2.1. Hydrogenation of Na2O
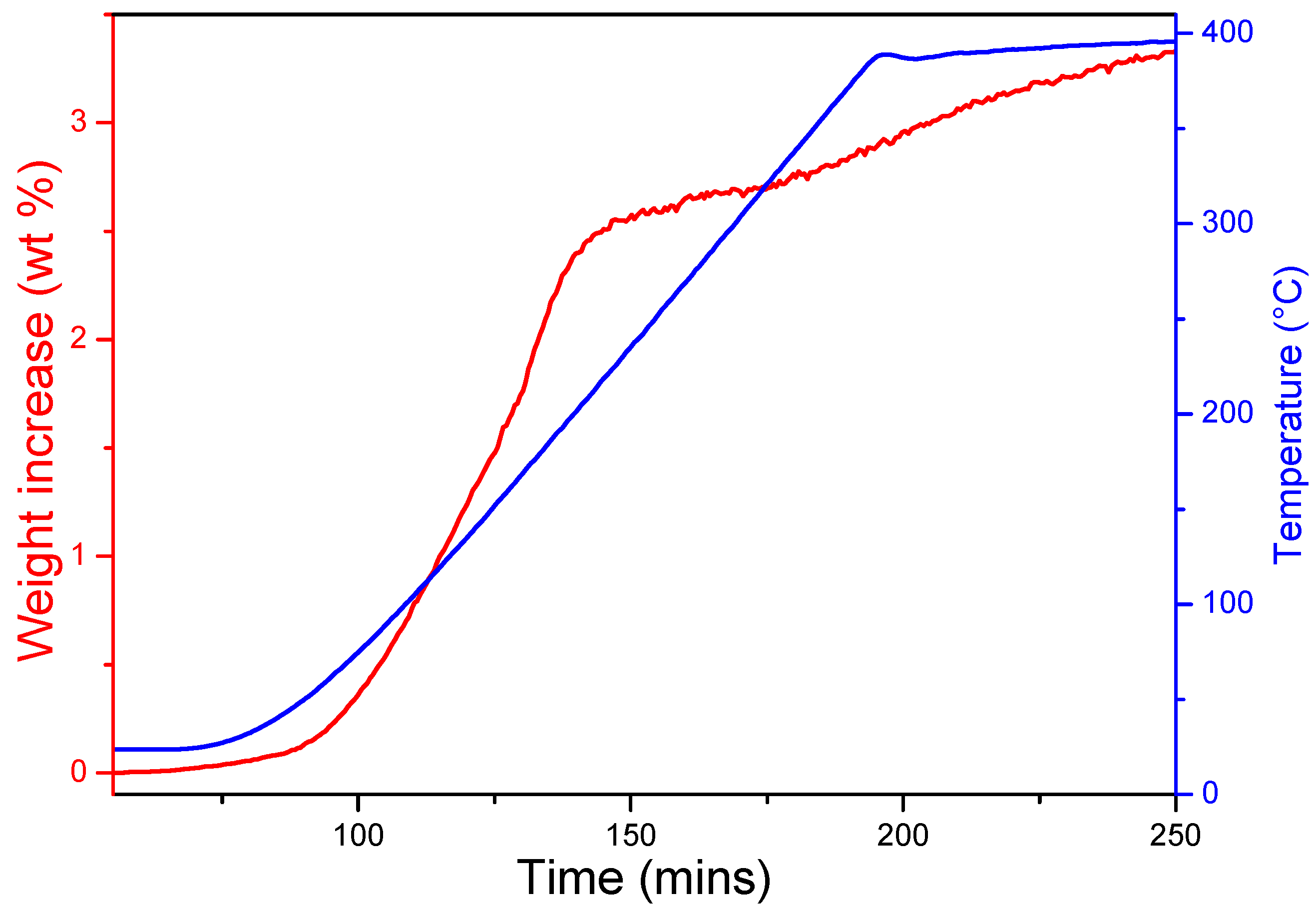
The time-resolved hydrogenation profile of as-milled Na
2O with temperature (at 18 bar) is shown in
Figure 1. On heating at 3 °C min
−1, hydrogen uptake is initiated close to room temperature (30–50 °C). Upon heating to 140 °C, the hydrogen uptake rate increases significantly and 2.5 wt% H
2 can be stored under these conditions. Subsequently, hydrogenation is relatively slow and a total of 3.3 wt% hydrogen is absorbed by 400 °C. These uptake results are consistent with previous studies [
16,
17], although it should be noted that the experimental figure of 3.3 wt% slightly exceeds the theoretical capacity. In principle, this could be possible due to the partial reaction of Na
2O with moisture during hydrogen uptake, but powder X-ray diffraction (PXD) patterns before and after hydrogenation suggest that the reaction of an impurity in the starting material with hydrogen may also contribute as discussed below.
