HIV-1 RT Inhibitors with a Novel Mechanism of Action: NNRTIs that Compete with the Nucleotide Substrate
Abstract
:1. Introduction

2. Indolopyridones (INDOPYs)
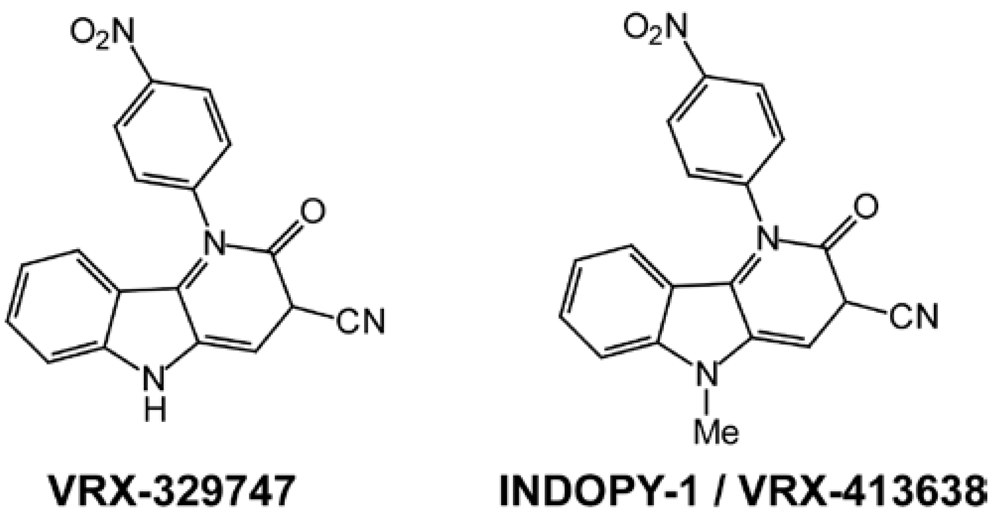
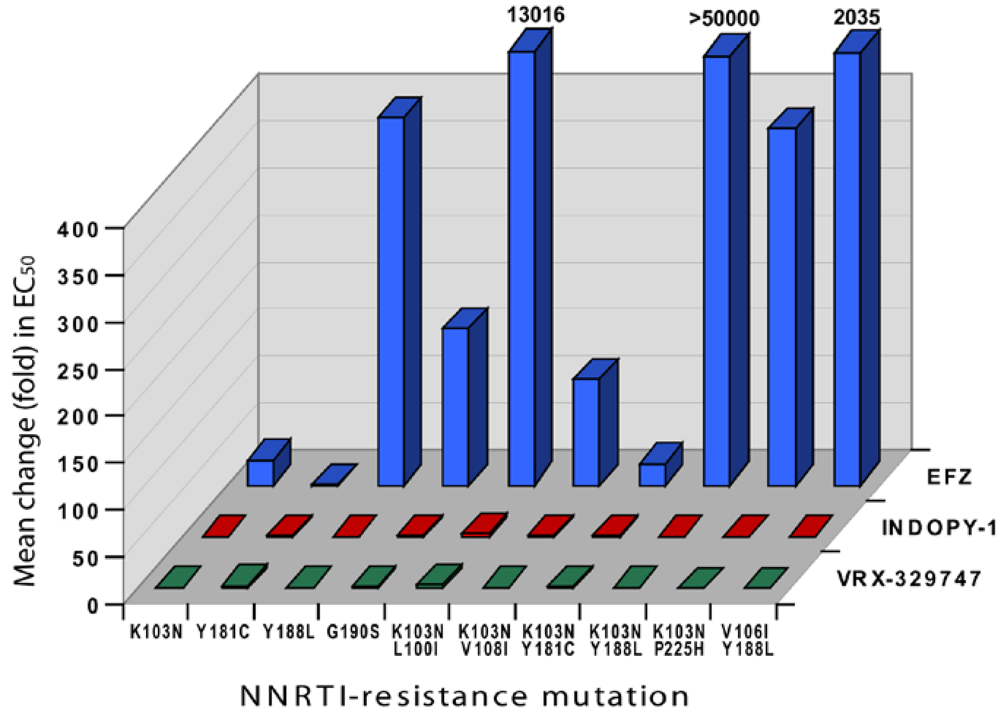
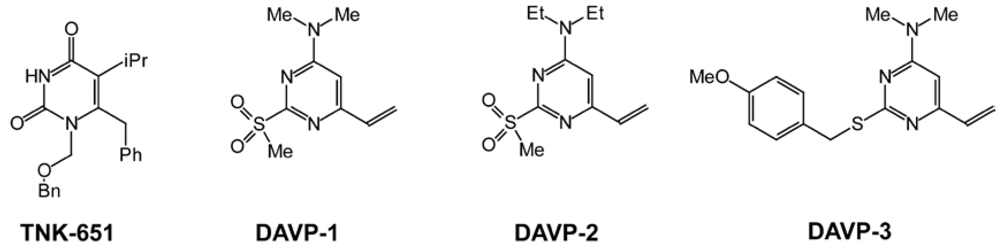
3. Dimethylamino-6-vinylpyrimidines (DAVPs)

| Ki (nM) | kon (M−1 s−1)a | koff (M−1 s−1)b | |
|---|---|---|---|
| Free RT | 8 | 1.04 × 104 | 8.4 × 10−5 |
| RT-primer/template | 8 | 14 × 104 | 1.1 × 10−5 |
| RT-primer/template-dNTP | 16 | 0.1 × 104 | 1.6 × 10−5 |
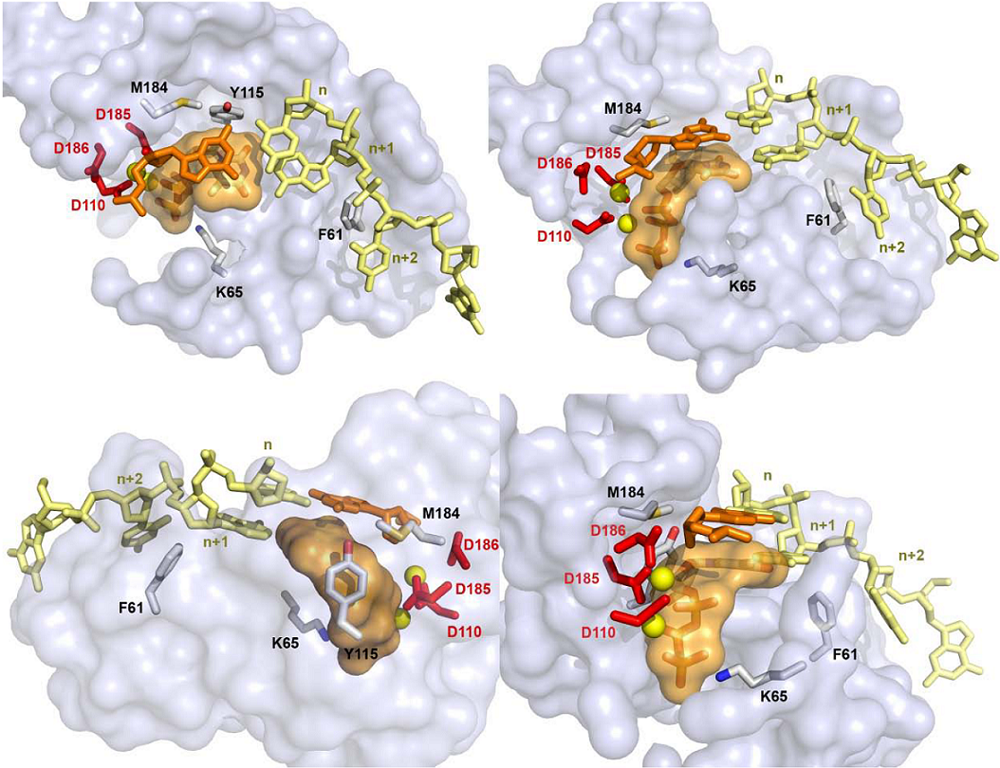
4. Structural Biology Studies of NcRTIs

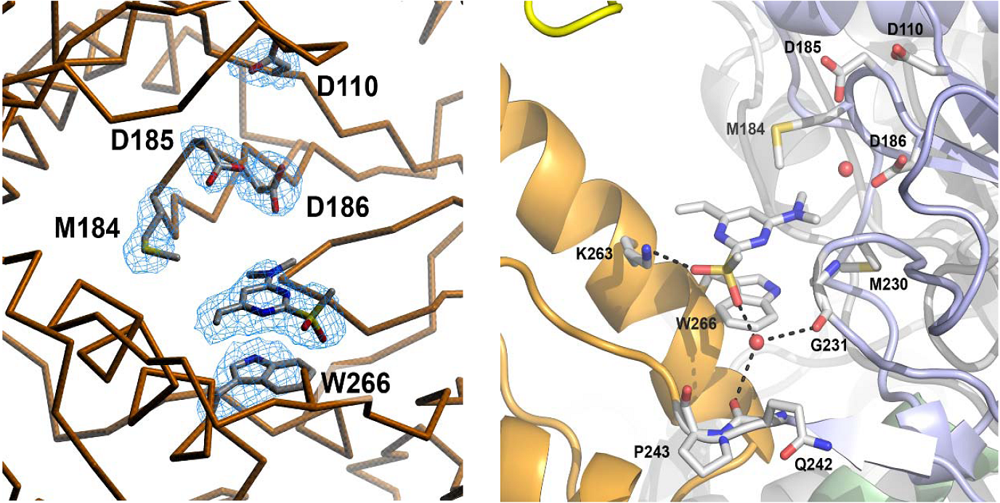
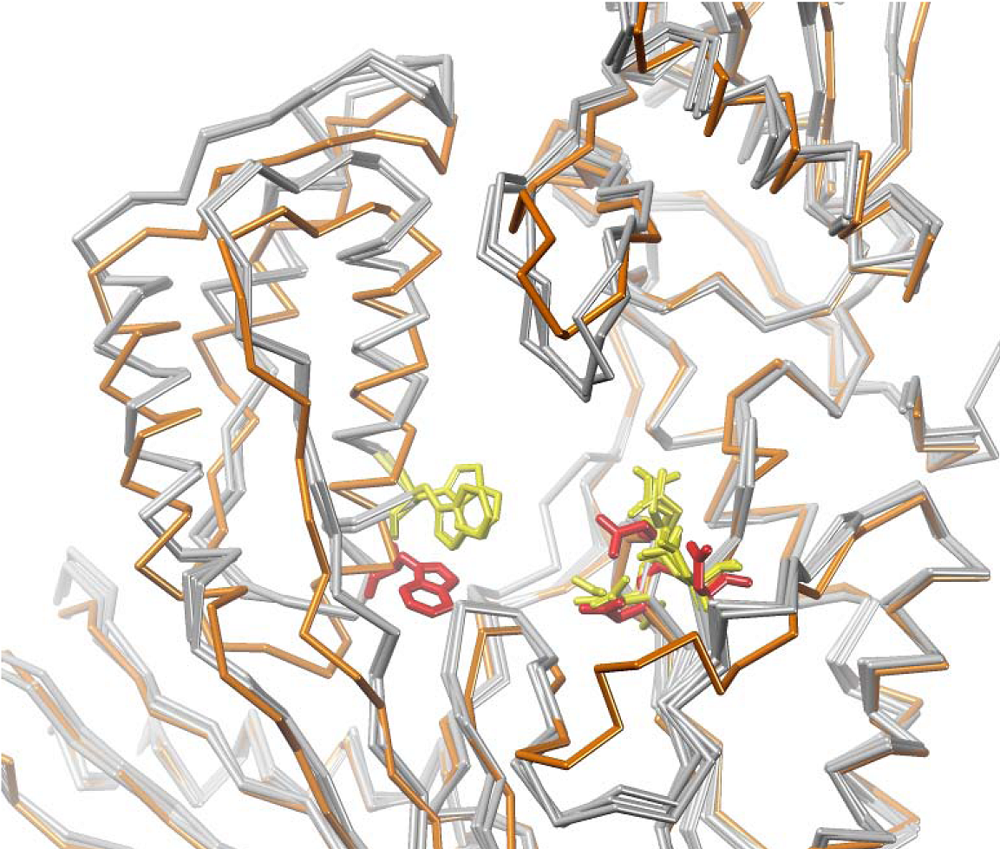
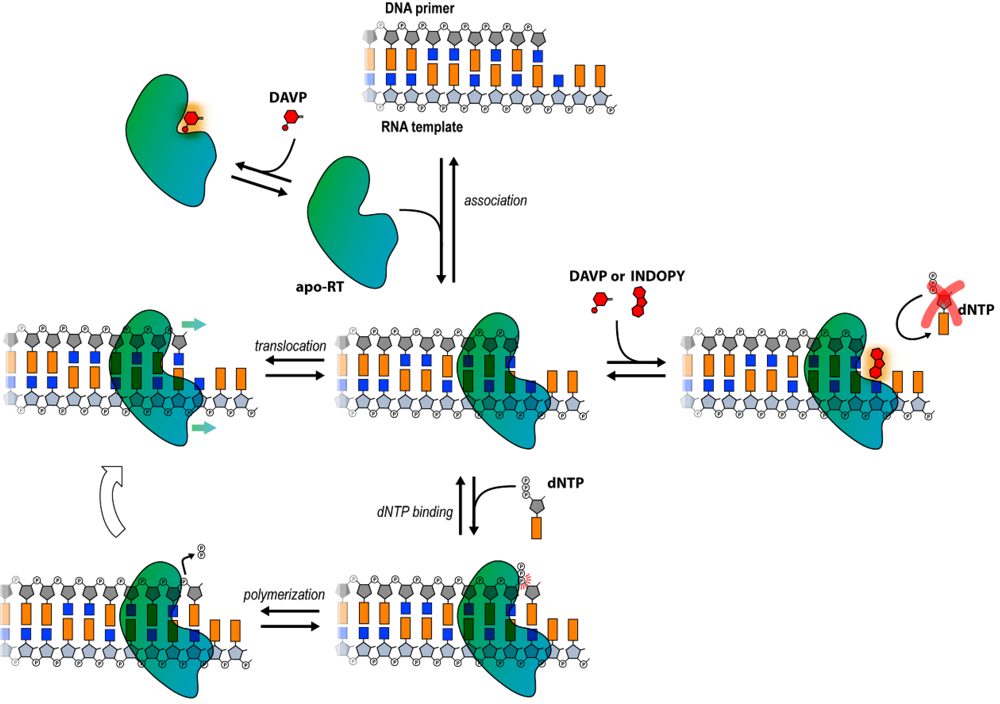
5. Conclusions
Acknowledgments
References
- Greene, W.C.; Debyser, Z.; Ikeda, Y.; Freed, E.O.; Stephens, E.; Yonemoto, W.; Buckheit, R.W.; Este, J.A.; Cihlar, T. Novel targets for HIV therapy. Antiviral Res. 2008, 80, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Mitsuya, H.; Weinhold, K.J.; Furman, P.A.; St Clair, M.H.; Lehrman, S.N.; Gallo, R.C.; Bolognesi, D.; Barry, D.W.; Broder, S. 3'-Azido-3'-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc. Natl. Acad. Sci. USA 1985, 82, 7096–7100. [Google Scholar] [CrossRef]
- St Clair, M.H.; Richards, C.A.; Spector, T.; Weinhold, K.J.; Miller, W.H.; Langlois, A.J.; Furman, P.A. 3'-Azido-3'-deoxythymidine triphosphate as an inhibitor and substrate of purified human immunodeficiency virus reverse transcriptase. Antimicrob. Agents Chemother. 1987, 31, 1972–1977. [Google Scholar] [PubMed]
- Esnouf, R.; Ren, J.; Ross, C.; Jones, Y.; Stammers, D.; Stuart, D. Mechanism of inhibition of HIV-1 reverse transcriptase by non-nucleoside inhibitors. Nat. Struct. Biol. 1995, 2, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Kohlstaedt, L.A.; Wang, J.; Friedman, J.M.; Rice, P.A.; Steitz, T.A. Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science 1992, 256, 1783–1790. [Google Scholar] [PubMed]
- Ren, J.; Esnouf, R.; Garman, E.; Somers, D.; Ross, C.; Kirby, I.; Keeling, J.; Darby, G.; Jones, Y.; Stuart, D.; et al. High resolution structures of HIV-1 RT from four RT-inhibitor complexes. Nat. Struct. Biol. 1995, 2, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Smerdon, S.J.; Jager, J.; Wang, J.; Kohlstaedt, L.A.; Chirino, A.J.; Friedman, J.M.; Rice, P.A.; Steitz, T.A. Structure of the binding site for nonnucleoside inhibitors of the reverse transcriptase of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 1994, 91, 3911–3915. [Google Scholar] [CrossRef]
