Serological Assays Based on Recombinant Viral Proteins for the Diagnosis of Arenavirus Hemorrhagic Fevers
Abstract
:1. Introduction
2. Currently Used Diagnostic Techniques for VHFs
4. Antigen-Capture ELISA
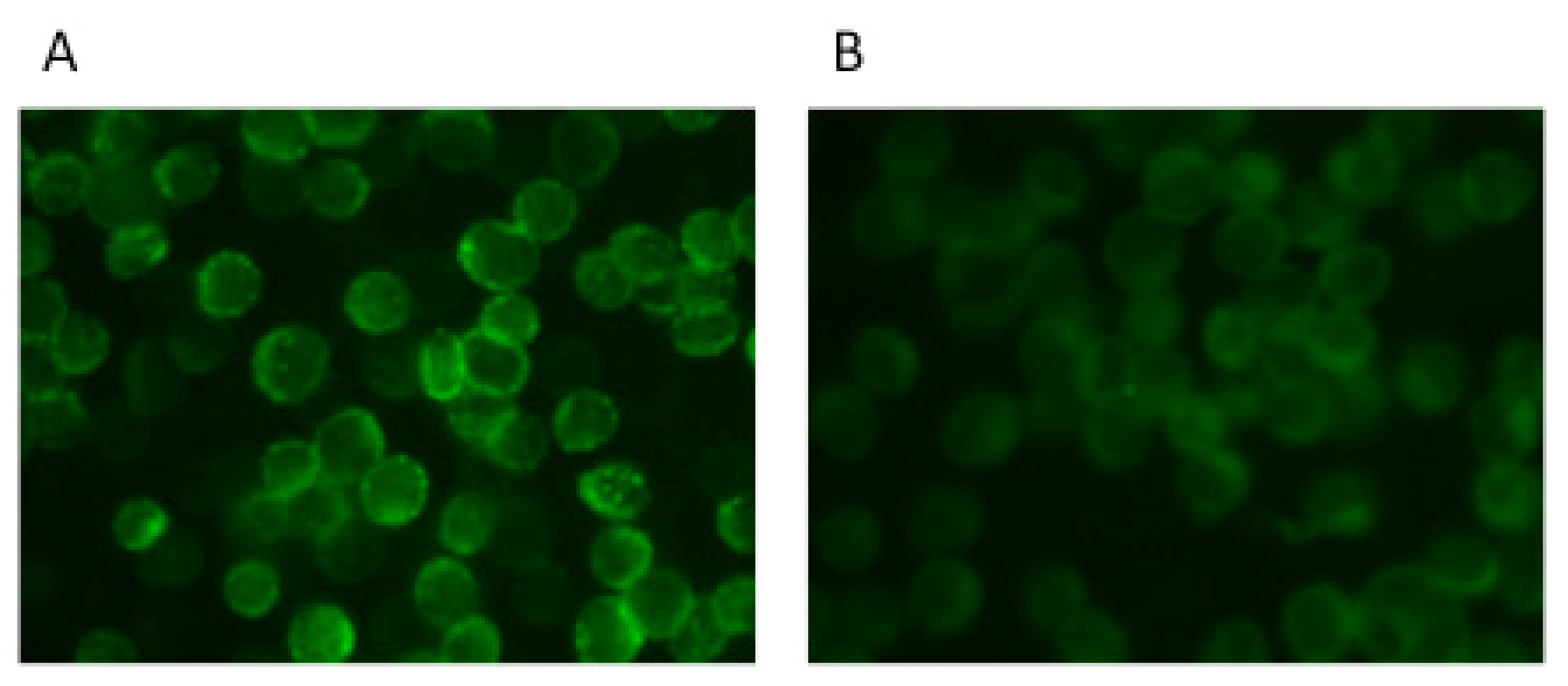
5. Neutralization Assays Based on VSV Pseudotypes
6. Conclusions
Conflict of Interest
Acknowledgements
References and Notes
- Pfau, C.J.; Bergold, G.H.; Casals, J.; Johnson, K.M.; Murphy, F.A.; Pedersen, I.R.; Rawls, W.E.; Rowe, W.P.; Webb, P.A.; Weissenbacher, M.C. Arenaviruses. Intervirology 1974, 4, 207–214. [Google Scholar] [CrossRef]
- Charrel, R.N.; de Lamballerie, X. Arenaviruses other than Lassa virus. Antivir. Res. 2003, 57, 89–100. [Google Scholar]
- Jahrling, P.B.; Peters, C.J. Lymphocytic choriomeningitis virus. A neglected pathogen of man. Arch. Pathol. Lab. Med. 1992, 116, 486–488. [Google Scholar]
- Barton, L.L.; Mets, M.B.; Beauchamp, C.L. Lymphocytic choriomeningitis virus: Emerging fetal teratogen. Am. J. Obstet. Gynecol. 2002, 187, 1715–1716. [Google Scholar] [CrossRef]
- Wright, R.; Johnson, D.; Neumann, M.; Ksiazek, T.G.; Rollin, P.; Keech, R.V.; Bonthius, D.J.; Hitchon, P.; Grose, C.F.; Bell, W.E.; et al. Congenital lymphocytic choriomeningitis virus syndrome: A disease that mimics congenital toxoplasmosis or Cytomegalovirus infection. Pediatrics 1997, 100, E9. [Google Scholar]
- Salazar-Bravo, J.; Ruedas, L.A.; Yates, T.L. Mammalian reservoirs of arenaviruses. Curr. Top. Microbiol. Immunol. 2002, 262, 25–63. [Google Scholar]
- Carey, D.E.; Kemp, G.E.; White, H.A.; Pinneo, L.; Addy, R.F.; Fom, A.L.; Stroh, G.; Casals, J.; Henderson, B.E. Lassa fever. Epidemiological aspects of the 1970 epidemic, Jos, Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1972, 66, 402–408. [Google Scholar] [CrossRef]
- Lukashevich, I.S.; Clegg, J.C.; Sidibe, K. Lassa virus activity in Guinea: Distribution of human antiviral antibody defined using enzyme-linked immunosorbent assay with recombinant antigen. J. Med. Virol. 1993, 40, 210–217. [Google Scholar] [CrossRef]
- McCormick, J.B.; Webb, P.A.; Krebs, J.W.; Johnson, K.M.; Smith, E.S. A prospective study of the epidemiology and ecology of Lassa fever. J. Infect. Dis. 1987, 155, 437–444. [Google Scholar] [CrossRef]
- Monath, T.P. Lassa fever: Review of epidemiology and epizootiology. Bull. World Health Organ. 1975, 52, 577–592. [Google Scholar]
- Monath, T.P. Lassa fever: A new appraisal. Niger. Med. J. 1973, 3, 162–163. [Google Scholar]
- Monson, M.H.; Frame, J.D.; Jahrling, P.B.; Alexander, K. Endemic Lassa fever in Liberia. I. Clinical and epidemiological aspects at Curran Lutheran Hospital, Zorzor, Liberia. Trans. R. Soc. Trop. Med. Hyg. 1984, 78, 549–553. [Google Scholar] [CrossRef]
- Ehichioya, D.U.; Hass, M.; Becker-Ziaja, B.; Ehimuan, J.; Asogun, D.A.; Fichet-Calvet, E.; Kleinsteuber, K.; Lelke, M.; ter Meulen, J.; Akpede, G.O.; et al. Current molecular epidemiology of Lassa virus in Nigeria. J. Clin. Microbiol. 2011, 49, 1157–1161. [Google Scholar] [CrossRef]
- Peters, C.J. Human infection with arenaviruses in the Americas. Curr. Top. Microbiol. Immunol. 2002, 262, 65–74. [Google Scholar]
- Tesh, R.B. Viral hemorrhagic fevers of South America. Biomedica 2002, 22, 287–295. [Google Scholar]
- McCormick, J.B.; Fisher-Hoch, S.P. Lassa fever. Curr. Top. Microbiol. Immunol. 2002, 262, 75–109. [Google Scholar]