Figure 1.
Hydrogen uptake of as-milled Na2O under 18 bar of hydrogen.
Figure 1.
Hydrogen uptake of as-milled Na2O under 18 bar of hydrogen.
PXD was performed to clarify the chemical reactions that occur on hydrogenation.
Figure 2 shows the PXD patterns of the Na
2O starting material after mechanical milling (sample 1), the material after hydrogenation (sample 2) and finally after subsequent dehydrogenation (sample 3). Although Na
2O was the main phase in the ball milled starting material as expected, an impurity phase of Na
2O
2 was also detected, and analysis of the diffraction pattern yielded a phase fraction of
ca. 10 wt%. After hydrogenation at 400 °C, NaH and NaOH were formed and the diffraction peaks from Na
2O and Na
2O
2 were no longer present. Upon dehydrogenation at 400 °C, Na
2O reformed and was present as the majority phase. Overall, therefore, the material system demonstrated a reversible reaction as described by Equation 1. The presence of NaOH in the dehydrogenated product, sample 3 could suggest partial hydrolysis of Na
2O, but the more likely origin of the hydroxide is from hydrogenation of the original Na
2O
2 impurity in the starting material (since Na
2O
2 + H
2→2NaOH). This leads to an excess of NaOH in the hydrogenated product, sample 2 (
i.e., NaH:NaOH is not in the expected 1:1 molar ratio following hydrogen uptake) which persists during the dehydrogenation process at 400 °C However, Rietveld refinement for hydrogenated Na
2O shows that the molar ratio of NaOH and NaH in the sample is 1.66:1. The phase fraction of NaOH exceeds the amount expected solely from the hydrogenation of 10 wt% Na
2O
2. Thus, both the hydrolysis of Na
2O (and possibly of NaH) and the hydrogenation of the original Na
2O
2 impurity lead to the observed excess of NaOH.
Figure 2.
XRD patterns of Na2O after ball milling (sample 1), after hydrogenation at 400 °C/18 bar H2 (sample 2), and after subsequent dehydrogenation at 400 °C (sample 3).
Figure 2.
XRD patterns of Na2O after ball milling (sample 1), after hydrogenation at 400 °C/18 bar H2 (sample 2), and after subsequent dehydrogenation at 400 °C (sample 3).
