HIV-1 Ribonuclease H: Structure, Catalytic Mechanism and Inhibitors
Abstract
:1. Introduction
2. RNase H Structure and Function
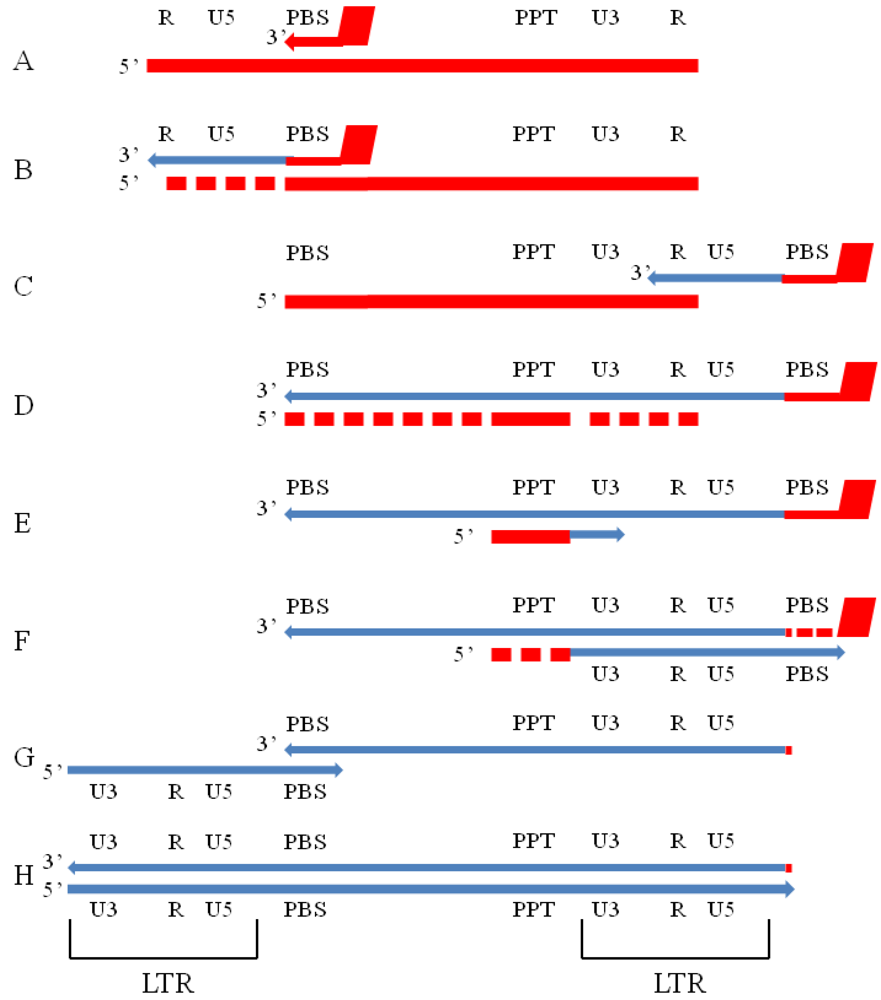
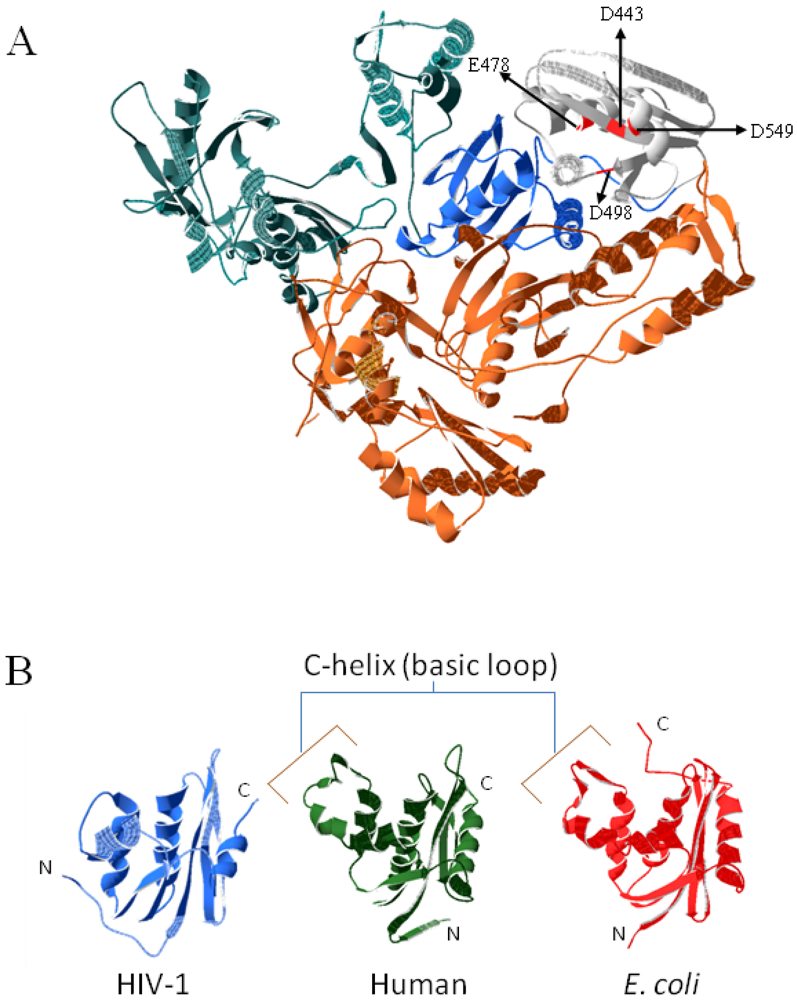
3. Substrate Binding
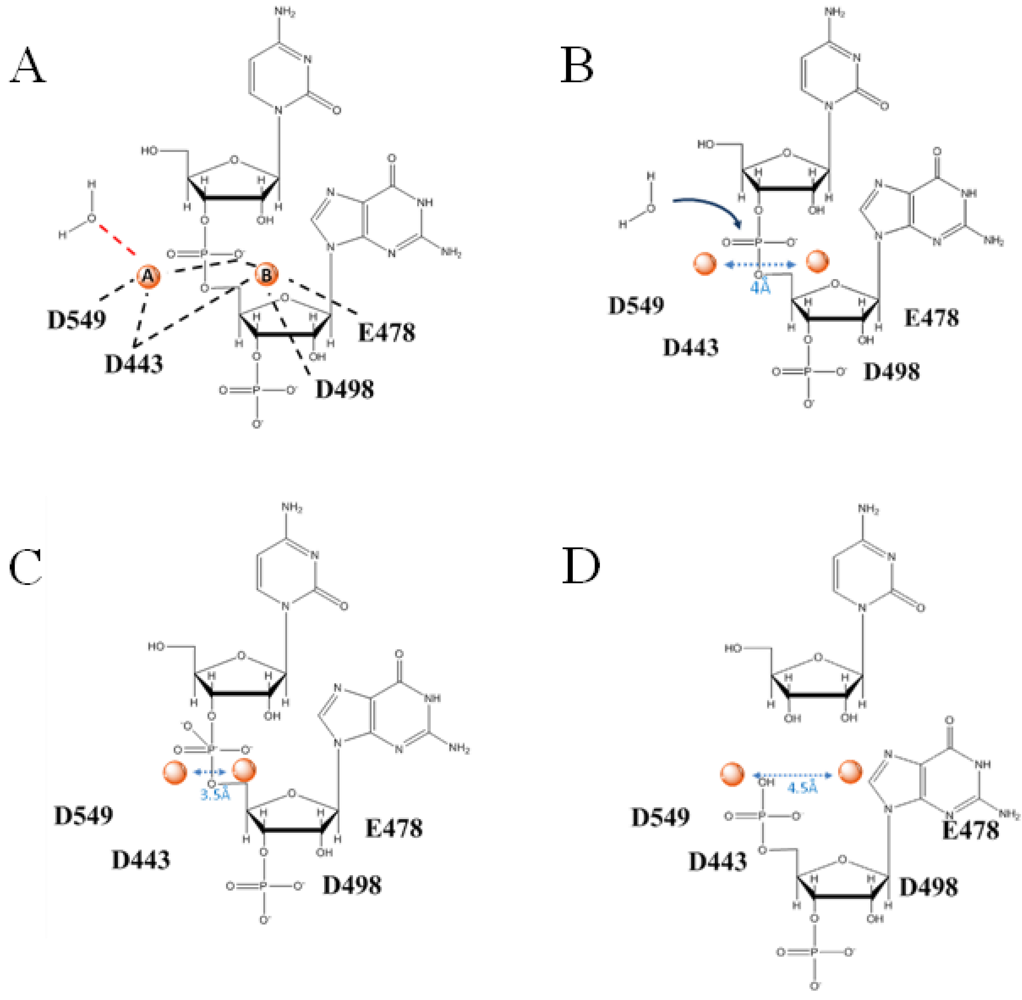
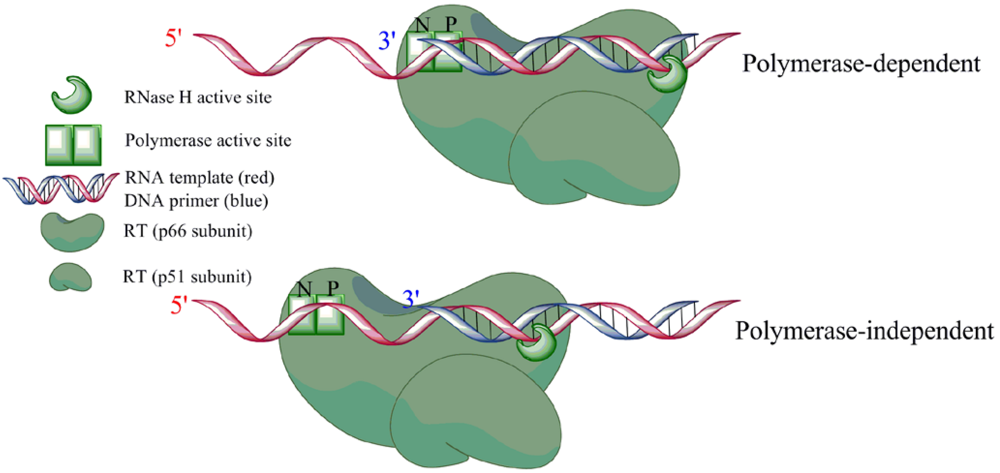
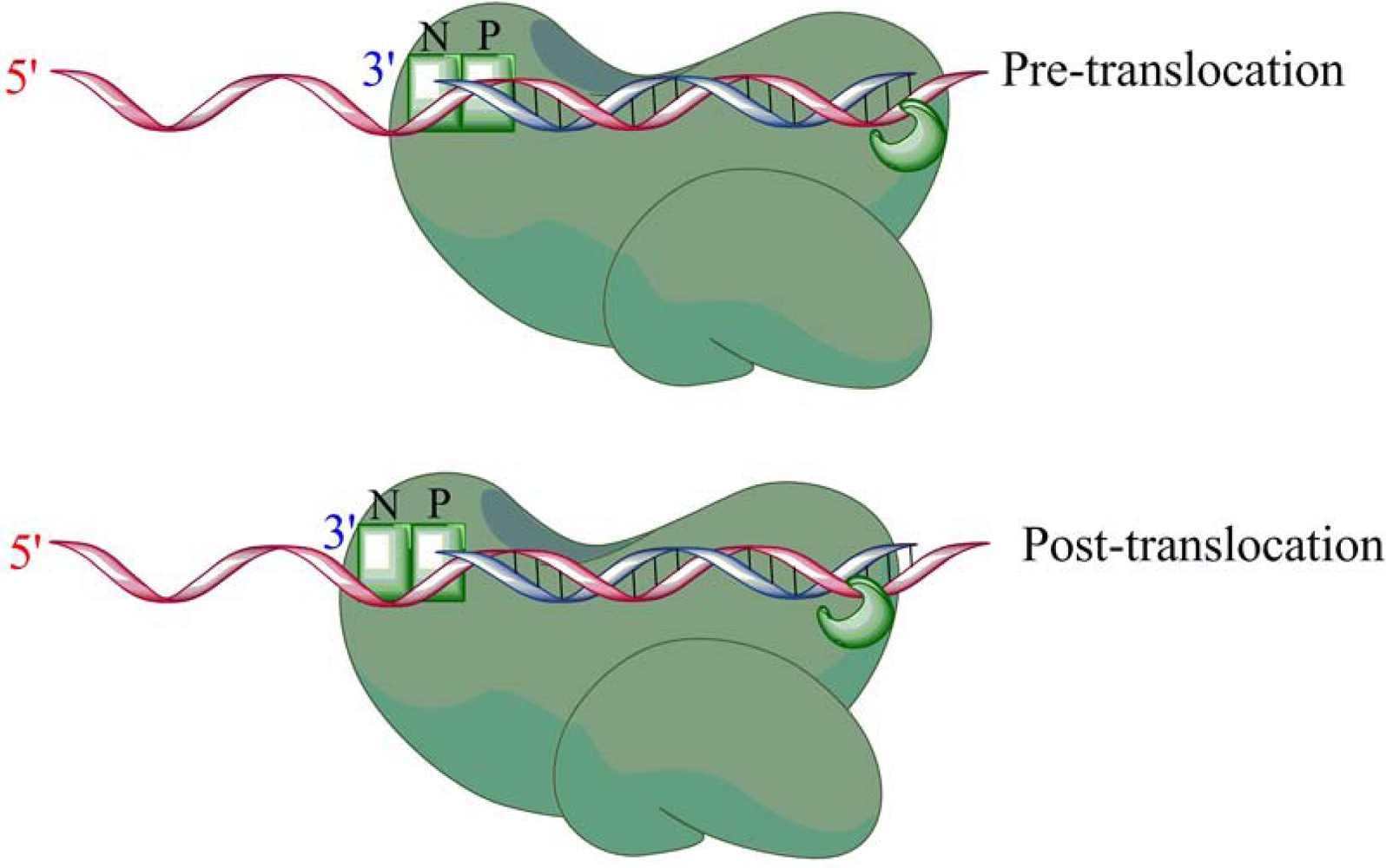
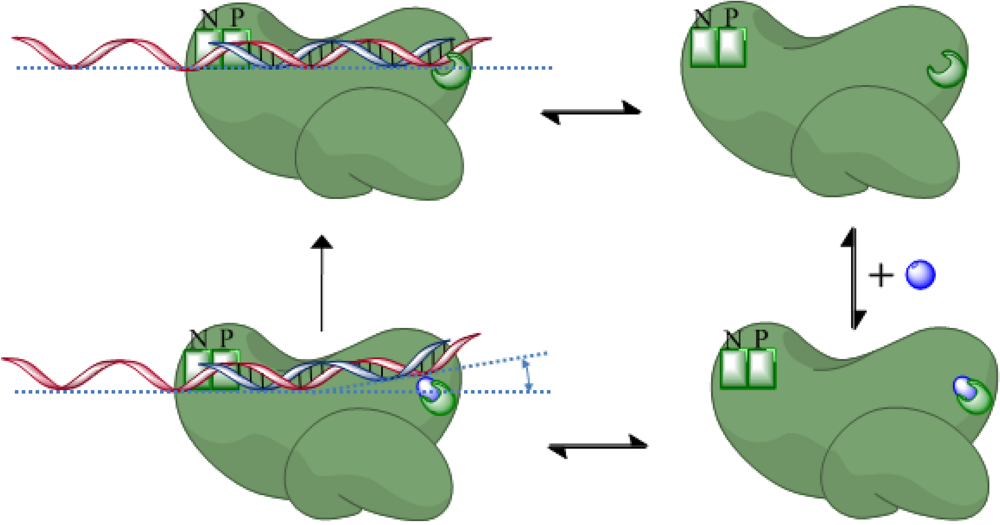
4. Role of RNase H in (+)-strand priming

5. Role of RNase H in strand transfer and (-)-strand primer removal
6. Role of RNase H activity in drug resistance
7. Inhibitors of HIV-1 RT-associated RNase H activity

8. Conclusion: Discovery and development of bona fide RNase H inhibitors
Acknowledgments
References
- Telesnitsky, A.; Goff, S. Retroviruses. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1997; pp. 121–160. [Google Scholar]
- De Clercq, E. Anti-HIV drugs: 25 compounds approved within 25 years after the discovery of HIV. Int. J. Antimicrob. Agents 2009, 33, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Davies 2nd, J.F.; Hostomska, Z.; Hostomsky, Z.; Jordan, S.R.; Matthews, D.A. Crystal structure of the ribonuclease H domain of HIV-1 reverse transcriptase. Science 1991, 252, 88–95. [Google Scholar] [PubMed]
- Ding, J.; Das, K.; Hsiou, Y.; Sarafianos, S.G.; Clark Jr., A.D.; Jacobo-Molina, A.; Tantillo, C.; Hughes, S.H.; Arnold, E. Structure and functional implications of the polymerase active site region in a complex of HIV-1 RT with a double-stranded DNA template-primer and an antibody Fab fragment at 2.8 A resolution. J. Mol. Biol. 1998, 284, 1095–1111. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chopra, R.; Verdine, G.L.; Harrison, S.C. Structure of a covalently trapped catalytic complex of HIV-1 reverse transcriptase: implications for drug resistance. Science 1998, 282, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Molina, A.; Ding, J.; Nanni, R.G.; Clark Jr. , A.D.; Lu, X.; Tantillo, C.; Williams, R.L.; Kamer, G.; Ferris, A.L.; Clark Jr., P.; et al. Crystal structure of human immunodeficiency virus type 1 reverse transcriptase complexed with double-stranded DNA at 3.0 A resolution shows bent DNA. Proc. Natl. Acad. Sci. U.S.A. 1993, 90, 6320–6324. [Google Scholar] [CrossRef] [PubMed]
- Sarafianos, S.G.; Das, K.; Tantillo, C.; Clark Jr., A.D.; Ding, J.; Whitcomb, J.M.; Boyer, P.L.; Hughes, S.H.; Arnold, E. Crystal structure of HIV-1 reverse transcriptase in complex with a polypurine tract RNA:DNA. EMBO J. 2001, 20, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Gregorio, G.G.; Bingman, C.; Martinez-Hackert, E.; Hendrickson, W.A.; Goff, S.P. Crystal structure of the moloney murine leukemia virus RNase H domain. J. Virol. 2006, 80, 8379–8389. [Google Scholar] [CrossRef] [PubMed]
- Katayanagi, K.; Miyagawa, M.; Matsushima, M.; Ishikawa, M.; Kanaya, S.; Ikehara, M.; Matsuzaki, T.; Morikawa, K. Three-dimensional structure of ribonuclease H from E. coli. Nature 1990, 347, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Hendrickson, W.A.; Crouch, R.J.; Satow, Y. Structure of ribonuclease H phased at 2 A resolution by MAD analysis of the selenomethionyl protein. Science 1990, 249, 1398–1405. [Google Scholar] [PubMed]
- Nowotny, M.; Gaidamakov, S.A.; Crouch, R.J.; Yang, W. Crystal structures of RNase H bound to an RNA/DNA hybrid: substrate specificity and metal-dependent catalysis. Cell 2005, 121, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, M.; Gaidamakov, S.A.; Ghirlando, R.; Cerritelli, S.M.; Crouch, R.J.; Yang, W. Structure of human RNase H1 complexed with an RNA/DNA hybrid: insight into HIV reverse transcription. Mol. Cell 2007, 28, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Keck, J.L.; Marqusee, S. The putative substrate recognition loop of Escherichia coli ribonuclease H is not essential for activity. J. Biol. Chem. 1996, 271, 19883–19887. [Google Scholar] [CrossRef] [PubMed]