- Rittinger, K.; Divita, G.; Goody, R.S. Human immunodeficiency virus reverse transcriptase substrate-induced conformational changes and the mechanism of inhibition by nonnucleoside inhibitors. Proc. Natl. Acad. Sci. USA 1995, 92, 8046–8049. [Google Scholar] [CrossRef]
- Spence, R.A.; Kati, W.M.; Anderson, K.S.; Johnson, K.A. Mechanism of inhibition of HIV-1 reverse transcriptase by nonnucleoside inhibitors. Science 1995, 267, 988–993. [Google Scholar] [PubMed]
- Xia, Q.; Radzio, J.; Anderson, K.S.; Sluis-Cremer, N. Probing nonnucleoside inhibitor-induced active-site distortion in HIV-1 reverse transcriptase by transient kinetic analyses. Protein Sci. 2007, 16, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Andries, K.; Azijn, H.; Thielemans, T.; Ludovici, D.; Kukla, M.; Heeres, J.; Janssen, P.; De Corte, B.; Vingerhoets, J.; Pauwels, R.; de Bethune, M.P. TMC125, a novel next-generation nonnucleoside reverse transcriptase inhibitor active against nonnucleoside reverse transcriptase inhibitor-resistant human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 2004, 48, 4680–4686. [Google Scholar] [CrossRef] [PubMed]
- Lazzarin, A.; Campbell, T.; Clotet, B.; Johnson, M.; Katlama, C.; Moll, A.; Towner, W.; Trottier, B.; Peeters, M.; Vingerhoets, J.; de Smedt, G.; Baeten, B.; Beets, G.; Sinha, R.; Woodfall, B. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-2: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet 2007, 370, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Bauman, J.D.; Clark, A.D.; Frenkel, Y.V.; Lewi, P.J.; Shatkin, A.J.; Hughes, S.H.; Arnold, E. High-resolution structures of HIV-1 reverse transcriptase/TMC278 complexes: strategic flexibility explains potency against resistance mutations. Proc. Natl. Acad. Sci. USA 2008, 105, 1466–1471. [Google Scholar] [CrossRef]
- Janssen, P.A.; Lewi, P.J.; Arnold, E.; Daeyaert, F.; de Jonge, M.; Heeres, J.; Koymans, L.; Vinkers, M.; Guillemont, J.; Pasquier, E.; Kukla, M.; Ludovici, D.; Andries, K.; de Bethune, M.P.; Pauwels, R.; Das, K.; Clark, A.D.; Frenkel, Y.V.; Hughes, S.H.; Medaer, B.; De Knaep, F.; Bohets, H.; De Clerck, F.; Lampo, A.; Williams, P.; Stoffels, P. In search of a novel anti-HIV drug: multidisciplinary coordination in the discovery of 4-[[4-[[4-[(1E)-2-cyanoethenyl]-2,6-dimethylphenyl]amino]-2- pyrimidinyl]amino]benzonitrile (R278474, rilpivirine). J. Med. Chem. 2005, 48, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Arasteh, K.; Rieger, A.; Yeni, P.; Pozniak, A.; Boogaerts, G.; van Heeswijk, R.; de Bethune, M.P.; Peeters, M.; Woodfall, B. Short-term