2.2. Dehydrogenation of NaH-NaOH
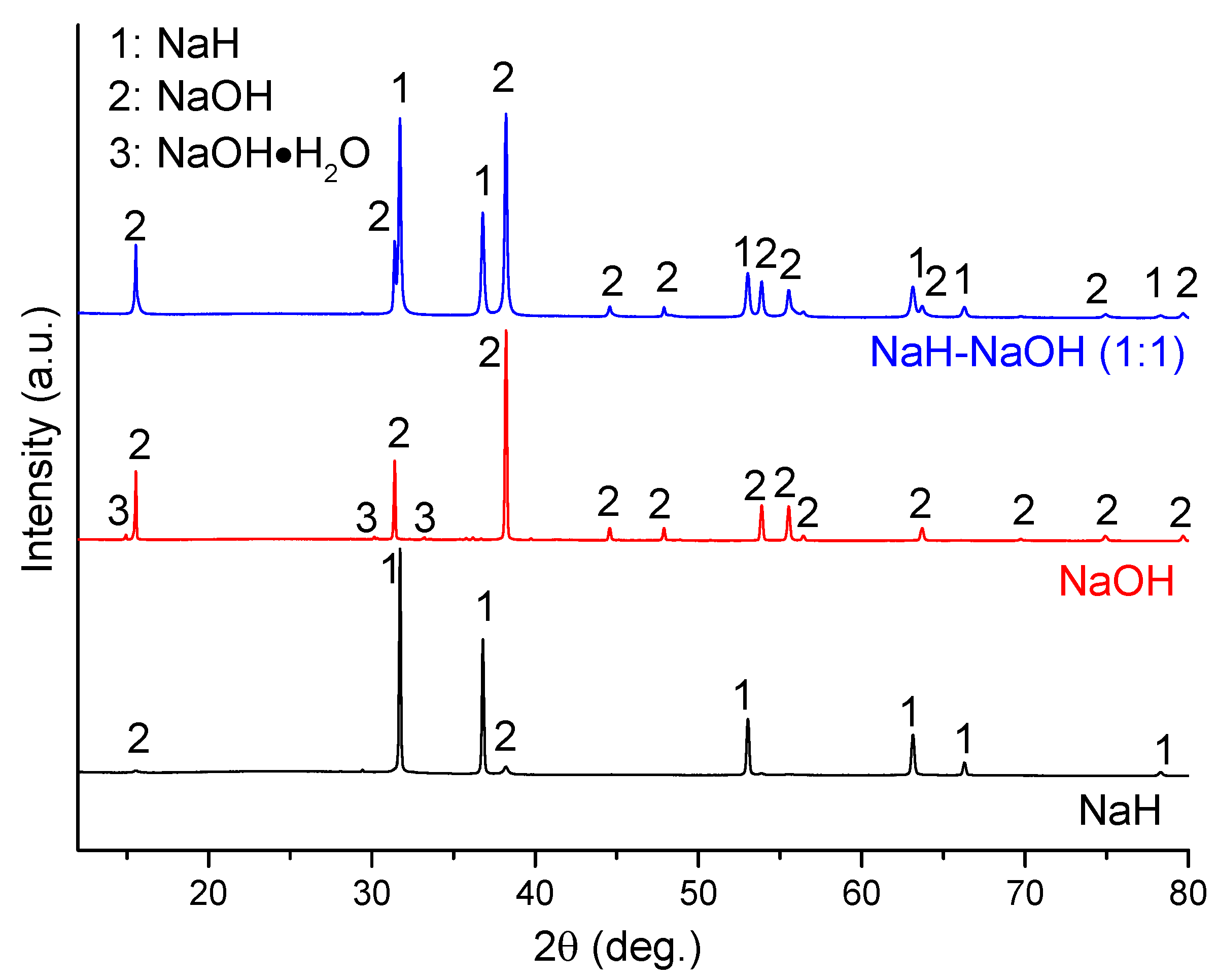
The dehydrogenation process in the NaH-NaOH system was investigated further.
Figure 3 shows a PXD pattern of the as-milled mixture of NaH-NaOH (molar ratio 1:1; sample 4) as compared with the individual commercial starting materials NaH and α-NaOH. It can be seen that the as-received NaH contains a small amount of NaOH impurity, while the as-received NaOH contains a minor phase of the hydrated hydroxide, NaOH·H
2O. By comparison, only NaH and NaOH were detected in sample 4. The absence of the NaOH·H
2O impurity in sample 4 is attributed to dehydration during ball milling.
Figure 3.
PXD patterns of as-received NaH and NaOH, and as-milled NaH-NaOH (1:1), sample 4.
Figure 3.
PXD patterns of as-received NaH and NaOH, and as-milled NaH-NaOH (1:1), sample 4.
