945–1948. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.J.; Pomeroy, P.P.; Harwood, J. The descriptive epizootiology of phocine distemper in the UK during 1988/89. Sci. Total Environ. 1992, 115, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Harding, K.C.; Hansen, B.J.; Goodman, S.J. Acquired immunity and stochasticity in epidemic intervals impede the evolution of host disease resistance. Am. Nat. 2005, 166, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Postnikov, E.B.; Sokolov, I.M. Continuum description of a contact infection spread in a SIR model. Math. Biosci. 2007, 208, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Klepac, P.; Pomeroy, L.W.; Bjornstad, O.N.; Kuiken, T.; Osterhaus, A.D.; Rijks, J.M. Stage-structured transmission of phocine distemper virus in the Dutch 2002 outbreak. Proc. Biol. Sci. 2009, 276, 2469–2476. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.G. Using simple models to review the application and implications of different approaches used to simulate transmission of pathogens among aquatic animals. Prev. Vet. Med. 2009, 88, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.M.; Thompson, H.; Hall, A.J. Prevalence of morbillivirus antibodies in Scottish harbour seals. Vet. Rec. 2002, 151, 609–610. [Google Scholar] [CrossRef] [PubMed]
- Grenfell, B.T.; Lonergan, M.E.; Harwood, J. Quantitative investigations of the epidemiology of phocine distemper virus (PDV) in European common seal populations. Sci. Total Environ. 1992, 115, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Pomeroy, P.P.; Hammond, J.A.; Hall, A.J.; Lonergan, M.; Duck, C.D.; Smith, V.J.; Thompson, H. Morbillivirus neutralizing antibodies in Scottish grey seals Halichoerus grypus: Assessing the effects of the 1988 and 2002 PDV epizootics. Mar. Ecol. Prog. Ser. 2005, 287, 241–250. [Google Scholar] [CrossRef]
- Kreutzer, M.; Kreutzer, R.; Siebert, U.; Muller, G.; Reijnders, P.; Brasseur, S.; Harkonen, T.; Dietz, R.; Sonne, C.; Born, E.W.; et al. In search of virus carriers of the 1988 and 2002 phocine distemper virus outbreaks in European harbour seals. Arch. Virol. 2008, 153, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.M.; Travis, J.M.; Harwood, J. Evaluating the influence of epidemiological parameters and host ecology on the spread of phocine distemper virus through populations of harbour seals. PLoS One 2008, 3, e2710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenhalgh, D. Some results for an SEIR epidemic model with density dependence in the death rate. IMA J. Math. Appl. Med. Biol. 1992, 9, 67–106. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.M. Harbour seal movement patterns. In Symposium of the Zoological Society of London; Boyd, I.L., Ed.; Oxford University Press: Oxford, UK, 1993; pp. 225–239. [Google Scholar]
- Harkonen, T.; Harding, K.C.; Heide-Jorgensen, M.P. Rates of increase in age-structured populations: A lesson from the European harbour seals. Can. J. Zool. 2002, 80, 1498–1510. [Google Scholar] [CrossRef]
- Harkonen, T.; Harding, K.; Rasmussen, T.D.; Teilmann, J.; Dietz, R. Age- and sex-specific mortality patterns in an emerging wildlife epidemic: The phocine distemper in European harbour seals. PLoS One 2007, 2, e887. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Lonergan, M.; Duck, C. Population dynamics of harbour seals Phoca vitulina in England: Monitoring growth and catastrophic declines. J. Appl. Ecol. 2005, 42, 638–648. [Google Scholar] [CrossRef]
- Harding, K.C.; Harkonen, T.; Caswell, H. The 2002 European seal plague: Epidemiology and population consequences. Ecol. Lett. 2002, 5, 727–732. [Google Scholar] [CrossRef]