randomized proof-of-principle trial of TMC278 in patients with HIV type-1 who have previously failed antiretroviral therapy. Antivir. Ther. 2009, 14, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Garvey, L.; Winston, A. Rilpivirine: a novel non-nucleoside reverse transcriptase inhibitor. Expert Opin. Investig. Drugs 2009, 18, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chopra, R.; Verdine, G.L.; Harrison, S.C. Structure of a covalently trapped catalytic complex of HIV-1 reverse transcriptase: implications for drug resistance. Science 1998, 282, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Budihas, S.R.; Gorshkova, I.; Gaidamakov, S.; Wamiru, A.; Bona, M.K.; Parniak, M.A.; Crouch, R.J.; McMahon, J.B.; Beutler, J.A.; Le Grice, S.F. Selective inhibition of HIV-1 reverse transcriptase-associated ribonuclease H activity by hydroxylated tropolones. Nucleic Acids Res. 2005, 33, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Himmel, D.M.; Sarafianos, S.G.; Dharmasena, S.; Hossain, M.M.; McCoy-Simandle, K.; Ilina, T.; Clark, A.D.; Knight, J.L.; Julias, J.G.; Clark, P.K.; Krogh-Jespersen, K.; Levy, R.M.; Hughes, S.H.; Parniak, M.A.; Arnold, E. HIV-1 reverse transcriptase structure with RNase H inhibitor dihydroxy benzoyl naphthyl hydrazone bound at a novel site. ACS Chem. Biol. 2006, 1, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Kirschberg, T.A.; Balakrishnan, M.; Squires, N.H.; Barnes, T.; Brendza, K.M.; Chen, X.; Eisenberg, E.J.; Jin, W.; Kutty, N.; Leavitt, S.; Liclican, A.; Liu, Q.; Liu, X.; Mak, J.; Perry, J.K.; Wang, M.; Watkins, W.J.; Lansdon, E.B. RNase H active site inhibitors of human immunodeficiency virus type 1 reverse transcriptase: design, biochemical activity, and structural information. J. Med. Chem. 2009, 52, 5781–5784. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, K.; Hang, J.Q.; Rajendran, S.; Yang, Y.; Derosier, A.; Wong Kai In, P.; Overton, H.; Parkes, K.E.; Cammack, N.; Martin, J.A. Two-metal ion mechanism of RNA cleavage by HIV RNase H and mechanism-based design of selective HIV RNase H inhibitors. Nucleic Acids Res. 2003, 31, 6852–6859. [Google Scholar] [CrossRef] [PubMed]
- Jochmans, D.; Deval, J.; Kesteleyn, B.; Van Marck, H.; Bettens, E.; De Baere, I.; Dehertogh, P.; Ivens, T.; Van Ginderen, M.; Van Schoubroeck, B.; Ehteshami, M.; Wigerinck, P.; Gotte, M.; Hertogs, K. Indolopyridones inhibit human immunodeficiency virus reverse transcriptase with a novel mechanism of action. J. Virol. 2006, 80, 12283–12292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Walker, M.; Xu, W.; Shim, J.H.; Girardet, J.L.; Hamatake, R.K.; Hong, Z. Novel nonnucleoside inhibitors that select nucleoside inhibitor resistance mutations in human immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. 2006, 50, 2772–2781. [Google Scholar] [CrossRef] [PubMed]