The thermal decomposition behavior of sample 4 compared to NaH and NaOH was investigated by DTA, as shown in
Figure 4. The DTA profile for NaH shows one endothermic peak at
ca. 360 °C, which can be assigned to the decomposition of NaH to Na metal and hydrogen. For NaOH, the DTA profile shows two endothermic peaks at 299 and 319 °C, which can be assigned to the α-β (orthorhombic-monoclinic) phase transition and the melting point, respectively [
25,
26]. By contrast, sample 4 shows different features, displaying multiple endothermic peaks. The first endothermic event occurs at 171 °C and is relatively well-defined while the second is more complex (consisting of perhaps three or more individual processes) and reaches a maximum in the DTA profile at 333 °C. The second endothermic event can be attributed to the reaction of NaH and NaOH culminating in the formation of Na
2O and hydrogen. The results further confirmed that the dehydrogenation pathway of the NaH-NaOH mixture is entirely different to that observed for NaH. The first endothermic event is not observed in either NaH or NaOH. It would appear therefore that the synergic interaction of hydrogen species in NaH and NaOH not only contributes to the lower dehydrogenation temperature
vs. NaH itself, but also leads to likely reaction in the solid state and structural changes during the heating period prior to dehydrogenation. To clarify the structural changes that occur before dehydrogenation, variable temperature,
in-situ synchrotron PXD experiments were conducted on the NaH-NaOH mixture and are discussed below.
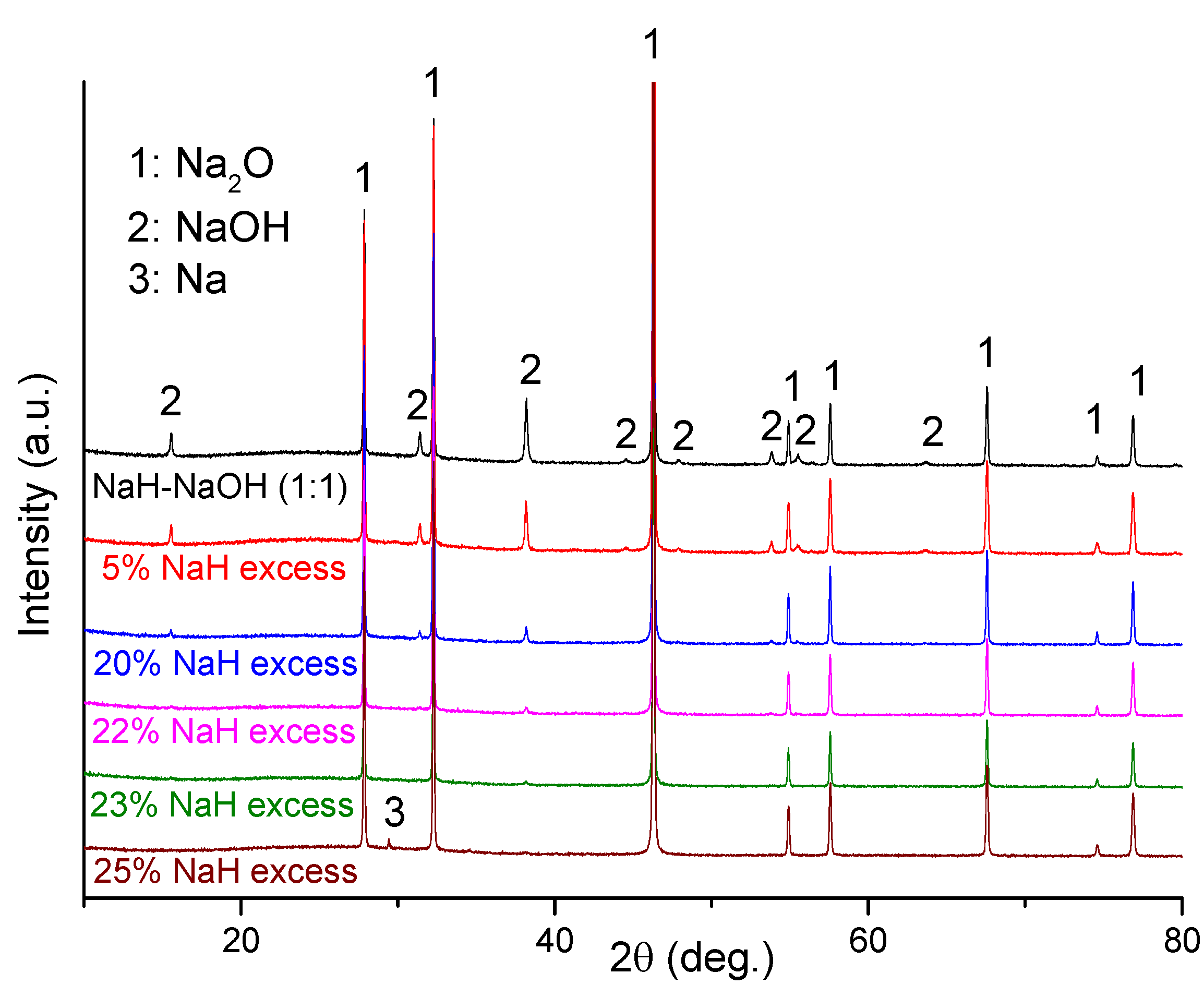
Ex-situ XRD characterization was performed on several dehydrogenated hydride-hydroxide mixtures of varying molar ratio after heating each to 400 °C (
