Medium-Term Function of a 3D Printed TCP/HA Structure as a New Osteoconductive Scaffold for Vertical Bone Augmentation: A Simulation by BMP-2 Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
Samples
| Healing period | Samples | Number of specimen analyzed (n) |
|---|---|---|
| 8 weeks | Coagulated blood | 4 |
| 3D-printed block (OF-plain) | 8 | |
| 3D-printed block + BMP-2 (OF-BMP-2) | 8 | |
| 16 weeks | Coagulated blood | 4 |
| 3D-printed block (OF-plain) | 8 | |
| 3D-printed block + BMP-2 (OF-BMP-2) | 8 | |
| Total | Total placed: 40 | Total analyzed: 40 |
2.2. Hemispheres and Placement of Bone Substitutes
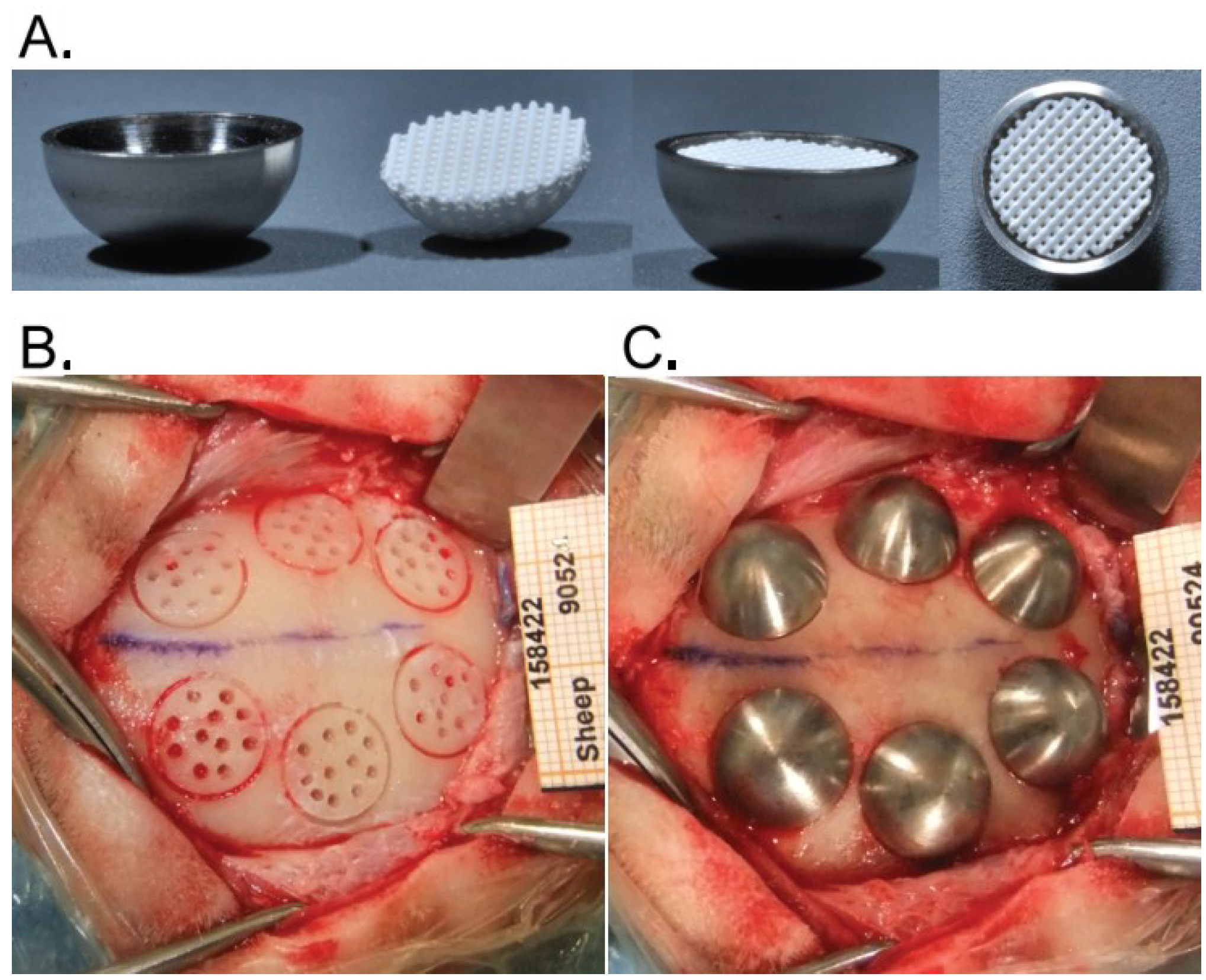
2.3. Animals
2.4. Surgical Procedure
2.5. Histological Preparation, Histopathologic and Histomorphometric Analysis
2.6. Statistical Analysis
3. Results
3.1. Histopathologic Evaluation