- Briese, T.; Paweska, J.T.; McMullan, L.K.; Hutchison, S.K.; Street, C.; Palacios, G.; Khristova, M.L.; Weyer, J.; Swanepoel, R.; Egholm, M.; et al. Genetic detection and characterization of Lujo virus, a new hemorrhagic fever-associated arenavirus from southern Africa. PLoS Pathog. 2009, 5, e1000455. [Google Scholar] [CrossRef]
- Cajimat, M.N.; Milazzo, M.L.; Bradley, R.D.; Fulhorst, C.F. Catarina virus, an arenaviral species principally associated with Neotoma micropus (southern plains woodrat) in Texas. Am. J. Trop. Med. Hyg. 2007, 77, 732–736. [Google Scholar]
- Cajimat, M.N.; Milazzo, M.L.; Borchert, J.N.; Abbott, K.D.; Bradley, R.D.; Fulhorst, C.F. Diversity among Tacaribe serocomplex viruses (family Arenaviridae) naturally associated with the Mexican woodrat (Neotoma mexicana). Virus Res. 2008, 133, 211–217. [Google Scholar] [CrossRef]
- Delgado, S.; Erickson, B.R.; Agudo, R.; Blair, P.J.; Vallejo, E.; Albarino, C.G.; Vargas, J.; Comer, J.A.; Rollin, P.E.; Ksiazek, T.G.; et al. Chapare virus, a newly discovered arenavirus isolated from a fatal hemorrhagic fever case in Bolivia. PLoS Pathog. 2008, 4, e1000047. [Google Scholar] [CrossRef]
- Gunther, S.; Hoofd, G.; Charrel, R.; Roser, C.; Becker-Ziaja, B.; Lloyd, G.; Sabuni, C.; Verhagen, R.; van der Groen, G.; Kennis, J.; et al. Mopeia virus-related arenavirus in natal multimammate mice, Morogoro, Tanzania. Emerg. Infect. Dis. 2009, 15, 2008–2012. [Google Scholar] [CrossRef]
- Lecompte, E.; ter Meulen, J.; Emonet, S.; Daffis, S.; Charrel, R.N. Genetic identification of Kodoko virus, a novel arenavirus of the African pigmy mouse (Mus Nannomys minutoides) in West Africa. Virology 2007, 364, 178–183. [Google Scholar] [CrossRef]
- Milazzo, M.L.; Cajimat, M.N.; Haynie, M.L.; Abbott, K.D.; Bradley, R.D.; Fulhorst, C.F. Diversity among tacaribe serocomplex viruses (family Arenaviridae) naturally associated with the white-throated woodrat (Neotoma albigula) in the southwestern United States. Vector Borne Zoonotic Dis. 2008, 8, 523–450. [Google Scholar] [CrossRef]
- Palacios, G.; Druce, J.; Du, L.; Tran, T.; Birch, C.; Briese, T.; Conlan, S.; Quan, P.L.; Hui, J.; Marshall, J.; et al. A new arenavirus in a cluster of fatal transplant-associated diseases. N. Engl. J. Med. 2008, 358, 991–998. [Google Scholar] [CrossRef]
- Palacios, G.; Savji, N.; Hui, J.; Travassos da Rosa, A.; Popov, V.; Briese, T.; Tesh, R.; Lipkin, W.I. Genomic and phylogenetic characterization of Merino Walk virus, a novel arenavirus isolated in South Africa. J. Gen. Virol. 2010, 91, 1315–1324. [Google Scholar] [CrossRef]
- Ishii, A.; Thomas, Y.; Moonga, L.; Nakamura, I.; Ohnuma, A.; Hang'ombe, B.; Takada, A.; Mweene, A.; Sawa, H. Novel arenavirus, Zambia. Emerg. Infect. Dis. 2011, 17, 1921–1924. [Google Scholar] [CrossRef]
- Frame, J.D. Surveillance of Lassa fever in missionaries stationed in West Africa. Bull. World Health Organ. 1975, 52, 593–598. [Google Scholar]
- Richmond, J.K.; Baglole, D.J. Lassa fever: Epidemiology, clinical features, and social consequences. BMJ 2003, 327, 1271–1275. [Google Scholar] [CrossRef]
- Gunther, S.; Emmerich, P.; Laue, T.; Kuhle, O.; Asper, M.; Jung, A.; Grewing, T.; ter Meulen, J.; Schmitz, H. Imported lassa fever in Germany: Molecular characterization of a new lassa virus strain. Emerg. Infect. Dis. 2000, 6, 466–476. [Google Scholar] [CrossRef]
- Hirabayashi, Y.; Oka, S.; Goto, H.; Shimada, K.; Kurata, T.; Fisher-Hoch, S.P.; McCormick, J.B. An imported case of Lassa fever with late appearance of polyserositis. J. Infect. Dis. 1988, 158, 872–875. [Google Scholar]
- Jeffs, B. A clinical guide to viral haemorrhagic fevers: Ebola, Marburg and Lassa. Trop. Doct. 2006, 36, 1–4. [Google Scholar] [CrossRef]