Figure 5). Clearly, Na
2O was the main phase in the dehydrogenated NaH-NaOH (1:1) sample, but some residual NaOH remains also detected. In this case, the presence of residual NaOH can be attributed to the fact that the NaH and NaOH starting materials contain low levels of NaOH and NaOH·H
2O impurities, respectively. Hence the NaH:NaOH ratio departs from the ideal stoichiometric 1:1 value in the mixture. In order to obtain single phase Na
2O as a dehydrogenation product, we optimized the quantity of NaH and NaOH in the starting mixture. As shown in
Figure 5, NaOH impurity reflections diminished as the NaH:NaOH ratio increased, reaching a minimum when a 23 wt% excess of NaH was used. On adding a 25 wt% excess of NaH, no NaOH was observed in diffraction patterns but reflections originating from Na metal appeared. Therefore, use of 23 wt% excess of NaH was found to be the optimum reactant composition required to obtain Na
2O with minimal impurities (sample 5).
Figure 4.
DTA profiles of NaH, NaOH, and as-milled NaH-NaOH (1:1) mixture.
Figure 4.
DTA profiles of NaH, NaOH, and as-milled NaH-NaOH (1:1) mixture.
Figure 5.
PXD patterns of NaH-NaOH with varying hydride:hydroxide molar ratio after dehydrogenation at 400 °C.
Figure 5.
PXD patterns of NaH-NaOH with varying hydride:hydroxide molar ratio after dehydrogenation at 400 °C.
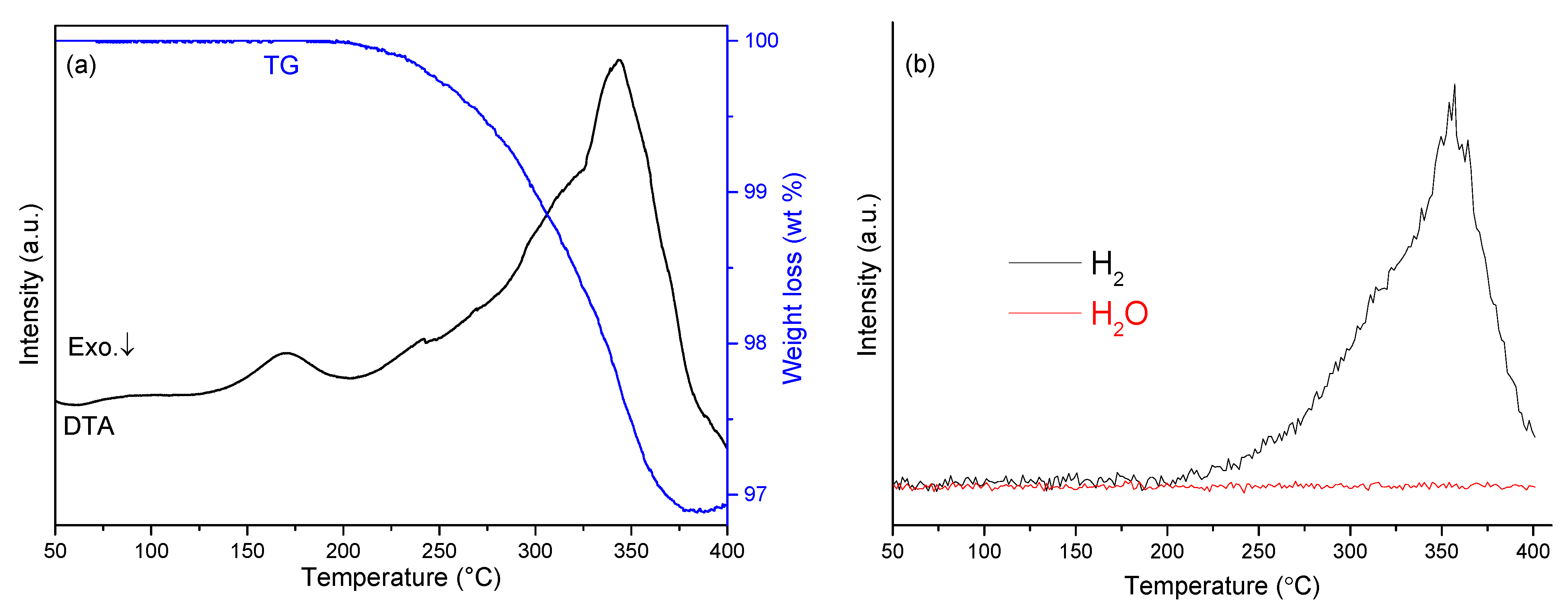
The thermal behavior of sample 5 was investigated by TG-DTA-MS (
Figure 6). TG results showed that the mixture released gas from 200–378 °C with a corresponding weight loss of 3.1 wt%. Moreover, it was evident from mass spectra collected simultaneously while heating that hydrogen was the only evolved gaseous product, consistent with the reversible reaction