- Keck, J.L.; Marqusee, S. Substitution of a highly basic helix/loop sequence into the RNase H domain of human immunodeficiency virus reverse transcriptase restores its Mn(2+)-dependent RNase H activity. Proc. Natl. Acad. Sci. U. S. A. 1995, 92, 2740–2744. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Gritsman, K.; Roth, M.J. Contributions of DNA polymerase subdomains to the RNase H activity of human immunodeficiency virus type 1 reverse transcriptase. J. Virol. 1994, 68, 5721–5729. [Google Scholar] [PubMed]
- Klumpp, K.; Hang, J.Q.; Rajendran, S.; Yang, Y.; Derosier, A.; Wong Kai In, P.; Overton, H.; Parkes, K.E.; Cammack, N.; Martin, J.A. Two-metal ion mechanism of RNA cleavage by HIV RNase H and mechanism-based design of selective HIV RNase H inhibitors. Nucleic Acids Res. 2003, 31, 6852–6859. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Lee, J.Y.; Nowotny, M. Making and breaking nucleic acids: two-Mg2+-ion catalysis and substrate specificity. Mol. Cell 2006, 22, 5–13. [Google Scholar] [CrossRef]
- Nowotny, M.; Yang, W. Stepwise analyses of metal ions in RNase H catalysis from substrate destabilization to product release. EMBO J. 2006, 25, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Bochner, R.; Duvshani, A.; Adir, N.; Hizi, A. Mutagenesis of Gln294 of the reverse transcriptase of human immunodeficiency virus type-2 and its effects on the ribonuclease H activity. FEBS Lett. 2008, 582, 2799–2805. [Google Scholar] [CrossRef] [PubMed]
- Sevilya, Z.; Loya, S.; Adir, N.; Hizi, A. The ribonuclease H activity of the reverse transcriptases of human immunodeficiency viruses type 1 and type 2 is modulated by residue 294 of the small subunit. Nucleic Acids Res. 2003, 31, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Gotte, M.; Maier, G.; Gross, H.J.; Heumann, H. Localization of the active site of HIV-1 reverse transcriptase-associated RNase H domain on a DNA template using site-specific generated hydroxyl radicals. J. Biol. Chem. 1998, 273, 10139–10146. [Google Scholar] [CrossRef] [PubMed]
- Bohlayer, W.P.; DeStefano, J.J. Tighter binding of HIV reverse transcriptase to RNA-DNA versus DNA-DNA results mostly from interactions in the polymerase domain and requires just a small stretch of RNA-DNA. Biochemistry 2006, 45, 7628–7638. [Google Scholar] [CrossRef] [PubMed]
- Cote, M.L.; Roth, M.J. Murine leukemia virus reverse transcriptase: structural comparison with HIV-1 reverse transcriptase. Virus Res. 2008, 134, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Julias, J.G.; McWilliams, M.J.; Sarafianos, S.G.; Alvord, W.G.; Arnold, E.; Hughes, S.H. Mutation of amino acids in the connection domain of human immunodeficiency virus type 1 reverse transcriptase that contact the template-primer affects RNase H activity. J. Virol. 2003, 77, 8548–8554. [Google Scholar] [CrossRef] [PubMed]
- Julias, J.G.; McWilliams, M.J.; Sarafianos, S.G.; Arnold, E.; Hughes, S.H. Mutations in the RNase H domain of HIV-1 reverse transcriptase affect the initiation of DNA synthesis and the specificity of RNase H cleavage in vivo. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 9515–9520. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Rivas, M.; Menendez-Arias, L. A mutation in the primer grip region of HIV-1 reverse transcriptase that confers reduced fidelity of DNA synthesis. Nucleic Acids Res. 2001, 29, 4963–4972. [Google Scholar] [CrossRef] [PubMed]
- Rausch, J.W.; Lener, D.; Miller, J.T.; Julias, J.G.; Hughes, S.H.; Le Grice, S.F. Altering the RNase H primer grip of human immunodeficiency virus reverse transcriptase modifies cleavage specificity. Biochemistry 2002, 41, 4856–4865. [Google Scholar] [CrossRef] [PubMed]