- Macher, A.M.; Wolfe, M.S. Historical Lassa fever reports and 30-year clinical update. Emerg. Infect. Dis. 2006, 12, 835–837. [Google Scholar] [CrossRef]
- Mahdy, M.S.; Chiang, W.; McLaughlin, B.; Derksen, K.; Truxton, B.H.; Neg, K. Lassa fever: The first confirmed case imported into Canada. Can. Dis. Wkly. Rep. 1989, 15, 193–198. [Google Scholar]
- Buchmeier, M.J.; Lewicki, H.A.; Tomori, O.; Oldstone, M.B. Monoclonal antibodies to lymphocytic choriomeningitis and pichinde viruses: Generation, characterization, and cross-reactivity with other arenaviruse. Virology 1981, 113, 73–85. [Google Scholar] [CrossRef]
- Ruo, S.L.; Mitchell, S.W.; Kiley, M.P.; Roumillat, L.F.; Fisher-Hoch, S.P.; McCormick, J.B. Antigenic relatedness between arenaviruses defined at the epitope level by monoclonal antibodies. J. Gen. Virol. 1991, 72, 549–555. [Google Scholar] [CrossRef]
- Sanchez, A.; Pifat, D.Y.; Kenyon, R.H.; Peters, C.J.; McCormick, J.B.; Kiley, M.P. Junin virus monoclonal antibodies: Characterization and cross-reactivity with other arenaviruses. J. Gen. Virol. 1989, 70, 1125–1132. [Google Scholar] [CrossRef]
- Charrel, R.N.; de Lamballerie, X. Zoonotic aspects of arenavirus infections. Vet. Microbiol. 2010, 140, 213–220. [Google Scholar] [CrossRef]
- Drosten, C.; Kummerer, B.M.; Schmitz, H.; Gunther, S. Molecular diagnostics of viral hemorrhagic fevers. Antivir. Res. 2003, 57, 61–87. [Google Scholar]
- Ogawa, H.; Miyamoto, H.; Ebihara, H.; Ito, K.; Morikawa, S.; Feldmann, H.; Takada, A. Detection of all known filovirus species by reverse transcription-polymerase chain reaction using a primer set specific for the viral nucleoprotein gene. J. Virol. Meth. 2011, 171, 310–313. [Google Scholar] [CrossRef]
- Olschlager, S.; Lelke, M.; Emmerich, P.; Panning, M.; Drosten, C.; Hass, M.; Asogun, D.; Ehichioya, D.; Omilabu, S.; Gunther, S. Improved detection of Lassa virus by reverse transcription-PCR targeting the 5' region of S RNA. J. Clin. Microbiol. 2010, 48, 2009–2013. [Google Scholar] [CrossRef]
- Palacios, G.; Quan, P.L.; Jabado, O.J.; Conlan, S.; Hirschberg, D.L.; Liu, Y.; Zhai, J.; Renwick, N.; Hui, J.; Hegyi, H.; et al. Panmicrobial oligonucleotide array for diagnosis of infectious diseases. Emerg. Infect. Dis. 2007, 13, 73–81. [Google Scholar] [CrossRef]
- Ksiazek, T.G.; Rollin, P.E.; Williams, A.J.; Bressler, D.S.; Martin, M.L.; Swanepoel, R.; Burt, F.J.; Leman, P.A.; Khan, A.S.; Rowe, A.K.; et al. Clinical virology of Ebola hemorrhagic fever (EHF): Virus, virus antigen, and IgG and IgM antibody findings among EHF patients in Kikwit, Democratic Republic of the Congo, 1995. J. Infect. Dis. 1999, 179, S177–S187. [Google Scholar]
- Ksiazek, T.G.; West, C.P.; Rollin, P.E.; Jahrling, P.B.; Peters, C.J. ELISA for the detection of antibodies to Ebola viruses. J. Infect. Dis. 1999, 179, S192–S198. [Google Scholar] [CrossRef]
- Bausch, D.G.; Rollin, P.E.; Demby, A.H.; Coulibaly, M.; Kanu, J.; Conteh, A.S.; Wagoner, K.D.; McMullan, L.K.; Bowen, M.D.; Peters, C.J.; et al. Diagnosis and clinical virology of Lassa fever as evaluated by enzyme-linked immunosorbent assay, indirect fluorescent-antibody test, and virus isolation. J. Clin. Microbiol. 2000, 38, 2670–2607. [Google Scholar]
- Emmerich, P.; Thome-Bolduan, C.; Drosten, C.; Gunther, S.; Ban, E.; Sawinsky, I.; Schmitz, H. Reverse ELISA for IgG and IgM antibodies to detect Lassa virus infections in Africa. J. Clin. Virol. 2006, 37, 277–281. [Google Scholar] [CrossRef]