| Cell type/response | Score | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Polymorphonuclear cells (PMN) | 0 | Rare, 1–5/phf | 5–10/phf | Heavy infiltrate | Dense |
| Lymphocytes (Lc) | 0 | Rare, 1–5/phf | 5–10/phf | Heavy infiltrate | Dense |
| Plasma cells (Plc) | 0 | Rare, 1–5/phf | 5–10/phf | Heavy infiltrate | Dense |
| Macrophages (Ma) | 0 | Rare, 1–5/phf | 5–10/phf | Heavy infiltrate | Dense |
| Giant cells/osteoclastic cells (Gc/Oc) | 0 | Rare, 1–2/phf | 3–5/phf | Heavy infiltrate | Sheets |
| Osteoblasts (Ob) | 0 | Slight, equivalent to normal bone | Moderate, >normal bone | Marked, >normal bone | Highly marked |
| phf = per high powered (400×) field | |||||
| Time | Samples n = 8 | Polymorphomuclear cells | Lymphocytes | Plasma cells | Macrophages | Giant Cells/ osteoclastic cells | Osteoblastic cells |
|---|---|---|---|---|---|---|---|
| 8 weeks | OF-plain | 0 | 0 | 0 | 1.1 ± 0.3 | 0.9 ± 0.3 | 2.6 ± 0.5 |
| OF-BMP-2 | 0 | 0 | 0 | 2.6 ± 0.5 | 1 | 3 | |
| 16 weeks | OF-plain | 0 | 0 | 0 | 1.3 ± 0.4 | 1 | 2 ± 0.5 |
| OF-BMP-2 | 0 | 0 | 0 | 1.6 ± 0.5 | 1.1 ± 0.3 | 1.8 ± 0.7 |
3.2. Histologic Evaluation
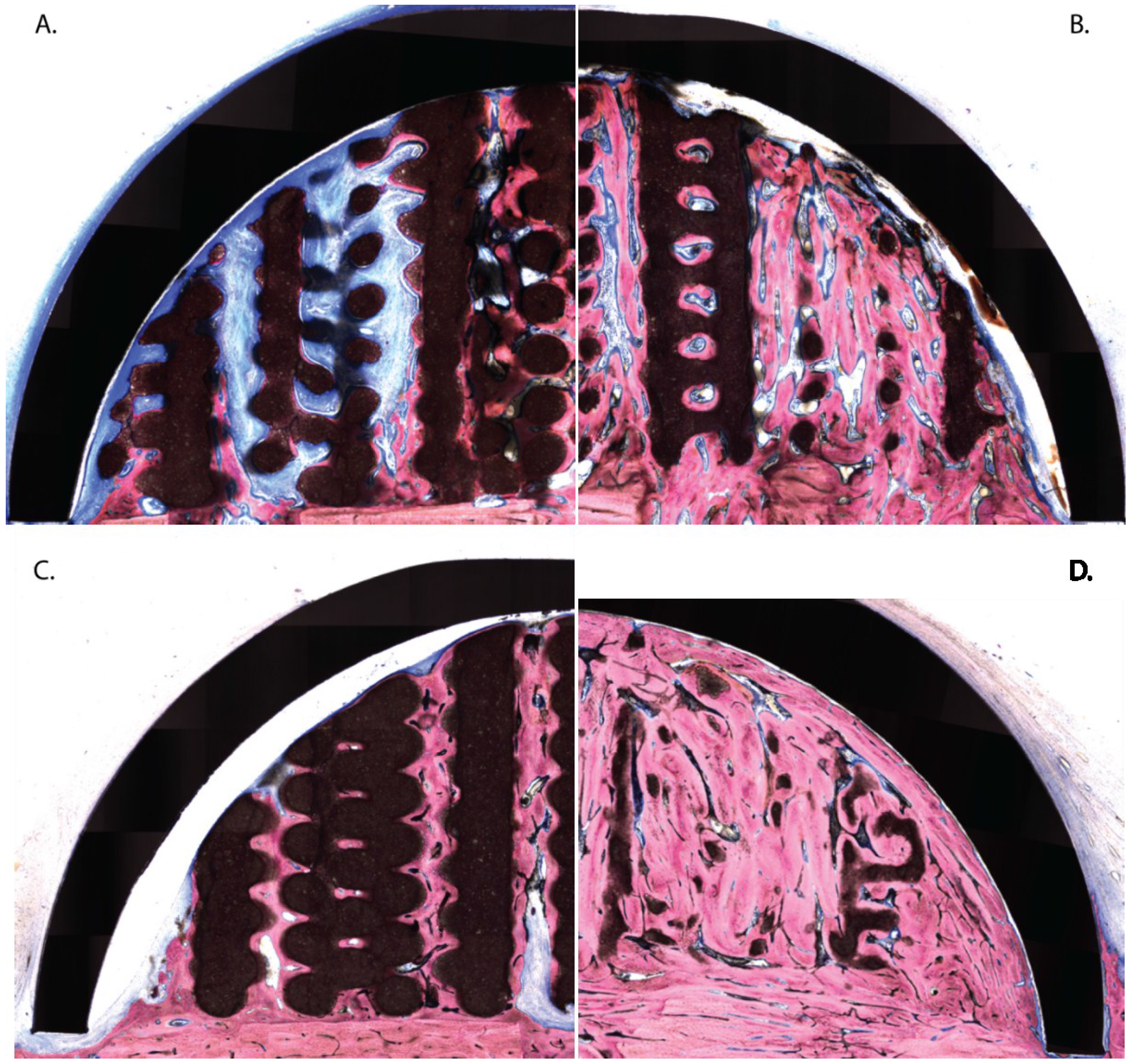
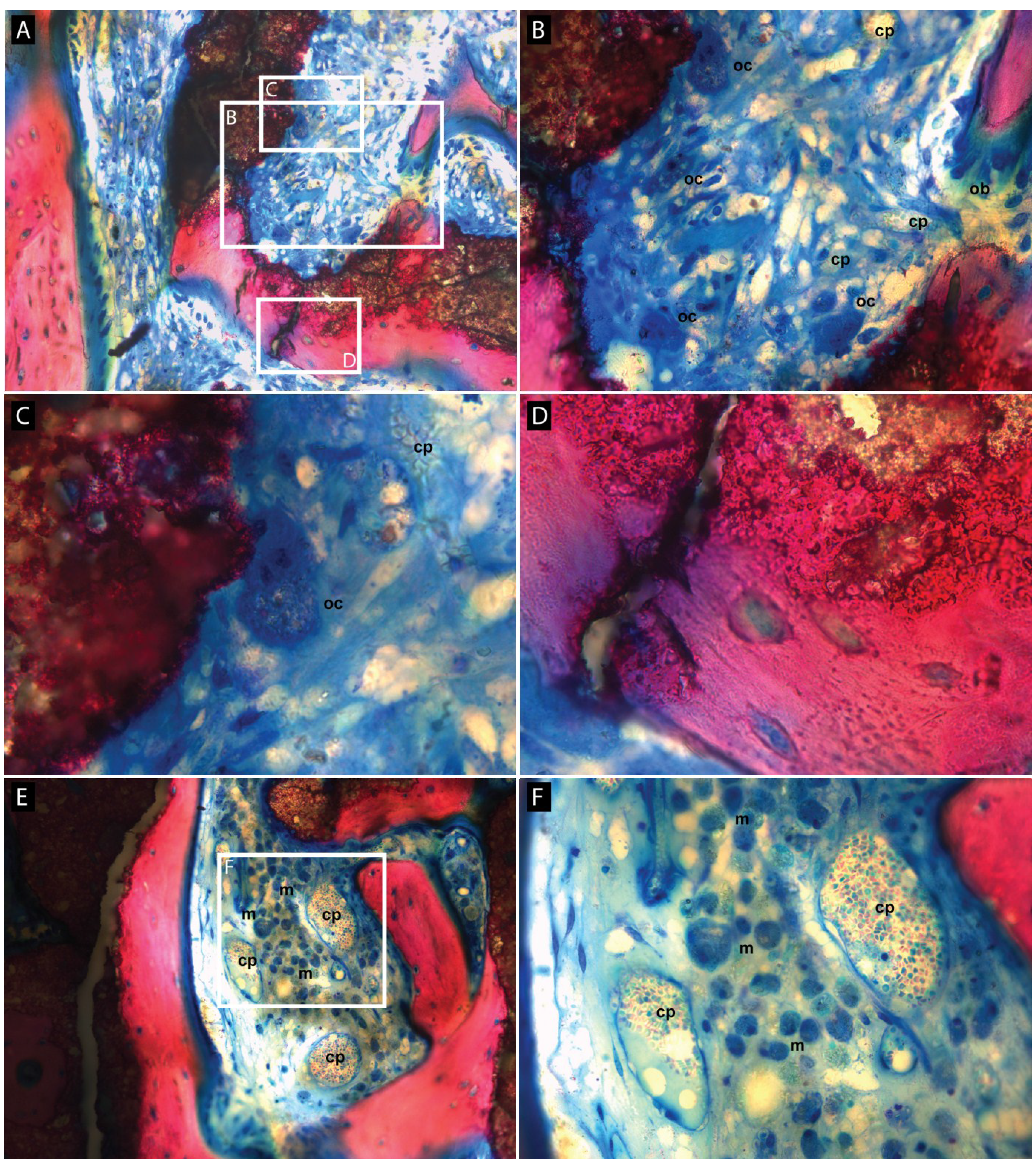
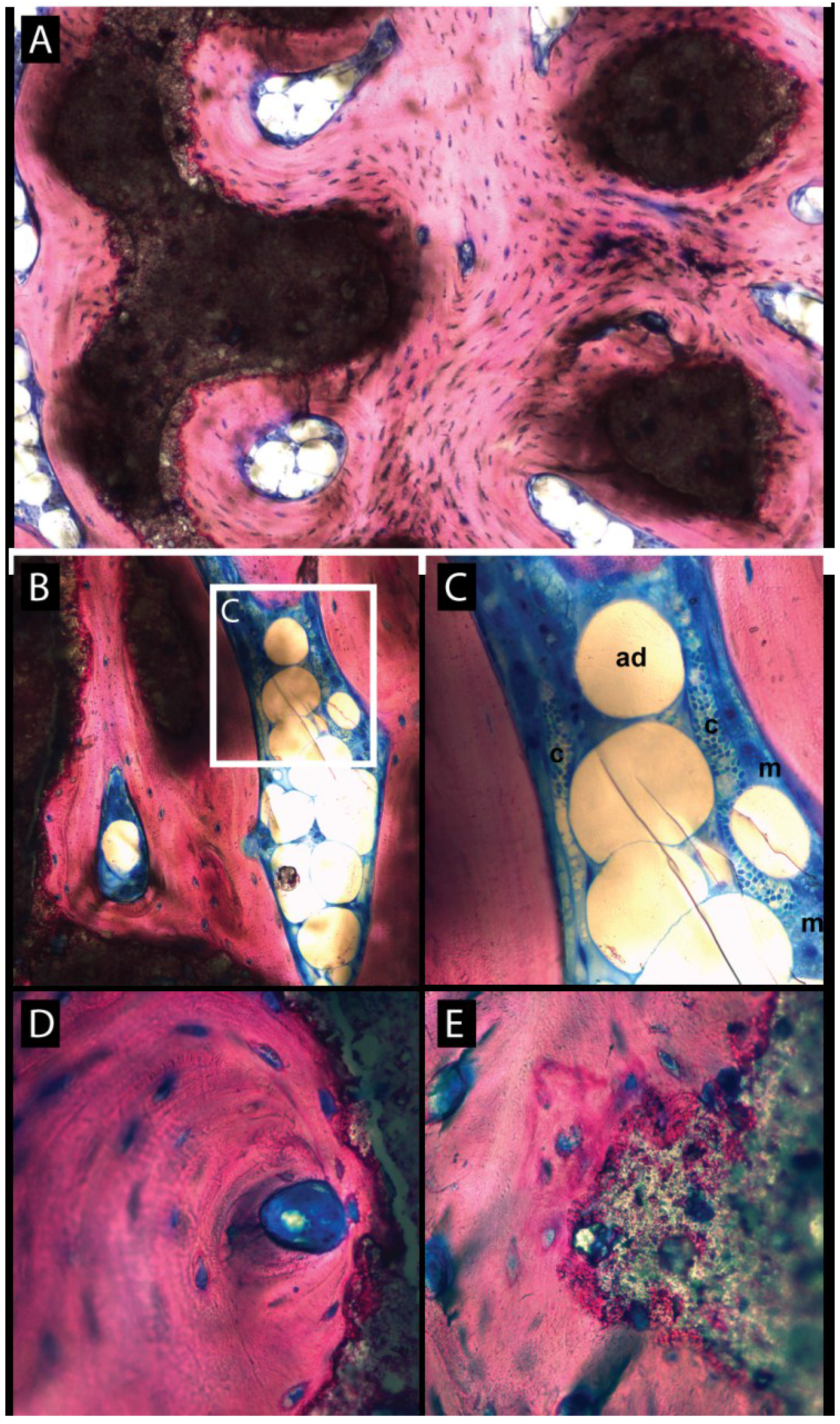
3.3. Histomorphometric Evaluation

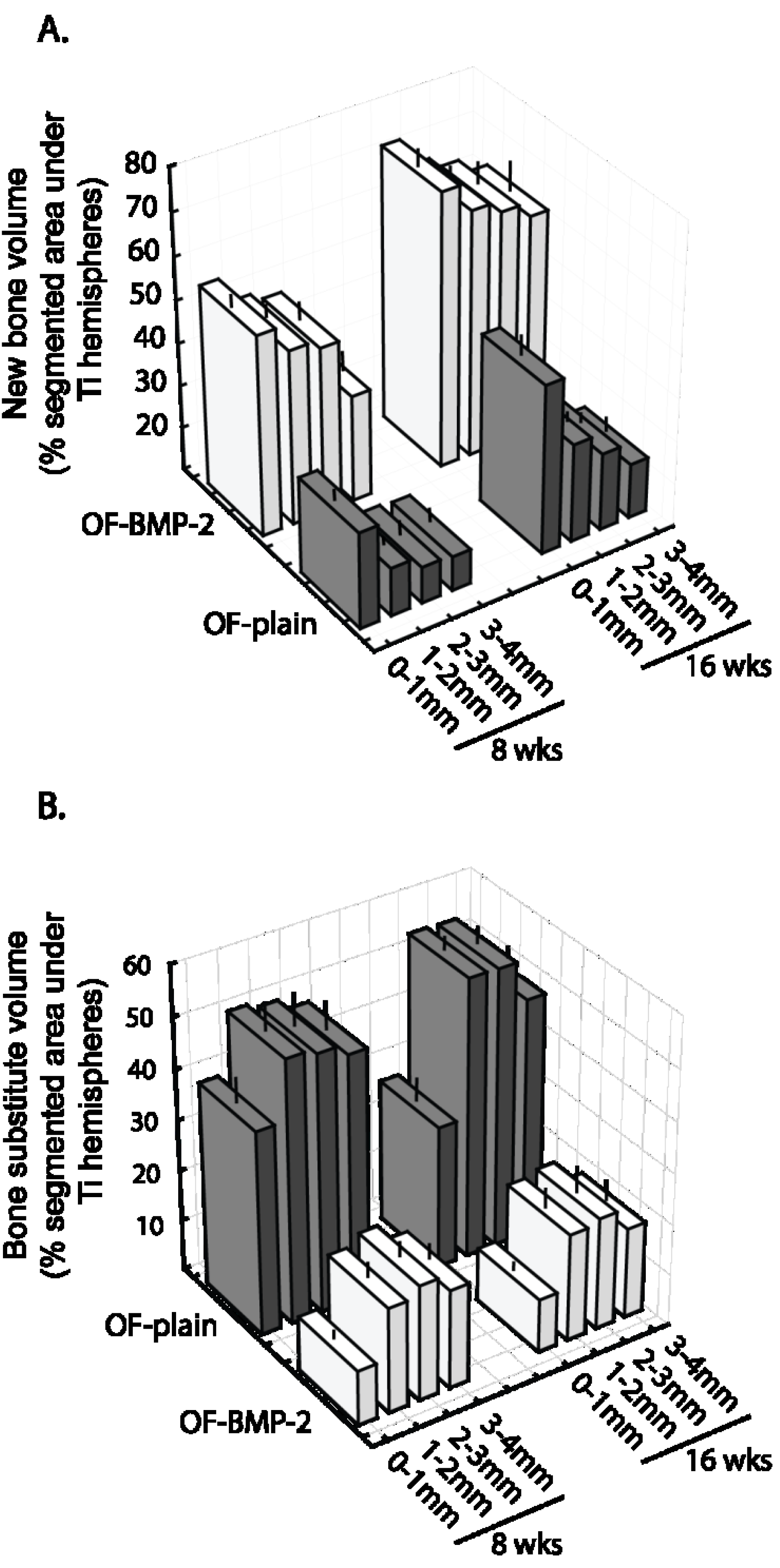
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Draenert, F.G.; Huetzen, D.; Neff, A.; Mueller, W.E. Vertical bone augmentation procedures: Basics and techniques in dental implantology. J. Biomed. Mater. Res. A 2014, 102, 1605–1613. [Google Scholar] [CrossRef]
- Sato, M.; Webster, T.J. Designing orthopedic implant surfaces: Harmonization of nanotopographical and chemical aspects. Nanomedicine (Lond.) 2006, 1, 351–354. [Google Scholar] [CrossRef]