- DeStefano, J.J.; Mallaber, L.M.; Fay, P.J.; Bambara, R.A. Determinants of the RNase H cleavage specificity of human immunodeficiency virus reverse transcriptase. Nucleic Acids Res. 1993, 21, 4330–4338. [Google Scholar] [CrossRef] [PubMed]
- Furfine, E.S.; Reardon, J.E. Reverse transcriptase RNase H from the human immunodeficiency virus. Relationship of the DNA polymerase and RNA hydrolysis activities . J. Biol. Chem. 1991, 266, 406–412. [Google Scholar] [PubMed]
- Gopalakrishnan, V.; Peliska, J.A.; Benkovic, S.J. Human immunodeficiency virus type 1 reverse transcriptase: spatial and temporal relationship between the polymerase and RNase H activities. Proc. Natl. Acad. Sci. U.S.A. 1992, 89, 10763–10767. [Google Scholar] [CrossRef] [PubMed]
- Gotte, M.; Fackler, S.; Hermann, T.; Perola, E.; Cellai, L.; Gross, H.J.; Le Grice, S.F.; Heumann, H. HIV-1 reverse transcriptase-associated RNase H cleaves RNA/RNA in arrested complexes: implications for the mechanism by which RNase H discriminates between RNA/RNA and RNA/DNA. EMBO J. 1995, 14, 833–841. [Google Scholar] [PubMed]
- Wohrl, B.M.; Volkmann, S.; Moelling, K. Mutations of a conserved residue within HIV-1 ribonuclease H affect its exo- and endonuclease activities. J. Mol. Biol. 1991, 220, 801–818. [Google Scholar] [CrossRef] [PubMed]
- Kati, W.M.; Johnson, K.A.; Jerva, L.F.; Anderson, K.S. Mechanism and fidelity of HIV reverse transcriptase. J. Biol. Chem. 1992, 267, 25988–25997. [Google Scholar] [PubMed]
- Gotte, M. Effects of nucleotides and nucleotide analogue inhibitors of HIV-1 reverse transcriptase in a ratchet model of polymerase translocation. Curr. Pharm. Des. 2006, 12, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Marchand, B.; Gotte, M. Site-specific footprinting reveals differences in the translocation status of HIV-1 reverse transcriptase. Implications for polymerase translocation and drug resistance. J. Biol. Chem. 2003, 278, 35362–35372. [Google Scholar] [CrossRef] [PubMed]
- Guajardo, R.; Sousa, R. A model for the mechanism of polymerase translocation. J. Mol. Biol. 1997, 265, 8–19. [Google Scholar] [CrossRef]
- Sarafianos, S.G.; Clark Jr., A.D.; Tuske, S.; Squire, C.J.; Das, K.; Sheng, D.; Ilankumaran, P.; Ramesha, A.R.; Kroth, H.; Sayer, J.M.; Jerina, D.M.; Boyer, P.L.; Hughes, S.H.; Arnold, E. Trapping HIV-1 reverse transcriptase before and after translocation on DNA. J. Biol. Chem. 2003, 278, 16280–16288. [Google Scholar] [CrossRef] [PubMed]
- Marchand, B.; Tchesnokov, E.P.; Gotte, M. The pyrophosphate analogue foscarnet traps the pre-translocational state of HIV-1 reverse transcriptase in a Brownian ratchet model of polymerase translocation. J. Biol. Chem. 2007, 282, 3337–3346. [Google Scholar] [CrossRef] [PubMed]
- Beilhartz, G.L.; Wendeler, M.; Baichoo, N.; Rausch, J.; Le Grice, S.; Gotte, M. HIV-1 Reverse Transcriptase Can Simultaneously Engage Its DNA/RNA Substrate at Both DNA Polymerase and RNase H Active Sites: Implications for RNase H Inhibition. J. Mol. Biol. 2009. [Google Scholar]
- Tong, W.; Lu, C.D.; Sharma, S.K.; Matsuura, S.; So, A.G.; Scott, W.A. Nucleotide-induced stable complex formation by HIV-1 reverse transcriptase. Biochemistry 1997, 36, 5749–5757. [Google Scholar] [CrossRef] [PubMed]
- Sarafianos, S.G.; Clark Jr., A.D.; Das, K.; Tuske, S.; Birktoft, J.J.; Ilankumaran, P.; Ramesha, A.R.; Sayer, J.M.; Jerina, D.M.; Boyer, P.L.; Hughes, S.H.; Arnold, E. Structures of HIV-1 reverse transcriptase with pre- and post-translocation AZTMP-terminated DNA. EMBO J. 2002, 21, 6614–6624. [Google Scholar] [CrossRef] [PubMed]