- Meyer, B.J.; de la Torre, J.C.; Southern, P.J. Arenaviruses: Genomic RNAs, transcription, and replication. Curr. Top. Microbiol. Immunol. 2002, 262, 139–157. [Google Scholar]
- Ter Meulen, J.; Koulemou, K.; Wittekindt, T.; Windisch, K.; Strigl, S.; Conde, S.; Schmitz, H. Detection of Lassa virus antinucleoprotein immunoglobulin G (IgG) and IgM antibodies by a simple recombinant immunoblot assay for field use. J. Clin. Microbiol. 1998, 36, 3143–3148. [Google Scholar]
- Takimoto, K.; Taharaguchi, M.; Morikawa, S.; Ike, F.; Yamada, Y.K. Detection of the antibody to lymphocytic choriomeningitis virus in sera of laboratory rodents infected with viruses of laboratory and newly isolated strains by ELISA using purified recombinant nucleoprotein. Exp. Anim. 2008, 57, 357–365. [Google Scholar] [CrossRef]
- Ure, A.E.; Ghiringhelli, P.D.; Possee, R.D.; Morikawa, S.; Romanowski, V. Argentine hemorrhagic fever diagnostic test based on recombinant Junin virus N protein. J. Med. Virol. 2008, 80, 2127–2133. [Google Scholar] [CrossRef]
- Nakauchi, M.; Fukushi, S.; Saijo, M.; Mizutani, T.; Ure, A.E.; Romanowski, V.; Kurane, I.; Morikawa, S. Characterization of monoclonal antibodies to Junin virus nucleocapsid protein and application to the diagnosis of hemorrhagic fever caused by South American arenaviruses. Clin. Vaccine Immunol. 2009, 16, 1132–1138. [Google Scholar] [CrossRef]
- Saijo, M.; Georges-Courbot, M.C.; Marianneau, P.; Romanowski, V.; Fukushi, S.; Mizutani, T.; Georges, A.J.; Kurata, T.; Kurane, I.; Morikawa, S. Development of recombinant nucleoprotein-based diagnostic systems for Lassa fever. Clin. Vaccine Immunol. 2007, 14, 1182–1189. [Google Scholar] [CrossRef]
- Saijo, M.; Niikura, M.; Morikawa, S.; Ksiazek, T.G.; Meyer, R.F.; Peters, C.J.; Kurane, I. Enzyme-linked immunosorbent assays for detection of antibodies to Ebola and Marburg viruses using recombinant nucleoproteins. J. Clin. Microbiol. 2001, 39, 1–7. [Google Scholar] [CrossRef]
- Ikegami, T.; Niikura, M.; Saijo, M.; Miranda, M.E.; Calaor, A.B.; Hernandez, M.; Acosta, L.P.; Manalo, D.L.; Kurane, I.; Yoshikawa, Y.; et al. Antigen capture enzyme-linked immunosorbent assay for specific detection of Reston Ebola virus nucleoprotein. Clin. Diagn. Lab. Immunol. 2003, 10, 552–557. [Google Scholar]
- Saijo, M.; Tang, Q.; Shimayi, B.; Han, L.; Zhang, Y.; Asiguma, M.; Tianshu, D.; Maeda, A.; Kurane, I.; Morikawa, S. Antigen-capture enzyme-linked immunosorbent assay for the diagnosis of crimean-congo hemorrhagic fever using a novel monoclonal antibody. J. Med. Virol. 2005, 77, 83–88. [Google Scholar] [CrossRef]
- Saijo, M.; Qing, T.; Niikura, M.; Maeda, A.; Ikegami, T.; Prehaud, C.; Kurane, I.; Morikawa, S. Recombinant nucleoprotein-based enzyme-linked immunosorbent assay for detection of immunoglobulin G antibodies to Crimean-Congo hemorrhagic fever virus. J. Clin. Microbiol. 2002, 40, 1587–1591. [Google Scholar] [CrossRef]
- Matsuura, Y.; Possee, R.D.; Overton, H.A.; Bishop, D.H. Baculovirus expression vectors: The requirements for high level expression of proteins, including glycoproteins. J. Gen. Virol. 1987, 68, 1233–1250. [Google Scholar] [CrossRef]
- Tang, Q.; Saijo, M.; Zhang, Y.; Asiguma, M.; Tianshu, D.; Han, L.; Shimayi, B.; Maeda, A.; Kurane, I.; Morikawa, S. A patient with Crimean-Congo hemorrhagic fever serologically diagnosed by recombinant nucleoprotein-based antibody detection systems. Clin. Diagn. Lab. Immunol. 2003, 10, 489–491. [Google Scholar]
- Saijo, M. National Institute of Infectious Diseases, Tokyo, Japan. Unpublished work, 2012.