- Maga, G.; Radi, M.; Zanoli, S.; Manetti, F.; Cancio, R.; Hubscher, U.; Spadari, S.; Falciani, C.; Terrazas, M.; Vilarrasa, J.; Botta, M. Discovery of non-nucleoside inhibitors of HIV-1 reverse transcriptase competing with the nucleotide substrate. Angew. Chem. Int. Ed. Engl. 2007, 46, 1810–1813. [Google Scholar] [CrossRef] [PubMed]
- Ehteshami, M.; Scarth, B.J.; Tchesnokov, E.P.; Dash, C.; Le Grice, S.F.; Hallenberger, S.; Jochmans, D.; Gotte, M. Mutations M184V and Y115F in HIV-1 reverse transcriptase discriminate against "nucleotide-competing reverse transcriptase inhibitors". J. Biol. Chem. 2008, 283, 29904–29911. [Google Scholar] [CrossRef] [PubMed]
- Kellam, P.; Boucher, C.A.; Larder, B.A. Fifth mutation in human immunodeficiency virus type 1 reverse transcriptase contributes to the development of high-level resistance to zidovudine. Proc. Natl. Acad. Sci. USA 1992, 89, 1934–1938. [Google Scholar] [CrossRef]
- Larder, B.A.; Kemp, S.D. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT). Science 1989, 246, 1155–1158. [Google Scholar] [PubMed]
- Marcelin, A.G.; Delaugerre, C.; Wirden, M.; Viegas, P.; Simon, A.; Katlama, C.; Calvez, V. Thymidine analogue reverse transcriptase inhibitors resistance mutations profiles and association to other nucleoside reverse transcriptase inhibitors resistance mutations observed in the context of virological failure. J. Med. Virol. 2004, 72, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Parikh, U.M.; Barnas, D.C.; Faruki, H.; Mellors, J.W. Antagonism between the HIV-1 reverse-transcriptase mutation K65R and thymidine-analogue mutations at the genomic level. J. Infect. Dis. 2006, 194, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Cases-Gonzalez, C.E.; Franco, S.; Martinez, M.A.; Menendez-Arias, L. Mutational patterns associated with the 69 insertion complex in multi-drug-resistant HIV-1 reverse transcriptase that confer increased excision activity and high-level resistance to zidovudine. J. Mol. Biol. 2007, 365, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Hertogs, K.; Bloor, S.; De Vroey, V.; van Den Eynde, C.; Dehertogh, P.; van Cauwenberge, A.; Sturmer, M.; Alcorn, T.; Wegner, S.; van Houtte, M.; Miller, V.; Larder, B.A. A novel human immunodeficiency virus type 1 reverse transcriptase mutational pattern confers phenotypic lamivudine resistance in the absence of mutation 184V. Antimicrob. Agents Chemother. 