- Champoux, J.J.; Schultz, S.J. Ribonuclease H: properties, substrate specificity and roles in retroviral reverse transcription. FEBS J. 2009, 276, 1506–1516. [Google Scholar] [CrossRef] [PubMed]
- Schultz, S.J.; Zhang, M.; Champoux, J.J. Recognition of internal cleavage sites by retroviral RNases H. J. Mol. Biol. 2004, 344, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Schultz, S.J.; Champoux, J.J. RNase H activity: structure, specificity, and function in reverse transcription. Virus Res. 2008, 134, 86–103. [Google Scholar] [CrossRef] [PubMed]
- Schultz, S.J.; Zhang, M.; Champoux, J.J. Sequence, distance, and accessibility are determinants of 5'-end-directed cleavages by retroviral RNases H. J. Biol. Chem. 2006, 281, 1943–1955. [Google Scholar] [CrossRef] [PubMed]
- Schultz, S.J.; Zhang, M.; Champoux, J.J. Preferred sequences within a defined cleavage window specify DNA 3' end-directed cleavages by retroviral RNases H. J. Biol. Chem. 2009, 284, 32225–32238. [Google Scholar] [CrossRef] [PubMed]
- Abbondanzieri, E.A.; Bokinsky, G.; Rausch, J.W.; Zhang, J.X.; Le Grice, S.F.; Zhuang, X. Dynamic binding orientations direct activity of HIV reverse transcriptase. Nature 2008, 453, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, G.M.; Rodriguez-Rodriguez, L.; Fay, P.J.; Bambara, R.A. Use of an oligoribonucleotide containing the polypurine tract sequence as a primer by HIV reverse transcriptase. J. Biol. Chem. 1995, 270, 28169–28176. [Google Scholar] [CrossRef] [PubMed]
- Gotte, M.; Maier, G.; Onori, A.M.; Cellai, L.; Wainberg, M.A.; Heumann, H. Temporal coordination between initiation of HIV (+)-strand DNA synthesis and primer removal. J. Biol. Chem. 1999, 274, 11159–11169. [Google Scholar] [CrossRef] [PubMed]
- Huber, H.E.; Richardson, C.C. Processing of the primer for plus strand DNA synthesis by human immunodeficiency virus 1 reverse transcriptase. J. Biol. Chem. 1990, 265, 10565–10573. [Google Scholar] [PubMed]
- Palaniappan, C.; Fay, P.J.; Bambara, R.A. Nevirapine alters the cleavage specificity of ribonuclease H of human immunodeficiency virus 1 reverse transcriptase. J. Biol. Chem. 1995, 270, 4861–4869. [Google Scholar] [CrossRef] [PubMed]
- Pullen, K.A.; Champoux, J.J. Plus-strand origin for human immunodeficiency virus type 1: implications for integration. J. Virol. 1990, 64, 6274–6277. [Google Scholar] [PubMed]
- Julias, J.G.; McWilliams, M.J.; Sarafianos, S.G.; Alvord, W.G.; Arnold, E.; Hughes, S.H. Effects of mutations in the G tract of the human immunodeficiency virus type 1 polypurine tract on virus replication and RNase H cleavage. J. Virol. 2004, 78, 13315–13324. [Google Scholar] [CrossRef] [PubMed]
- Grobler, J.A.; Dornadula, G.; Rice, M.R.; Simcoe, A.L.; Hazuda, D.J.; Miller, M.D. HIV-1 reverse transcriptase plus-strand initiation exhibits preferential sensitivity to non-nucleoside reverse transcriptase inhibitors in vitro. J. Biol. Chem. 2007, 282, 8005–8010. [Google Scholar] [CrossRef] [PubMed]
- Basu, V.P.; Song, M.; Gao, L.; Rigby, S.T.; Hanson, M.N.; Bambara, R.A. Strand transfer events during HIV-1 reverse transcription. Virus Res. 2008, 134, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Pullen, K.A.; Ishimoto, L.K.; Champoux, J.J. Incomplete removal of the RNA primer for minus-strand DNA synthesis by human immunodeficiency virus type 1 reverse transcriptase. J. Virol. 1992, 66, 367–373. [Google Scholar] [PubMed]
- Gao, L.; Balakrishnan, M.; Roques, B.P.; Bambara, R.A. Insights into the multiple roles of pausing in HIV-1 reverse transcriptase-promoted strand transfers. J. Biol. Chem. 2007, 282, 6222–6231. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Balakrishnan, M.; Roques, B.P.; Bambara, R.A. Acceptor RNA cleavage profile supports an invasion mechanism for HIV-1 minus strand transfer. J. Biol. Chem. 2005, 280, 14443–14452. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.K.; Svarovskaia, E.S.; Pathak, V.K. Dynamic copy choice: steady state between murine leukemia virus polymerase and polymerase-dependent RNase H activity determines frequency of in vivo template switching. Proc. Natl. Acad. Sci. U. S. A. 2001, 98, 12209–12214. [Google Scholar] [CrossRef] [PubMed]