- van der Groen, G.; Piot, P.; Desmyter, J.; Colaert, J.; Muylle, L.; Tkachenko, E.A.; Ivanov, A.P.; Verhagen, R.; van Ypersele de Strihou, C. Seroepidemiology of Hantaan-related virus infections in Belgian populations. Lancet 1983, 2, 1493–1494. [Google Scholar]
- Morrill, J.C.; Soliman, A.K.; Imam, I.Z.; Botros, B.A.; Moussa, M.I.; Watts, D.M. Serological evidence of Crimean-Congo haemorrhagic fever viral infection among camels imported into Egypt. J. Trop. Med. Hyg. 1990, 93, 201–204. [Google Scholar]
- Emmerich, P.; Gunther, S.; Schmitz, H. Strain-specific antibody response to Lassa virus in the local population of west Africa. J. Clin. Virol. 2008, 42, 40–44. [Google Scholar] [CrossRef]
- Xia, H.; Li, P.; Yang, J.; Pan, L.; Zhao, J.; Wang, Z.; Li, Y.; Zhou, H.; Dong, Y.; Guo, S.; et al. Epidemiological survey of Crimean-Congo hemorrhagic fever virus in Yunnan, China, 2008. Int. J. Infect. Dis. 2011, 15, e459–e463. [Google Scholar] [CrossRef]
- Heinrich, N.; Saathoff, E.; Weller, N.; Clowes, P.; Kroidl, I.; Ntinginya, E.; Machibya, H.; Maboko, L.; Loscher, T.; Dobler, G.; et al. High seroprevalence of Rift Valley FEVER AND EVIDENCE FOR ENDEMIC circulation in Mbeya region, Tanzania, in a cross-sectional study. PLoS Negl. Trop. Dis. 2012, 6, e1557. [Google Scholar] [CrossRef] [Green Version]
- Saijo, M.; Niikura, M.; Morikawa, S.; Kurane, I. Immunofluorescence method for detection of Ebola virus immunoglobulin g, using HeLa cells which express recombinant nucleoprotein. J. Clin. Microbiol. 2001, 39, 776–778. [Google Scholar] [CrossRef]
- Ikegami, T.; Saijo, M.; Niikura, M.; Miranda, M.E.; Calaor, A.B.; Hernandez, M.; Manalo, D.L.; Kurane, I.; Yoshikawa, Y.; Morikawa, S. Development of an immunofluorescence method for the detection of antibodies to Ebola virus subtype Reston by the use of recombinant nucleoprotein-expressing HeLa cells. Microbiol. Immunol. 2002, 46, 633–638. [Google Scholar]
- Sayama, Y.; Demetria, C.; Saito, M.; Azul, R.R.; Taniguchi, S.; Fukushi, S.; Yoshikawa, T.; Iizuka, I.; Mizutani, T.; Kurane, I.; et al. A seroepidemiologic study of Reston ebolavirus in swine in the Philippines. BMC Vet. Res. 2012, 8, 82. [Google Scholar] [CrossRef]
- Taniguchi, S.; Watanabe, S.; Masangkay, J.S.; Omatsu, T.; Ikegami, T.; Alviola, P.; Ueda, N.; Iha, K.; Fujii, H.; Ishii, Y.; et al. Reston Ebolavirus antibodies in bats, the Philippines. Emerg. Infect. Dis. 2011, 17, 1559–1560. [Google Scholar]
- Saijo, M.; Tang, Q.; Shimayi, B.; Han, L.; Zhang, Y.; Asiguma, M.; Tianshu, D.; Maeda, A.; Kurane, I.; Morikawa, S. Recombinant nucleoprotein-based serological diagnosis of Crimean-Congo hemorrhagic fever virus infections. J. Med. Virol. 2005, 75, 295–299. [Google Scholar] [CrossRef]
- Morrison, H.G.; Goldsmith, C.S.; Regnery, H.L.; Auperin, D.D. Simultaneous expression of the Lassa virus N and GPC genes from a single recombinant vaccinia virus. Virus Res. 1991, 18, 231–241. [Google Scholar] [CrossRef]
- Iha, K.; Nakauchi, M.; Taniguchi, S.; Fukushi, S.; Mizutani, T.; Ogata, M.; Saijo, M.; Romanowski, V.; Enria, D.A.; Kyuwa, S.; et al. Establishment of serological diagnosis of Argentine hemorrhagic fever using recombinant antigens. National Institute of Infectious Diseases, Tokyo, Japan. To be submitted for publication, 2012.