- Fini, M.; Giardino, R.; Borsari, V.; Torricelli, P.; Rimondini, L.; Giavaresi, G.; Nicoli Aldini, N. In vitro behaviour of osteoblasts cultured on orthopaedic biomaterials with different surface roughness, uncoated and fluorohydroxyapatite-coated, relative to the in vivo osteointegration rate. Int. J. Artif. Organs 2003, 26, 520–528. [Google Scholar]
- Seitz, H.; Rieder, W.; Irsen, S.; Leukers, B.; Tille, C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 74, 782–788. [Google Scholar] [CrossRef]
- Hollister, S.J.; Lin, C.Y.; Saito, E.; Schek, R.D.; Taboas, J.M.; Williams, J.M.; Partee, B.; Flanagan, C.L.; Diggs, A.; Wilke, E.N.; et al. Engineering craniofacial scaffolds. Orthod Craniofac Res. 2005, 8, 162–173. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef]
- Barrere, F.; van Blitterswijk, C.A.; de Groot, K. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomed. 2006, 1, 317–332. [Google Scholar]
- Daculsi, G.; Laboux, O.; Malard, O.; Weiss, P. Current state of the art of biphasic calcium phosphate bioceramics. J. Mater. Sci. Mater. Med. 2003, 14, 195–200. [Google Scholar] [CrossRef]
- Daculsi, G. Biphasic calcium phosphate concept applied to artificial bone, implant coating and injectable bone substitute. Biomaterials 1998, 19, 1473–1478. [Google Scholar] [CrossRef]
- Carrel, J.P.; Wiskott, A.; Moussa, M.; Rieder, P.; Scherrer, S.; Durual, S. A 3D printed TCP/HA structure as a new osteoconductive scaffold for vertical bone augmentation. Clin. Oral Implants Res. 2014, 2014. [Google Scholar] [CrossRef]
- Carreira, A.C.; Lojudice, F.H.; Halcsik, E.; Navarro, R.D.; Sogayar, M.C.; Granjeiro, J.M. Bone morphogenetic proteins: Facts, challenges, and future perspectives. J. Dent Res. 2014, 93, 335–345. [Google Scholar] [CrossRef]
- Busenlechner, D.; Tangl, S.; Mair, B.; Fugger, G.; Gruber, R.; Redl, H.; Watzek, G. Simultaneous in vivo comparison of bone substitutes in a guided bone regeneration model. Biomaterials 2008, 29, 3195–3200. [Google Scholar] [CrossRef]
- ISO/IEC 17025:2005. In General Requirements for the Competence of Testing and Calibration Laboratories; International Organization for Standardization (ISO): Geneva, Switzerland, 2005.
- 2010/63/EU. In European Horizontal Legislation for the Protection of Animals Used for Scientific Purposes; European Commission: Brussels, Belgium, 2010.
- ISO 10993-6:2007. In Biological Evaluation of Medical Devices—Part 6: Tests for Local Effects after Implantation; International Organization for Standardization (ISO): Geneva, Switzerland, 2007.