- Telesnitsky, A.; Goff, S.P. Two defective forms of reverse transcriptase can complement to restore retroviral infectivity. EMBO J. 1993, 12, 4433–4438. [Google Scholar] [PubMed]
- Smith, J.S.; Roth, M.J. Specificity of human immunodeficiency virus-1 reverse transcriptase-associated ribonuclease H in removal of the minus-strand primer, tRNA(Lys3). J. Biol. Chem. 1992, 267, 15071–15079. [Google Scholar] [PubMed]
- Bushman, F.D.; Craigie, R. Activities of human immunodeficiency virus (HIV) integration protein in vitro: specific cleavage and integration of HIV DNA. Proc. Natl. Acad. Sci. U.S.A. 1991, 88, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; McWilliams, M.J.; Julias, J.G.; Hughes, S.H. Mutations in the U5 region adjacent to the primer binding site affect tRNA cleavage by human immunodeficiency virus type 1 reverse transcriptase in vivo. J. Virol. 2008, 82, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Kohlstaedt, L.A.; Wang, J.; Friedman, J.M.; Rice, P.A.; Steitz, T.A. Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science 1992, 256, 1783–1790. [Google Scholar] [PubMed]
- Ren, J.; Esnouf, R.; Garman, E.; Somers, D.; Ross, C.; Kirby, I.; Keeling, J.; Darby, G.; Jones, Y.; Stuart, D.; et al. High resolution structures of HIV-1 RT from four RT-inhibitor complexes. Nat. Struct. Biol. 1995, 2, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Archer, R.H.; Dykes, C.; Gerondelis, P.; Lloyd, A.; Fay, P.; Reichman, R.C.; Bambara, R.A.; Demeter, L.M. Mutants of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase resistant to nonnucleoside reverse transcriptase inhibitors demonstrate altered rates of RNase H cleavage that correlate with HIV-1 replication fitness in cell culture. J. Virol. 2000, 74, 8390–8401. [Google Scholar] [CrossRef] [PubMed]
- Brehm, J.H.; Koontz, D.; Meteer, J.D.; Pathak, V.; Sluis-Cremer, N.; Mellors, J.W. Selection of mutations in the connection and RNase H domains of human immunodeficiency virus type 1 reverse transcriptase that increase resistance to 3'-azido-3'-dideoxythymidine. J. Virol. 2007, 81, 7852–7859. [Google Scholar] [CrossRef] [PubMed]
- Cane, P.A.; Green, H.; Fearnhill, E.; Dunn, D. Identification of accessory mutations associated with high-level resistance in HIV-1 reverse transcriptase. Aids 2007, 21, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Gotte, M. Should we include connection domain mutations of HIV-1 reverse transcriptase in HIV resistance testing. PLoS Med. 2007, 4, e346. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, A.; Kodama, E.N.; Sarafianos, S.G.; Schuckmann, M.M.; Sakagami, Y.; Matsuoka, M.; Takiguchi, M.; Gatanaga, H.; Oka, S. Amino acid mutation N348I in the connection subdomain of human immunodeficiency virus type 1 reverse transcriptase confers multiclass resistance to nucleoside and nonnucleoside reverse transcriptase inhibitors. J. Virol. 2008, 82, 3261–3270. [Google Scholar] [CrossRef] [PubMed]
- Ntemgwa, M.; Wainberg, M.A.; Oliveira, M.; Moisi, D.; Lalonde, R.; Micheli, V.; Brenner, B.G. Variations in reverse transcriptase and RNase H domain mutations in human immunodeficiency virus type 1 clinical isolates are associated with divergent phenotypic resistance to zidovudine. Antimicrob. Agents Chemother. 2007, 51, 3861–3869. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Lengruber, R.B.; Soares, E.A.; Jere, A.; Sprinz, E.; Martinez, A.M.; Silveira, J.; Sion, F.S.; Pathak, V.K.; Soares, M.A. Conservation patterns of HIV-1 RT connection and RNase H domains: identification of new mutations in NRTI-treated patients. PLoS One 2008, 3, e1781. [Google Scholar] [CrossRef] [PubMed]