- Saijo, M.; Georges-Courbot, M.C.; Fukushi, S.; Mizutani, T.; Philippe, M.; Georges, A.J.; Kurane, I.; Morikawa, S. Marburgvirus nucleoprotein-capture enzyme-linked immunosorbent assay using monoclonal antibodies to recombinant nucleoprotein: Detection of authentic Marburgvirus. Jpn. J. Infect. Dis. 2006, 59, 323–325. [Google Scholar]
- Velumani, S.; Du, Q.; Fenner, B.J.; Prabakaran, M.; Wee, L.C.; Nuo, L.Y.; Kwang, J. Development of an antigen-capture ELISA for detection of H7 subtype avian influenza from experimentally infected chickens. J. Virol. Meth. 2008, 147, 219–225. [Google Scholar] [CrossRef]
- Ji, Y.; Guo, W.; Zhao, L.; Li, H.; Lu, G.; Wang, Z.; Wang, G.; Liu, C.; Xiang, W. Development of an antigen-capture ELISA for the detection of equine influenza virus nucleoprotein. J. Virol. Meth. 2011, 175, 120–124. [Google Scholar] [CrossRef]
- Jansen van Vuren, P.; Potgieter, A.C.; Paweska, J.T.; van Dijk, A.A. Preparation and evaluation of a recombinant Rift Valley fever virus N protein for the detection of IgG and IgM antibodies in humans and animals by indirect ELISA. J. Virol. Meth. 2007, 140, 106–114. [Google Scholar] [CrossRef]
- Fukushi, S.; Nakauchi, M.; Mizutani, T.; Saijo, M.; Kurane, I.; Morikawa, S. Antigen-capture ELISA for the detection of Rift Valley fever virus nucleoprotein using new monoclonal antibodies. J. Virol. Meth. 2012, 180, 68–74. [Google Scholar] [CrossRef]
- Ksiazek, T.G.; Rollin, P.E.; Jahrling, P.B.; Johnson, E.; Dalgard, D.W.; Peters, C.J. Enzyme immunosorbent assay for Ebola virus antigens in tissues of infected primates. J. Clin. Microbiol. 1992, 30, 947–950. [Google Scholar]
- Logan, T.M.; Linthicum, K.J.; Moulton, J.R.; Ksiazek, T.G. Antigen-capture enzyme-linked immunosorbent assay for detection and quantification of Crimean-Congo hemorrhagic fever virus in the tick, Hyalomma truncatum. J. Virol. Meth. 1993, 42, 33–44. [Google Scholar] [CrossRef]
- Mills, J.N.; Ellis, B.A.; McKee, K.T., Jr.; Ksiazek, T.G.; Oro, J.G.; Maiztegui, J.I.; Calderon, G.E.; Peters, C.J.; Childs, J.E. Junin virus activity in rodents from endemic and nonendemic loci in central Argentina. Am. J. Trop. Med. Hyg. 1991, 44, 589–597. [Google Scholar]
- Niklasson, B.; Peters, C.J.; Grandien, M.; Wood, O. Detection of human immunoglobulins G and M antibodies to Rift Valley fever virus by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 1984, 19, 225–229. [Google Scholar]
- Roehrig, J.T.; Hombach, J.; Barrett, A.D. Guidelines for plaque-reduction neutralization testing of human antibodies to dengue viruses. Viral Immunol. 2008, 21, 123–132. [Google Scholar] [CrossRef]
- Hassantoufighi, A.; Zhang, H.; Sandbulte, M.; Gao, J.; Manischewitz, J.; King, L.; Golding, H.; Straight, T.M.; Eichelberger, M.C. A practical influenza neutralization assay to simultaneously quantify hemagglutinin and neuraminidase-inhibiting antibody responses. Vaccine 2010, 28, 790–797. [Google Scholar] [CrossRef]
- Holzmann, H. Diagnosis of tick-borne encephalitis. Vaccine 2003, 21, S36–S40. [Google Scholar] [CrossRef]
- Daniels, P.; Ksiazek, T.; Eaton, B.T. Laboratory diagnosis of Nipah and Hendra virus infections. Microbes Infect. 2001, 3, 289–295. [Google Scholar] [CrossRef]
- Tomori, O.; Johnson, K.M.; Kiley, M.P.; Elliott, L.H. Standardization of a plaque assay for Lassa virus. J. Med. Virol. 1987, 22, 77–89. [Google Scholar] [CrossRef]
- Alche, L.E.; Coto, C.E. Differentiation of Junin virus and antigenic variants isolated in vivo by kinetic neutralization assays. J. Gen. Virol. 1988, 69, 2123–2127. [Google Scholar] [CrossRef]
- Fukushi, S.; Mizutani, T.; Saijo, M.; Kurane, I.; Taguchi, F.; Tashiro, M.; Morikawa, S. Evaluation of a novel vesicular stomatitis virus pseudotype-based assay for detection of neutralizing antibody responses to SARS-CoV. J. Med. Virol. 2006, 78, 1509–1512. [Google Scholar] [CrossRef]