2000, 44, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Kosalaraksa, P.; Kavlick, M.F.; Maroun, V.; Le, R.; Mitsuya, H. Comparative fitness of multi-dideoxynucleoside-resistant human immunodeficiency virus type 1 (HIV-1) in an In vitro competitive HIV-1 replication assay. J. Virol. 1999, 73, 5356–5363. [Google Scholar] [PubMed]
- Fisher, T.S.; Darden, T.; Prasad, V.R. Substitutions at Phe61 in the beta3-beta4 hairpin of HIV-1 reverse transcriptase reveal a role for the Fingers subdomain in strand displacement DNA synthesis. J. Mol. Biol. 2003, 325, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Fisher, T.S.; Prasad, V.R. Substitutions of Phe61 located in the vicinity of template 5'-overhang influence polymerase fidelity and nucleoside analog sensitivity of HIV-1 reverse transcriptase. J. Biol. Chem. 2002, 277, 22345–22352. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Arias, L. Mutation Rates and Intrinsic Fidelity of Retrovial Reverse Transcriptases. Viruses 2009, 1, 1137–1165. [Google Scholar] [CrossRef]
- Radi, M.; Petricci, E.; Maga, G.; Corelli, F.; Botta, M. Parallel solution-phase synthesis of 4-dialkylamino-2-methylsulfonyl-6-vinylpyrimidines. J. Comb. Chem. 2005, 7, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Radi, M.; Falciani, C.; Contemori, L.; Petricci, E.; Maga, G.; Samuele, A.; Zanoli, S.; Terrazas, M.; Castria, M.; Togninelli, A.; Este, J.A.; Clotet-Codina, I.; Armand-Ugon, M.; Botta, M. A multidisciplinary approach for the identification of novel HIV-1 non-nucleoside reverse transcriptase inhibitors: S-DABOCs and DAVPs. ChemMedChem 2008, 3, 573–593. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L.; Ren, J.; Esnouf, R.M.; Willcox, B.E.; Jones, E.Y.; Ross, C.; Miyasaka, T.; Walker, R.T.; Tanaka, H.; Stammers, D.K.; Stuart, D.I. Complexes of HIV-1 reverse transcriptase with inhibitors of the HEPT series reveal conformational changes relevant to the design of potent non-nucleoside inhibitors. J. Med. Chem. 1996, 39, 1589–1600. [Google Scholar] [CrossRef] [PubMed]
- Elinder, M.; Nordstrom, H.; Geitmann, M.; Hamalainen, M.; Vrang, L.; Oberg, B.; Danielson, U.H. Screening for NNRTIs with slow dissociation and high affinity for a panel of HIV-1 RT variants. J. Biomol. Screen. 2009, 14, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Geitmann, M.; Unge, T.; Danielson, U.H. Biosensor-based kinetic characterization of the interaction between HIV-1 reverse transcriptase and non-nucleoside inhibitors. J. Med. Chem. 2006, 49, 2367–2374. [Google Scholar] [CrossRef] [PubMed]