described by equation 1. The DTA profile for sample 5 reveals a similar feature with the sample NaH-NaOH (1:1). The first endothermic peak was observed at 170 °C, while the second cluster of overlapping endothermic peaks with a maximum at 342 °C is attributed to the dehydrogenation reaction of NaH with NaOH and might be expected to incorporate first the α-β phase transition and second the melting of NaOH.
Figure 6.
Simultaneous TG-DTA-MS profiles of sample 5: (a) TG-DTA traces; (b) mass spectra for hydrogen and water.
Figure 6.
Simultaneous TG-DTA-MS profiles of sample 5: (a) TG-DTA traces; (b) mass spectra for hydrogen and water.
2.3. In-Situ Synchrotron Powder Diffraction
In-situ synchrotron PXD methods were used to clarify the reaction mechanism underpinning the first endothermic event at
ca. 170 °C by heating sample 5 from room temperature to 260 °C.
Figure 7 shows selected regions of the
in-situ diffraction patterns for sample 5, the relevant peak indices and a plot of the resulting cell volume changes of the NaH and NaOH phases, respectively, as a function of temperature. Structure refinements performed against the synchrotron PXD data were conducted for each data set (see supplementary information). A fundamental parameter (FP) approach was employed in TOPAS to perform whole-pattern profile fitting of the diffraction data collected in transmission mode. The diffraction background was fitted with Chebychev functions. Structural data from the ICDD PDF4 (2014) database for NaH (02-0809) and α-NaOH (078-0188) were used as starting models in TOPAS. At room temperature NaH crystallizes in cubic space group
Fm3m (No.225;
a = 4.8826(1) Å), while α-NaOH is orthorhombic (
Cmcm, No. 63;
a = 3.4039(1) Å,
b = 3.4011(1) Å,
c = 11.3901(1) Å).