- Yap, S.H.; Sheen, C.W.; Fahey, J.; Zanin, M.; Tyssen, D.; Lima, V.D.; Wynhoven, B.; Kuiper, M.; Sluis-Cremer, N.; Harrigan, P.R.; Tachedjian, G. N348I in the connection domain of HIV-1 reverse transcriptase confers zidovudine and nevirapine resistance. PLoS Med. 2007, 4, e335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delviks-Frankenberry, K.A.; Nikolenko, G.N.; Barr, R.; Pathak, V.K. Mutations in human immunodeficiency virus type 1 RNase H primer grip enhance 3'-azido-3'-deoxythymidine resistance. J. Virol. 2007, 81, 6837–6845. [Google Scholar] [CrossRef] [PubMed]
- Delviks-Frankenberry, K A.; Nikolenko, G.N.; Boyer, P.L.; Hughes, S.H.; Coffin, J.M.; Jere, A.; Pathak, V.K. HIV-1 reverse transcriptase connection subdomain mutations reduce template RNA degradation and enhance AZT excision. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 10943–10948. [Google Scholar] [CrossRef] [PubMed]
- Nikolenko, G.N.; Delviks-Frankenberry, K.A.; Palmer, S.; Maldarelli, F.; Fivash, M.J.; Coffin, J.M.; Pathak, V.K. Mutations in the connection domain of HIV-1 reverse transcriptase increase 3'-azido-3'-deoxythymidine resistance. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Nikolenko, G.N.; Palmer, S.; Maldarelli, F.; Mellors, J.W.; Coffin, J.M.; Pathak, V.K. Mechanism for nucleoside analog-mediated abrogation of HIV-1 replication: balance between RNase H activity and nucleotide excision. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Radzio, J.; Sluis-Cremer, N. Efavirenz accelerates HIV-1 reverse transcriptase ribonuclease H cleavage, leading to diminished zidovudine excision. Mol. Pharmacol. 2008, 73, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Ehteshami, M.; Beilhartz, G.L.; Scarth, B.J.; Tchesnokov, E.P.; McCormick, S.; Wynhoven, B.; Harrigan, P.R.; Gotte, M. Connection domain mutations N348I and A360V in HIV-1 reverse transcriptase enhance resistance to AZT through both RNase H dependent and independent mechanisms. J. Biol. Chem 2008. [Google Scholar]
- Brehm, J.H.; Mellors, J.W.; Sluis-Cremer, N. Mechanism by which a glutamine to leucine substitution at residue 509 in the ribonuclease H domain of HIV-1 reverse transcriptase confers zidovudine resistance. Biochemistry 2008, 47, 14020–14027. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, A.; Shimane, K.; Sarafianos, S.G.; Kodama, E.N.; Sakagami, Y.; Negishi, F.; Koizumi, H.; Gatanaga, H.; Matsuoka, M.; Takiguchi, M.; Oka, S. Clinical relevance of substitutions in the connection subdomain and RNase H domain of HIV-1 reverse transcriptase from a cohort of antiretroviral treatment-naive patients. Antiviral Res. 2009, 82, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Ehteshami, M.; Gotte, M. Effects of mutations in the connection and RNase H domains of HIV-1 reverse transcriptase on drug susceptibility. AIDS Rev. 2008, 10, 224–235. [Google Scholar] [PubMed]
- Parkes, K.E.; Ermert, P.; Fassler, J.; Ives, J.; Martin, J.A.; Merrett, J.H.; Obrecht, D.; Williams, G.; Klumpp, K. Use of a pharmacophore model to discover a new class of influenza endonuclease inhibitors. J. Med. Chem. 2003, 46, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Hang, J.Q.; Rajendran, S.; Yang, Y.; Li, Y.; In, P.W.; Overton, H.; Parkes, K.E.; Cammack, N.; Martin, J.A.; Klumpp, K. Activity of the isolated HIV RNase H domain and specific inhibition by N-hydroxyimides. Biochem. Biophys. Res. Commun. 