- Ludovici, D.W.; De Corte, B.L.; Kukla, M.J.; Ye, H.; Ho, C.Y.; Lichtenstein, M.A.; Kavash, R.W.; Andries, K.; de Bethune, M.P.; Azijn, H.; Pauwels, R.; Lewi, P.J.; Heeres, J.; Koymans, L.M.; de Jonge, M.R.; Van Aken, K.J.; Daeyaert, F.F.; Das, K.; Arnold, E.; Janssen, P.A. Evolution of anti-HIV drug candidates. Part 3: Diarylpyrimidine (DAPY) analogues. Bioorg. Med. Chem. Lett. 2001, 11, 2235–2239. [Google Scholar] [CrossRef] [PubMed]
- de Bethune, M.P. Non-nucleoside reverse transcriptase inhibitors (NNRTIs), their discovery, development, and use in the treatment of HIV-1 infection: A review of the last 20 years (1989-2009). Antiviral Res. 2009, 85, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Katlama, C.; Haubrich, R.; Lalezari, J.; Lazzarin, A.; Madruga, J.V.; Molina, J.M.; Schechter, M.; Peeters, M.; Picchio, G.; Vingerhoets, J.; Woodfall, B.; De Smedt, G. Efficacy and safety of etravirine in treatment-experienced, HIV-1 patients: pooled 48 week analysis of two randomized, controlled trials. AIDS 2009, 23, 2289–2300. [Google Scholar] [CrossRef] [PubMed]
- Vingerhoets, J.; Buelens, A.; Peeters, M.; Picchio, G.; Tambuyzer, L.; Van Marck, H.; De Smedt, G.; Woodfall, B.; De Bethune, M.P. Impact of baseline NNRTI mutations on the virological response to TMC125 in the DUET-1 and DUET-2 Phase III clinical trials. Antivir. Ther. 2007, 12. [Google Scholar]
- Johnson, V.A.; Brun-Vezinet, F.; Clotet, B.; Gunthard, H.F.; Kuritzkes, D.R.; Pillay, D.; Schapiro, J.M.; Richman, D.D. Update of the drug resistance mutations in HIV-1: 2007. Top HIV Med. 2007, 15, 119–125. [Google Scholar] [PubMed]
- Das, K.; Clark Jr., A.D.; Lewi, P.J.; Heeres, J.; De Jonge, M.R.; Koymans, L.M.; Vinkers, H.M.; Daeyaert, F.; Ludovici, D.W.; Kukla, M.J.; De Corte, B.; Kavash, R.W.; Ho, C.Y.; Ye, H.; Lichtenstein, M.A.; Andries, K.; Pauwels, R.; De Bethune, M.P.; Boyer, P.L.; Clark, P.; Hughes, S.H.; Janssen, P.A.; Arnold, E. Roles of conformational and positional adaptability in structure-based design of TMC125-R165335 (etravirine) and related non-nucleoside reverse transcriptase inhibitors that are highly potent and effective against wild-type and drug-resistant HIV-1 variants. J. Med. Chem. 2004, 47, 2550–2560. [Google Scholar] [CrossRef] [PubMed]
- Freisz, S.; Bec, G.; Radi, M.; Wolff, P.; Crespan, E.; Angeli, L.; Dumas, P.; Maga, G.; Botta, M.; Ennifar, E. Crystal Structure of HIV-1 Reverse Transcriptase Bound to a Non-Nucleoside Inhibitor with a Novel Mechanism of Action. Angew. Chem. Int. Ed. Engl.