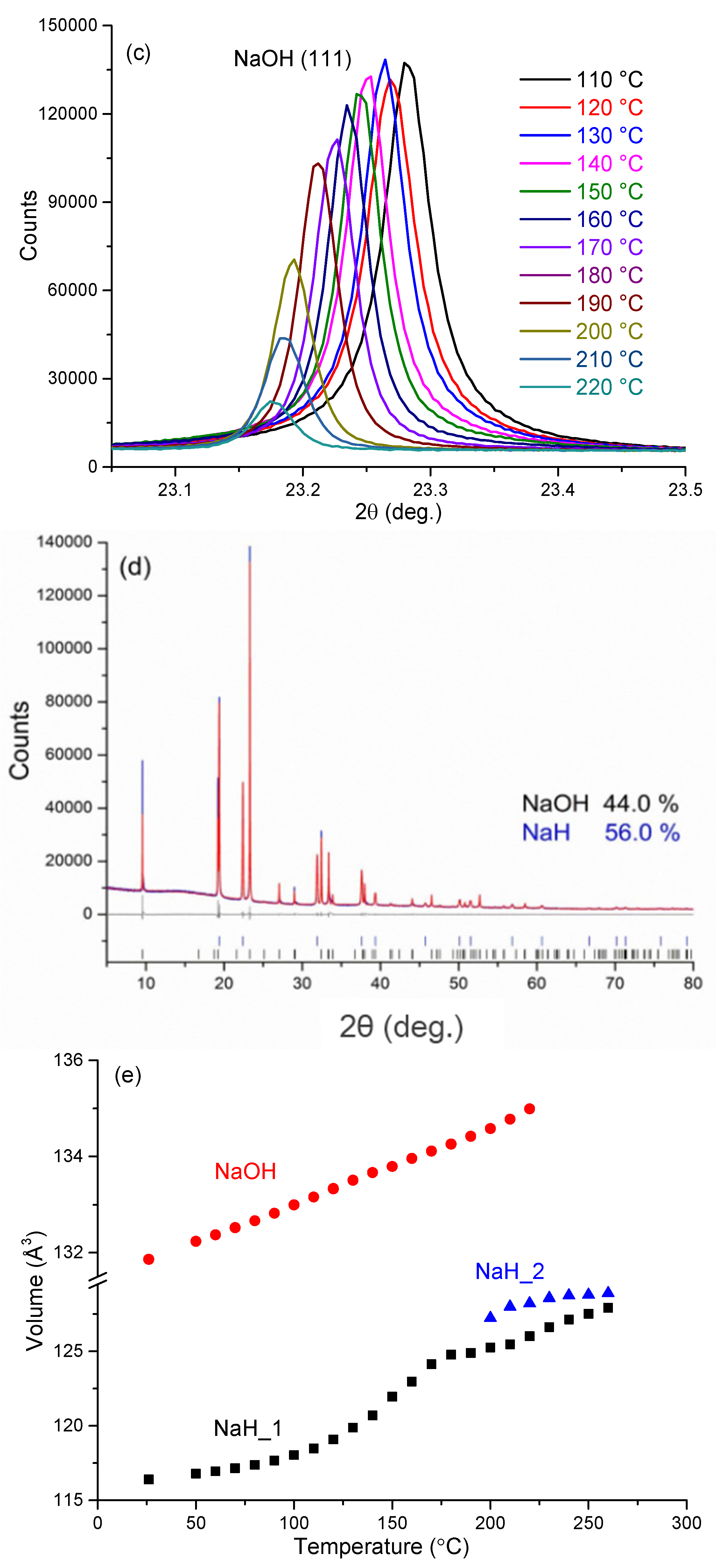
Before heating, room temperature PXD data show that sample 5 consists only of NaH (44 wt%) and NaOH (56 wt%). There are no impurity diffraction peaks observed in the PXD pattern. As shown in
Figure 7, below 110 °C, reflections for both NaH and NaOH shift slightly to lower angle with increasing temperature. This shift corresponds to the expected thermal expansion of the NaH and NaOH lattices. The cell volume for NaH increases from
ca. 116.4 Å
3 to
ca. 118.5 Å
3 over this temperature range, corresponding to a volume expansion of approximately 1.8%. Over the same temperature range, the cell volume for NaOH increases by approximately 0.99% and the phase fractions of NaH and NaOH remain effectively constant, indicating that no reaction occurs between the hydride and hydroxide.
From 110 °C, all the NaH peaks become increasingly asymmetric with some evidence of reflections emerging at slightly higher 2θ values to the main peaks as manifested by a broadening tail to all NaH reflections. Meanwhile, the NaH peaks shift continuously to lower 2θ angles and the hydride cell volume increases significantly such that at 180 °C the value is approximately 7.2% larger compared to that at room temperature (
Figure 7e). Most significantly, however, starting from 190 °C, the NaH peaks become asymmetric at lower diffraction angle while simultaneously the NaOH diffraction peaks become weaker. As can be seen clearly for the NaH 200 reflection at 2θ ~ 22.4° (
Figure 7b), as the temperature increases a new peak starts to appear at a slightly lower 2θ angle. Given that every NaH peak splits in the same sense and that all the original reflections are also retained, the phenomenon indicates the formation of a slightly larger unit cell with a structure type common to the original NaH phase. This indicates that a new NaH-like phase with slightly larger lattice parameters is formed as NaOH becomes depleted.