2004, 317, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Kirschberg, T.A.; Balakrishnan, M.; Squires, N.H.; Barnes, T.; Brendza, K.M.; Chen, X.; Eisenberg, E.J.; Jin, W.; Kutty, N.; Leavitt, S.; Liclican, A.; Liu, Q.; Liu, X.; Mak, J.; Perry, J.K.; Wang, M.; Watkins, W.J.; Lansdon, E.B. RNase H active site inhibitors of human immunodeficiency virus type 1 reverse transcriptase: design, biochemical activity, and structural information. J. Med. Chem. 2009, 52, 5781–5784. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, E.; Esposito, F.; Badas, R.; Di Santo, R.; Costi, R.; La Colla, P. 6-[1-(4-Fluorophenyl)methyl-1H-pyrrol-2-yl)]-2,4-dioxo-5-hexenoic acid ethyl ester a novel diketo acid derivative which selectively inhibits the HIV-1 viral replication in cell culture and the ribonuclease H activity in vitro. Antiviral. Res. 2005, 65, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Wendeler, M.; Lee, H.F.; Bermingham, A.; Miller, J.T.; Chertov, O.; Bona, M.K.; Baichoo, N.S.; Ehteshami, M.; Beutler, J.; O'Keefe, B.R.; Gotte, M.; Kvaratskhelia, M.; Le Grice, S. Vinylogous ureas as a novel class of inhibitors of reverse transcriptase-associated ribonuclease H activity. ACS Chem. Biol. 2008, 3, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Himmel, D.M.; Sarafianos, S.G.; Dharmasena, S.; Hossain, M.M.; McCoy-Simandle, K.; Ilina, T.; Clark Jr., A.D.; Knight, J.L.; Julias, J.G.; Clark Jr., P.K.; Krogh-Jespersen, K.; Levy, R.M.; Hughes, S.H.; Parniak, M.A.; Arnold, E. HIV-1 reverse transcriptase structure with RNase H inhibitor dihydroxy benzoyl naphthyl hydrazone bound at a novel site. ACS Chem. Biol. 2006, 1, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Budihas, S.R.; Gorshkova, I.; Gaidamakov, S.; Wamiru, A.; Bona, M.K.; Parniak, M.A.; Crouch, R.J.; McMahon, J.B.; Beutler, J.A.; Le Grice, S.F. Selective inhibition of HIV-1 reverse transcriptase-associated ribonuclease H activity by hydroxylated tropolones. Nucleic Acids Res. 2005, 33, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Himmel, D.M.; Maegley, K.A.; Pauly, T.A.; Bauman, J.D.; Das, K.; Dharia, C.; Clark Jr., A.D.; Ryan, K.; Hickey, M.J.; Love, R.A.; Hughes, S.H.; Bergqvist, S.; Arnold, E. Structure of HIV-1 reverse transcriptase with the inhibitor beta-Thujaplicinol bound at the RNase H active site. Structure 2009, 17, 1625–1635. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, K.; Mirzadegan, T. Recent progress in the design of small molecule inhibitors of HIV RNase H. Curr. Pharm. Des. 2006, 12, 1909–1922. [Google Scholar] [CrossRef] [PubMed]
- Grobler, J.A.; Stillmock, K.; Hu, B.; Witmer, M.; Felock, P.; Espeseth, A.S.; Wolfe, A.; Egbertson, M.; Bourgeois, M.; Melamed, J.; Wai, J.S.; Young, S.; Vacca, J.; Hazuda, D.J. Diketo acid inhibitor mechanism and HIV-1 integrase: implications for metal binding in the active site of phosphotransferase enzymes. Proc. Natl. Acad. Sci. U.S.A. 2002, 99, 6661–6666. [Google Scholar] [CrossRef] [PubMed]
- Hazuda, D.J.; Felock, P.; Witmer, M.; Wolfe, A.; Stillmock, K.; Grobler, J.A.; Espeseth, A.; Gabryelski, L.; Schleif, W.; Blau, C.; Miller, M.D. Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science 2000, 287, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Shaw-Reid, C.A.; Munshi, V.; Graham, P.; Wolfe, A.; Witmer, M.; Danzeisen, R.; Olsen, D.B.; Carroll, S.S.; Embrey, M.; Wai, J.S.; Miller, M.D.; Cole, J.L.; Hazuda, D.J. Inhibition of HIV-1 ribonuclease H by a novel diketo acid, 4-[5-(benzoylamino)thien-2-yl]-2,4-dioxobutanoic acid. J. Biol. Chem. 2003, 278, 2777–2780. [Google Scholar] [CrossRef] [PubMed]