- Ceccherini-Silberstein, F.; Gago, F.; Santoro, M.; Gori, C.; Svicher, V.; Rodriguez-Barrios, F.; d'Arrigo, R.; Ciccozzi, M.; Bertoli, A.; d'Arminio Monforte, A.; Balzarini, J.; Antinori, A.; Perno, C.F. High sequence conservation of human immunodeficiency virus type 1 reverse transcriptase under drug pressure despite the continuous appearance of mutations. J. Virol. 2005, 79, 10718–10729. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Jacques, P.S.; Rodgers, D.W.; Ottman, M.; Darlix, J.L.; Le Grice, S.F. Alterations to the primer grip of p66 HIV-1 reverse transcriptase and their consequences for template-primer utilization. Biochemistry 1996, 35, 8553–8562. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Eickbush, T.H. Origin and evolution of retroelements based upon their reverse transcriptase sequences. EMBO J. 1990, 9, 3353–3362. [Google Scholar] [PubMed]
- Jacobo-Molina, A.; Ding, J.; Nanni, R.G.; Clark, A.D. Jr; Lu, X.; Tantillo, C.; Williams, R.L.; Kamer, G.; Ferris, A.L.; Clark, P.; et al. Crystal structure of human immunodeficiency virus type 1 reverse transcriptase complexed with double-stranded DNA at 3.0 A resolution shows bent DNA. Proc. Natl. Acad. Sci. USA 1993, 90, 6320–6324. [Google Scholar] [CrossRef]
- Ding, J.; Das, K.; Moereels, H.; Koymans, L.; Andries, K.; Janssen, P.A.; Hughes, S.H.; Arnold, E. Structure of HIV-1 RT/TIBO R 86183 complex reveals similarity in the binding of diverse nonnucleoside inhibitors. Nat. Struct. Biol. 1995, 2, 407–415. [Google Scholar] [CrossRef]
- Hermann, T.; Meier, T.; Gotte, M.; Heumann, H. The 'helix clamp' in HIV-1 reverse transcriptase: a new nucleic acid binding motif common in nucleic acid polymerases. Nucleic Acids Res. 1994, 22, 4625–4633. [Google Scholar] [CrossRef] [PubMed]
- Beard, W.A.; Bebenek, K.; Darden, T.A.; Li, L.; Prasad, R.; Kunkel, T.A.; Wilson, S.H. Vertical-scanning mutagenesis of a critical tryptophan in the minor groove binding track of HIV-1 reverse transcriptase. Molecular nature of polymerase-nucleic acid interactions. J. Biol. Chem. 1998, 273, 30435–30442. [Google Scholar] [CrossRef] [PubMed]
- Beard, W.A.; Minnick, D.T.; Wade, C.L.; Prasad, R.; Won, R.L.; Kumar, A.; Kunkel, T.A.; Wilson, S.H. Role of the "helix clamp" in HIV-1 reverse transcriptase catalytic cycling as revealed by alanine-scanning mutagenesis. J. Biol. Chem. 1996, 271, 12213–12220. [Google Scholar] [CrossRef] [PubMed]
- Latham, G.J.; Forgacs, E.; Beard, W.A.; Prasad, R.; Bebenek, K.; Kunkel, T.A.; Wilson, S.H.; Lloyd, R.S. Vertical-scanning mutagenesis of a critical tryptophan in the "minor groove binding track" of HIV-1 reverse transcriptase. Major groove DNA adducts identify specific protein interactions in the minor groove. J. Biol. Chem. 2000, 275, 15025–15033. [Google Scholar] [CrossRef] [PubMed]
- Delarue, M.; Poch, O.; Tordo, N.; Moras, D.; Argos, P. An attempt to unify the structure of polymerases. Protein Eng. 1990, 3, 461–467. [Google Scholar] [CrossRef]
- Poch, O.; Sauvaget, I.; Delarue, M.; Tordo, N. Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J. 1989, 8, 3867–3874. [Google Scholar] [PubMed]
- Larder, B.A.; Purifoy, D.J.; Powell, K.L.; Darby, G. Site-specific mutagenesis of AIDS virus reverse transcriptase. Nature 1987, 327, 716–717. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.N.; Kaushik, N.; Rege, N.; Sarafianos, S.G.; Yadav, P.N.; Modak, M.J. Role of methionine 184 of human immunodeficiency virus type-1 reverse transcriptase in the polymerase function and fidelity of DNA synthesis. Biochemistry 1996, 35, 2168–2179. [Google Scholar] [CrossRef] [PubMed]
- Sarafianos, S.G.; Marchand, B.; Das, K.; Himmel, D.M.; Parniak, M.A.; Hughes, S.H.; Arnold, E. Structure and function of HIV-1 reverse transcriptase: molecular mechanisms of polymerization and inhibition. J. Mol. Biol. 2009, 385, 693–713. [Google Scholar] [CrossRef] [PubMed]
- Hsiou, Y.; Ding, J.; Das, K.; Clark, A.D. Jr.; Boyer, P.L.; Lewi, P.; Janssen, P.A.; Kleim, J.P.; Rosner, M.; Hughes, S.H.; Arnold, E. The Lys103Asn mutation of HIV-1 RT: a novel mechanism of drug resistance. J. Mol. Biol. 2001, 309, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Hsiou, Y.; Ding, J.; Das, K.; Clark, A.D. Jr.; Hughes, S.H.; Arnold, E. Structure of unliganded HIV-1 reverse transcriptase at 2.7 A resolution: implications of conformational changes for polymerization and inhibition mechanisms. Structure 1996, 4, 853–860. [Google Scholar] [CrossRef]