Figure 7.
In-situ synchrotron PXD patterns for sample 5 heated from room temperature to 260 °C with a constant heating rate of 10 °C min–1 in a closed quartz capillary under Ar atmosphere (λ = 0.9533 Å) showing: (a) the region from 7° ≤ 2θ≤ 40°; (b) the change in the NaH (200) reflections from 110 to 220 °C; (c) the change in the NaOH (111) reflections from 110 to 220 °C; (d) the refinement profile at 100 °C; and (e) cell volume against temperature for NaH (“NaH_1”), “Na-O-H” (“NaH_2”) and NaOH, respectively, from room temperature to 260 °C.
Figure 7.
In-situ synchrotron PXD patterns for sample 5 heated from room temperature to 260 °C with a constant heating rate of 10 °C min–1 in a closed quartz capillary under Ar atmosphere (λ = 0.9533 Å) showing: (a) the region from 7° ≤ 2θ≤ 40°; (b) the change in the NaH (200) reflections from 110 to 220 °C; (c) the change in the NaOH (111) reflections from 110 to 220 °C; (d) the refinement profile at 100 °C; and (e) cell volume against temperature for NaH (“NaH_1”), “Na-O-H” (“NaH_2”) and NaOH, respectively, from room temperature to 260 °C.
By 240 °C there is no evidence of NaOH in the diffraction patterns and the NaH-like phase reflections become more symmetric and sharper, indicative of a single phase. Also noteworthy is that there is no evidence for the formation of Na2O over the entire temperature range of the experiment. The cell volume of the hydride at 240 °C is 127.1 Å3, which is approximately 9.2% larger as compared to the room temperature NaH structure. The results suggest that a structural change begins from 110 °C and that by 190 °C NaOH reacts appreciably with NaH in the solid state to form a complex hydride with an NaH-type structure. As the temperature increases further, a single composition of the NaH-like phase is formed. Thus we propose that an NaH-NaOH solid solution forms in which up to 50% of the hydride is replaced by hydroxide; NaH1-x(OH)x where x ≤ 0.5. Hence, one might reasonably speculate that the formation of the hydride-hydroxide is the vital precursor to an intraphase Hδ… Hδ– interaction and the hydrogen evolution step during desorption.
The process could thus be represented by the modified version of the reaction equation below:
When compared with other
AH-
AOH(
AOH)
2 systems (
A = Li, K, Mg), similar solid solution behavior has only been observed previously in the K-O-H system [
19]. On heating, however, KOH-KH does not apparently combine to form K
2O, but rather KH decomposes independently while KOH remains to 500 °C [
17]. By contrast, in the LiOH-LiH system, although the final decomposition reaction product is Li
2O, no solid solution phases are reported prior to oxide formation upon heating, which might be a consequence of the relatively low dehydrogenation temperature [
17,
27,
28]. There is no strong evidence for solid solution formation in the MgH-Mg(OH)
2 system prior to dehydrogenation [
29]. Na-O-H is unique among these four hydroxide-based combinations as the only system with the appropriate thermodynamics for reversible hydrogen storage. The present work suggests that for hydroxide-hydride materials not only is the inter-phase synergic interaction of protic hydrogen (in NaOH) and hydridic hydrogen (in NaH) important in the dehydrogenation mechanism, but that also an intra-phase H
δ+… H
δ– interaction may be a crucial step in the desorption process. Furthermore, the ensuing lattice expansion and anion disorder of the sodium hydride hydroxide could play a significant role in the diffusion of hydrogen in the solid state either or both as protons and hydride. The mobility of one or both of these species is likely to be key to mediating and controlling the hydrogen uptake and release kinetics in this and similar systems. Strategies involving nanostructuring, additives and catalysts are likely to be crucial in the development of cheap, abundant materials systems such as hydroxides into potentially useful hydrogen stores.