- Jahrling, P.B. Protection of Lassa virus-infected guinea pigs with Lassa-immune plasma of guinea pig, primate, and human origin. J. Med. Virol. 1983, 12, 93–102. [Google Scholar] [CrossRef]
- Takada, A.; Ebihara, H.; Jones, S.; Feldmann, H.; Kawaoka, Y. Protective efficacy of neutralizing antibodies against Ebola virus infection. Vaccine 2007, 25, 993–999. [Google Scholar] [CrossRef]
- McKee, K.T., Jr.; Oro, J.G.; Kuehne, A.I.; Spisso, J.A.; Mahlandt, B.G. Candid No. 1 Argentine hemorrhagic fever vaccine protects against lethal Junin virus challenge in rhesus macaques. Intervirology 1992, 34, 154–163. [Google Scholar] [CrossRef]
- Kretzschmar, E.; Buonocore, L.; Schnell, M.J.; Rose, J.K. High-efficiency incorporation of functional influenza virus glycoproteins into recombinant vesicular stomatitis viruses. J. Virol. 1997, 71, 5982–5989. [Google Scholar]
- Schnell, M.J.; Buonocore, L.; Kretzschmar, E.; Johnson, E.; Rose, J.K. Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc. Natl. Acad. Sci. U. S. A. 1996, 93, 11359–11365. [Google Scholar]
- Garbutt, M.; Liebscher, R.; Wahl-Jensen, V.; Jones, S.; Moller, P.; Wagner, R.; Volchkov, V.; Klenk, H.D.; Feldmann, H.; Stroher, U. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J. Virol. 2004, 78, 5458–5465. [Google Scholar]
- Fukushi, S.; Mizutani, T.; Sakai, K.; Saijo, M.; Taguchi, F.; Yokoyama, M.; Kurane, I.; Morikawa, S. Amino acid substitutions in the s2 region enhance severe acute respiratory syndrome coronavirus infectivity in rat angiotensin-converting enzyme 2-expressing cells. J. Virol. 2007, 81, 10831–10834. [Google Scholar] [CrossRef]
- Watanabe, R.; Matsuyama, S.; Shirato, K.; Maejima, M.; Fukushi, S.; Morikawa, S.; Taguchi, F. Entry from the cell surface of severe acute respiratory syndrome coronavirus with cleaved S protein as revealed by pseudotype virus bearing cleaved S protein. J. Virol. 2008, 82, 11985–11991. [Google Scholar] [CrossRef]
- Wang, Y.; Keck, Z.Y.; Foung, S.K. Neutralizing antibody response to hepatitis C virus. Viruses 2011, 3, 2127–2145. [Google Scholar] [CrossRef]
- Lin, H.X.; Feng, Y.; Tu, X.; Zhao, X.; Hsieh, C.H.; Griffin, L.; Junop, M.; Zhang, C. Characterization of the spike protein of human coronavirus NL63 in receptor binding and pseudotype virus entry. Virus Res. 2011, 160, 283–293. [Google Scholar] [CrossRef]
- Shah, P.P.; Wang, T.; Kaletsky, R.L.; Myers, M.C.; Purvis, J.E.; Jing, H.; Huryn, D.M.; Greenbaum, D.C.; Smith, A.B., 3rd; Bates, P.; et al. A small-molecule oxocarbazate inhibitor of human cathepsin L blocks severe acute respiratory syndrome and ebola pseudotype virus infection into human embryonic kidney 293T cells. Mol. Pharmacol. 2010, 78, 319–324. [Google Scholar] [CrossRef]
- Kolokoltsov, A.A.; Wang, E.; Colpitts, T.M.; Weaver, S.C.; Davey, R.A. Pseudotyped viruses permit rapid detection of neutralizing antibodies in human and equine serum against Venezuelan equine encephalitis virus. Am. J. Trop. Med. Hyg. 2006, 75, 702–709. [Google Scholar]
- Basu, A.; Li, B.; Mills, D.M.; Panchal, R.G.; Cardinale, S.C.; Butler, M.M.; Peet, N.P.; Majgier-Baranowska, H.; Williams, J.D.; Patel, I.; et al. Identification of a small-molecule entry inhibitor for filoviruses. J. Virol. 2011, 85, 3106–3119. [Google Scholar]
- Dylla, D.E.; Xie, L.; Michele, D.E.; Kunz, S.; McCray, P.B., Jr. Altering alpha-dystroglycan receptor affinity of LCMV pseudotyped lentivirus yields unique cell and tissue tropism. Genet. Vaccines Ther. 2011, 9, 8. [Google Scholar] [CrossRef]
- Matsuno, K.; Kishida, N.; Usami, K.; Igarashi, M.; Yoshida, R.; Nakayama, E.; Shimojima, M.; Feldmann, H.; Irimura, T.; Kawaoka, Y.; Takada, A. Different potential of C-type lectin-mediated entry between Marburg virus strains. J. Virol. 2010, 84, 5140–5147. [Google Scholar]