- Lonergan, M.; Harwood., J. The potential effects of repeated outbreaks of phocine distemper among harbour seals: A response to Harding et al. Ecol. Lett. 2003, 6, 889–893. [Google Scholar] [CrossRef]
- Reijnders, P.J.H.; Brasseur, S.M.J.M.; Tougaard, S.; Seibert, U.; Borchardt, T.; Stede, M. Population development and status of harbour seals (Phoca vitulia) in the Wadden Sea. NAMMCO Sci. Publ. 2010, 8, 95–106. [Google Scholar] [CrossRef]
- Reijnders, P.J.H.; Ries, E.H.; Tougaard, S.; Nørgaard, N.; Heidemann, G.; Schwarz, J.; Vareschi, E.; Traut, I.M. Population development of harbour seals Phoca vitulina in the Wadden Sea after the 1988 virus epizootic. J. Sea Res. 1997, 38, 161–168. [Google Scholar] [CrossRef]
- Lonergan, M.; Duck, C.D.; Thompson, D.; Mackey, B.L.; Cunningham, L.; Boyd, I.L. Using sparse survey data to investigate the declining abundance of British harbour seals. J. Zool. 2007, 271, 261–269. [Google Scholar] [CrossRef]
- SCOS (Special Committee on Seals). Scientific Advice on Matters Relating to the Management of Seal Populations: 2011; Sea Mammal Research Unit, University of St Andrews: St Andrews, Fife, UK, 2011; p. 127. [Google Scholar]
- Garnier, R.; Gandon, S.; Harding, K.C.; Boulinier, T. Length of intervals between epidemics: Evaluating the influence of maternal transfer of immunity. Ecol. Evol. 2014, 4, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Yorke, A.E. Status, Biology, and Ecology of Fur Seals. In Proceedings of the International Symposium and Workshop, Cambridge, England, 23-27 April 1984; Technical Report NMFS 51. Croxall, J.P., Gentry, R.L., Eds.; National Oceanic Atmospheric Administration: Seattle, WA, USA, 1987; pp. 9–21. [Google Scholar]
- Gulland, F.M.D. Impact of infectious diseases on wild animal populations—A review. In Ecology of Infectious Diseases in Natural Populations; Grenfell, B.T., Dobson, A.P., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 20–51. [Google Scholar]
- Kennedy, S.; Kuiken, T.; Jepson, P.D.; Deaville, R.; Forsyth, M.; Barrett, T.; van de Bildt, M.W.; Osterhaus, A.D.M.E.; Eybatov, T.; Duck, C.; et al. Mass die-Off of Caspian seals caused by canine distemper virus. Emerg. Infect. Dis. 2000, 6, 637–639. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duignan, P.J.; Van Bressem, M.-F.; Baker, J.D.; Barbieri, M.; Colegrove, K.M.; De Guise, S.; De Swart, R.L.; Di Guardo, G.; Dobson, A.; Duprex, W.P.; et al. Phocine Distemper Virus: Current Knowledge and Future Directions. Viruses 2014, 6, 5093-5134. https://doi.org/10.3390/v6125093
Duignan PJ, Van Bressem M-F, Baker JD, Barbieri M, Colegrove KM, De Guise S, De Swart RL, Di Guardo G, Dobson A, Duprex WP, et al. Phocine Distemper Virus: Current Knowledge and Future Directions. Viruses. 2014; 6(12):5093-5134. https://doi.org/10.3390/v6125093
Chicago/Turabian StyleDuignan, Pádraig J., Marie-Françoise Van Bressem, Jason D. Baker, Michelle Barbieri, Kathleen M. Colegrove, Sylvain De Guise, Rik L. De Swart, Giovanni Di Guardo, Andrew Dobson, W. Paul Duprex, and et al. 2014. "Phocine Distemper Virus: Current Knowledge and Future Directions" Viruses 6, no. 12: 5093-5134. https://doi.org/10.3390/v6125093
APA StyleDuignan, P. J., Van Bressem, M.-F., Baker, J. D., Barbieri, M., Colegrove, K. M., De Guise, S., De Swart, R. L., Di Guardo, G., Dobson, A., Duprex, W. P., Early, G., Fauquier, D., Goldstein, T., Goodman, S. J., Grenfell, B., Groch, K. R., Gulland, F., Hall, A., Jensen, B. A., ... Wellehan, J. F. X. (2014). Phocine Distemper Virus: Current Knowledge and Future Directions. Viruses, 6(12), 5093-5134. https://doi.org/10.3390/v6125093