- Sarafianos, S.G.; Das, K.; Clark, A.D.; Ding, J.; Boyer, P.L.; Hughes, S.H.; Arnold, E. Lamivudine (3TC) resistance in HIV-1 reverse transcriptase involves steric hindrance with beta-branched amino acids. Proc. Natl. Acad. Sci. USA 1999, 96, 10027–10032. [Google Scholar] [CrossRef]
- Das, K.; Sarafianos, S.G.; Clark, A.D. Jr.; Boyer, P.L.; Hughes, S.H.; Arnold, E. Crystal structures of clinically relevant Lys103Asn/Tyr181Cys double mutant HIV-1 reverse transcriptase in complexes with ATP and non-nucleoside inhibitor HBY 097. J. Mol. Biol. 2007, 365, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Nichols, C.; Bird, L.; Chamberlain, P.; Weaver, K.; Short, S.; Stuart, D.I.; Stammers, D.K. Structural mechanisms of drug resistance for mutations at codons 181 and 188 in HIV-1 reverse transcriptase and the improved resilience of second generation non-nucleoside inhibitors. J. Mol. Biol. 2001, 312, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Boyer, P.L.; Tantillo, C.; Jacobo-Molina, A.; Nanni, R.G.; Ding, J.; Arnold, E.; Hughes, S.H. Sensitivity of wild-type human immunodeficiency virus type 1 reverse transcriptase to dideoxynucleotides depends on template length; the sensitivity of drug-resistant mutants does not. Proc. Natl. Acad. Sci. USA 1994, 91, 4882–4886. [Google Scholar] [CrossRef]
- Kleim, J.P.; Rosner, M.; Winkler, I.; Paessens, A.; Kirsch, R.; Hsiou, Y.; Arnold, E.; Riess, G. Selective pressure of a quinoxaline nonnucleoside inhibitor of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase (RT) on HIV-1 replication results in the emergence of nucleoside RT-inhibitor-specific (RT Leu-74-->Val or Ile and Val-75-->Leu or Ile) HIV-1 mutants. Proc. Proc. Natl. Acad. Sci. USA 1996, 93, 34–38. [Google Scholar] [CrossRef]
- Blanca, G.; Baldanti, F.; Paolucci, S.; Skoblov, A.Y.; Victorova, L.; Hubscher, U.; Gerna, G.; Spadari, S.; Maga, G. Nevirapine resistance mutation at codon 181 of the HIV-1 reverse transcriptase confers stavudine resistance by increasing nucleotide substrate discrimination and phosphorolytic activity. J. Biol. Chem. 2003, 278, 15469–15472. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Harrison, S.C.; Verdine, G.L. Trapping of a catalytic HIV reverse transcriptase template:primer complex through a disulfide bond. Chem. Biol. 2000, 7, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Sarafianos, S.G.; Clark, A.D.; Das, K.; Tuske, S.; Birktoft, J.J.; Ilankumaran, P.; Ramesha, A.R.; Sayer, J.M.; Jerina, D.M.; Boyer, P.L.; Hughes, S.H.; Arnold, E. Structures of HIV-1 reverse transcriptase with pre- and post-translocation AZTMP-terminated DNA. EMBO J. 2002, 21, 6614–6624. [Google Scholar] [CrossRef] [PubMed]
- Sarafianos, S.G.; Clark, A.D.; Tuske, S.; Squire, C.J.; Das, K.; Sheng, D.; Ilankumaran, P.; Ramesha, A.R.; Kroth, H.; Sayer, J.M.; Jerina, D.M.; Boyer, P.L.; Hughes, S.H.; Arnold, E. Trapping HIV-1 reverse transcriptase before and after translocation on DNA. J. Biol. Chem. 2003, 278, 16280–16288. [Google Scholar] [CrossRef] [PubMed]
- Tuske, S.; Sarafianos, S.G.; Clark, A.D.; Ding, J.; Naeger, L.K.; White, K.L.; Miller, M.D.; Gibbs, C.S.; Boyer, P.L.; Clark, P.; Wang, G.; Gaffney, B.L.; Jones, R.A.; Jerina, D.M.; Hughes, S.H.; Arnold, E. Structures of HIV-1 RT-DNA complexes before and after incorporation of the anti-AIDS drug tenofovir. Nat. Struct. Mol. Biol. 2004, 11, 469–474. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Share and Cite
Maga, G.; Radi, M.; Gerard, M.-A.; Botta, M.; Ennifar, E. HIV-1 RT Inhibitors with a Novel Mechanism of Action: NNRTIs that Compete with the Nucleotide Substrate. Viruses 2010, 2, 880-899. https://doi.org/10.3390/v2040880
Maga G, Radi M, Gerard M-A, Botta M, Ennifar E. HIV-1 RT Inhibitors with a Novel Mechanism of Action: NNRTIs that Compete with the Nucleotide Substrate. Viruses. 2010; 2(4):880-899. https://doi.org/10.3390/v2040880
Chicago/Turabian StyleMaga, Giovanni, Marco Radi, Marie-Aline Gerard, Maurizio Botta, and Eric Ennifar. 2010. "HIV-1 RT Inhibitors with a Novel Mechanism of Action: NNRTIs that Compete with the Nucleotide Substrate" Viruses 2, no. 4: 880-899. https://doi.org/10.3390/v2040880