- Radoshitzky, S.R.; Kuhn, J.H.; Spiropoulou, C.F.; Albarino, C.G.; Nguyen, D.P.; Salazar-Bravo, J.; Dorfman, T.; Lee, A.S.; Wang, E.; Ross, S.R.; et al. Receptor determinants of zoonotic transmission of New World hemorrhagic fever arenaviruses. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 2664–2669. [Google Scholar]
- Vela, E.M.; Zhang, L.; Colpitts, T.M.; Davey, R.A.; Aronson, J.F. Arenavirus entry occurs through a cholesterol-dependent, non-caveolar, clathrin-mediated endocytic mechanism. Virology 2007, 369, 1–11. [Google Scholar] [CrossRef]
- Ogino, M.; Ebihara, H.; Lee, B.H.; Araki, K.; Lundkvist, A.; Kawaoka, Y.; Yoshimatsu, K.; Arikawa, J. Use of vesicular stomatitis virus pseudotypes bearing hantaan or seoul virus envelope proteins in a rapid and safe neutralization test. Clin. Diagn. Lab. Immunol. 2003, 10, 154–160. [Google Scholar]
- Fukushi, S.; Mizutani, T.; Saijo, M.; Matsuyama, S.; Miyajima, N.; Taguchi, F.; Itamura, S.; Kurane, I.; Morikawa, S. Vesicular stomatitis virus pseudotyped with severe acute respiratory syndrome coronavirus spike protein. J. Gen. Virol. 2005, 86, 2269–2274. [Google Scholar] [CrossRef]
- Moore, M.J.; Dorfman, T.; Li, W.; Wong, S.K.; Li, Y.; Kuhn, J.H.; Coderre, J.; Vasilieva, N.; Han, Z.; Greenough, T.C.; et al. Retroviruses pseudotyped with the severe acute respiratory syndrome coronavirus spike protein efficiently infect cells expressing angiotensin-converting enzyme 2. J. Virol. 2004, 78, 10628–10635. [Google Scholar]
- Nie, Y.; Wang, P.; Shi, X.; Wang, G.; Chen, J.; Zheng, A.; Wang, W.; Wang, Z.; Qu, X.; Luo, M.; et al. Highly infectious SARS-CoV pseudotyped virus reveals the cell tropism and its correlation with receptor expression. Biochem. Biophys. Res. Commun. 2004, 321, 994–1000. [Google Scholar] [CrossRef]
- Fukushi, S.; Watanabe, R.; Taguchi, F. Pseudotyped vesicular stomatitis virus for analysis of virus entry mediated by SARS coronavirus spike proteins. Meth. Mol. Biol. 2008, 454, 331–338. [Google Scholar]
- Kaku, Y.; Noguchi, A.; Marsh, G.A.; Barr, J.A.; Okutani, A.; Hotta, K.; Bazartseren, B.; Fukushi, S.; Broder, C.C.; Yamada, A.; et al. Second generation of pseudotype-based serum neutralization assay for Nipah virus antibodies: Sensitive and high-throughput analysis utilizing secreted alkaline phosphatase. J. Virol. Meth. 2012, 179, 226–232. [Google Scholar] [CrossRef]
- Coulibaly-N'Golo, D.; Allali, B.; Kouassi, S.K.; Fichet-Calvet, E.; Becker-Ziaja, B.; Rieger, T.; Olschlager, S.; Dosso, H.; Denys, C.; Ter Meulen, J.; et al. Novel arenavirus sequences in Hylomyscus sp. and Mus (Nannomys) setulosus from Cote d'Ivoire: Implications for evolution of arenaviruses in Africa. PLoS One 2011, 6, e20893. [Google Scholar]
- Irwin, N.R.; Bayerlova, M.; Missa, O.; Martinkova, N. Complex patterns of host switching in New World arenaviruses. Mol. Ecol. 2012, 21, 4137–4150. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fukushi, S.; Tani, H.; Yoshikawa, T.; Saijo, M.; Morikawa, S. Serological Assays Based on Recombinant Viral Proteins for the Diagnosis of Arenavirus Hemorrhagic Fevers. Viruses 2012, 4, 2097-2114. https://doi.org/10.3390/v4102097
Fukushi S, Tani H, Yoshikawa T, Saijo M, Morikawa S. Serological Assays Based on Recombinant Viral Proteins for the Diagnosis of Arenavirus Hemorrhagic Fevers. Viruses. 2012; 4(10):2097-2114. https://doi.org/10.3390/v4102097
Chicago/Turabian StyleFukushi, Shuetsu, Hideki Tani, Tomoki Yoshikawa, Masayuki Saijo, and Shigeru Morikawa. 2012. "Serological Assays Based on Recombinant Viral Proteins for the Diagnosis of Arenavirus Hemorrhagic Fevers" Viruses 4, no. 10: 2097-2114. https://doi.org/10.3390/v4102097
APA StyleFukushi, S., Tani, H., Yoshikawa, T., Saijo, M., & Morikawa, S. (2012). Serological Assays Based on Recombinant Viral Proteins for the Diagnosis of Arenavirus Hemorrhagic Fevers. Viruses, 4(10), 2097-2114. https://doi.org/10.3390/v4102097