- Shaw-Reid, C.A.; Feuston, B.; Munshi, V.; Getty, K.; Krueger, J.; Hazuda, D.J.; Parniak, M.A.; Miller, M.D.; Lewis, D. Dissecting the effects of DNA polymerase and ribonuclease H inhibitor combinations on HIV-1 reverse-transcriptase activities. Biochemistry 2005, 44, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Fletcher, R.S.; Barnard, J.; Arion, D.; Motakis, D.; Dmitrienko, G.I.; Parniak, M.A. Inhibition of the ribonuclease H and DNA polymerase activities of HIV-1 reverse transcriptase by N-(4-tert-butylbenzoyl)-2-hydroxy-1-naphthaldehyde hydrazone. Biochemistry 1997, 36, 3179–3185. [Google Scholar] [CrossRef] [PubMed]
- Keck, J.L.; Goedken, E.R.; Marqusee, S. Activation/attenuation model for RNase H. A one-metal mechanism with second-metal inhibition. J. Biol. Chem. 1998, 273, 34128–34133. [Google Scholar] [CrossRef] [PubMed]
- Tsunaka, Y.; Takano, K.; Matsumura, H.; Yamagata, Y.; Kanaya, S. Identification of single Mn(2+) binding sites required for activation of the mutant proteins of E.coli RNase HI at Glu48 and/or Asp134 by X-ray crystallography. J. Mol. Biol. 2005, 345, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Sluis-Cremer, N.; Arion, D.; Parniak, M.A. Destabilization of the HIV-1 reverse transcriptase dimer upon interaction with N-acyl hydrazone inhibitors. Mol. Pharmacol. 2002, 62, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Arion, D.; Sluis-Cremer, N.; Min, K.L.; Abram, M.E.; Fletcher, R.S.; Parniak, M.A. Mutational analysis of Tyr-501 of HIV-1 reverse transcriptase. Effects on ribonuclease H activity and inhibition of this activity by N-acylhydrazones. J. Biol. Chem. 2002, 277, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.Q.; Boyer, P.L.; Arnold, E.; Hughes, S.H. Effects of mutations in the polymerase domain on the polymerase, RNase H and strand transfer activities of human immunodeficiency virus type 1 reverse transcriptase. J. Mol. Biol. 1998, 277, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Jacques, P.S.; Wohrl, B.M.; Howard, K.J.; Le Grice, S.F. Modulation of HIV-1 reverse transcriptase function in "selectively deleted" p66/p51 heterodimers. J. Biol. Chem. 1994, 269, 1388–1393. [Google Scholar] [PubMed]
- Loya, S.; Tal, R.; Kashman, Y.; Hizi, A. Illimaquinone, a selective inhibitor of the RNase H activity of human immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. 1990, 34, 2009–2012. [Google Scholar] [PubMed]
- Min, B.S.; Miyashiro, H.; Hattori, M. Inhibitory effects of quinones on RNase H activity associated with HIV-1 reverse transcriptase. Phytother. Res. 2002, 16, S57–62. [Google Scholar] [CrossRef] [PubMed]
- Hannoush, R.N.; Carriero, S.; Min, K.L.; Damha, M.J. Selective inhibition of HIV-1 reverse transcriptase (HIV-1 RT) RNase H by small RNA hairpins and dumbbells. Chembiochem. 2004, 5, 527–533. [Google Scholar] [CrossRef] [PubMed]
- James, W. Aptamers in the virologists' toolkit. J. Gen. Virol. 2007, 88, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Kissel, J.D.; Held, D.M.; Hardy, R.W.; Burke, D.H. Single-stranded DNA aptamer RT1t49 inhibits RT polymerase and RNase H functions of HIV type 1, HIV type 2, and SIVCPZ RTs. AIDS Res. Hum. Retroviruses 2007, 23, 699–708. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Share and Cite
Beilhartz, G.L.; Götte, M. HIV-1 Ribonuclease H: Structure, Catalytic Mechanism and Inhibitors. Viruses 2010, 2, 900-926. https://doi.org/10.3390/v2040900
Beilhartz GL, Götte M. HIV-1 Ribonuclease H: Structure, Catalytic Mechanism and Inhibitors. Viruses. 2010; 2(4):900-926. https://doi.org/10.3390/v2040900
Chicago/Turabian StyleBeilhartz, Greg L., and Matthias Götte. 2010. "HIV-1 Ribonuclease H: Structure, Catalytic Mechanism and Inhibitors" Viruses 2, no. 4: 900-926. https://doi.org/10.3390/v2040900
APA StyleBeilhartz, G. L., & Götte, M. (2010). HIV-1 Ribonuclease H: Structure, Catalytic Mechanism and Inhibitors. Viruses, 2(4), 900-926. https://doi.org/10.3390/v2040900