- Yamada, Y.; Tamura, T.; Hariu, K.; Asano, Y.; Sato, S.; Ito, K. Angiogenesis in newly augmented bone observed in rabbit calvarium using a titanium cap. Clin. Oral Implants Res. 2008, 19, 1003–1009. [Google Scholar] [CrossRef]
- Yamada, Y.; Nanba, K.; Ito, K. Effects of occlusiveness of a titanium cap on bone generation beyond the skeletal envelope in the rabbit calvarium. Clin. Oral Implants Res. 2003, 14, 455–463. [Google Scholar] [CrossRef]
- Kuemmerle, J.M.; Oberle, A.; Oechslin, C.; Bohner, M.; Frei, C.; Boecken, I.; Von Rechenberg, B. Assessment of the suitability of a new brushite calcium phosphate cement for cranioplasty—An experimental study in sheep. J. Craniomaxillofac Surg. 2005, 33, 37–44. [Google Scholar] [CrossRef]
- Gosain, A.K.; Riordan, P.A.; Song, L.; Amarante, M.T.; Kalantarian, B.; Nagy, P.G.; Wilson, C.R.; Toth, J.M.; McIntyre, B.L. A 1-year study of osteoinduction in hydroxyapatite-derived biomaterials in an adult sheep model: Part ii. Bioengineering implants to optimize bone replacement in reconstruction of cranial defects. Plast Reconstr. Surg. 2004, 114, 1155–1163. [Google Scholar] [CrossRef]
- Eufinger, H.; Rasche, C.; Lehmbrock, J.; Wehmoller, M.; Weihe, S.; Schmitz, I.; Schiller, C.; Epple, M. Performance of functionally graded implants of polylactides and calcium phosphate/calcium carbonate in an ovine model for computer assisted craniectomy and cranioplasty. Biomaterials 2007, 28, 475–485. [Google Scholar] [CrossRef]
- Artzi, Z.; Weinreb, M.; Givol, N.; Rohrer, M.D.; Nemcovsky, C.E.; Prasad, H.S.; Tal, H. Biomaterial resorption rate and healing site morphology of inorganic bovine bone and beta-tricalcium phosphate in the canine: A 24-month longitudinal histologic study and morphometric analysis. Int. J. Oral Maxillofac Implants 2004, 19, 357–368. [Google Scholar]
- Walker, D.H.; Wright, N.M. Bone morphogenetic proteins and spinal fusion. Neurosurg. Focus 2002, 13, e3. [Google Scholar] [CrossRef]
- Ritting, A.W.; Weber, E.W.; Lee, M.C. Exaggerated inflammatory response and bony resorption from bmp-2 use in a pediatric forearm nonunion. J. Hand. Surg. Am. 2012, 37, 316–321. [Google Scholar] [CrossRef]
- Perri, B.; Cooper, M.; Lauryssen, C.; Anand, N. Adverse swelling associated with use of RH-BMP-2 in anterior cervical discectomy and fusion: A case study. Spine J. 2007, 7, 235–239. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, T.H.; Lee, J.H. Creating growth factor gradients in three dimensional porous matrix by centrifugation and surface immobilization. Biomaterials 2011, 32, 8254–8260. [Google Scholar] [CrossRef]
- Hagi, T.T.; Wu, G.; Liu, Y.; Hunziker, E.B. Cell-mediated BMP-2 liberation promotes bone formation in a mechanically unstable implant environment. Bone 2010, 46, 1322–1327. [Google Scholar] [CrossRef]
- Lu, J.; Descamps, M.; Dejou, J.; Koubi, G.; Hardouin, P.; Lemaitre, J.; Proust, J.P. The biodegradation mechanism of calcium phosphate biomaterials in bone. J. Biomed. Mater. Res. 2002, 63, 408–412. [Google Scholar] [CrossRef]
- Kamitakahara, M.; Ohtsuki, C.; Miyazaki, T. Review paper: Behavior of ceramic biomaterials derived from tricalcium phosphate in physiological condition. J. Biomater. Appl. 2008, 23, 197–212. [Google Scholar] [CrossRef]
- Kamiya, N.; Ye, L.; Kobayashi, T.; Mochida, Y.; Yamauchi, M.; Kronenberg, H.M.; Feng, J.Q.; Mishina, Y. Bmp signaling negatively regulates bone mass through sclerostin by inhibiting the canonical wnt pathway. Development 2008, 135, 3801–3811. [Google Scholar] [CrossRef]
- Glass, D.A., 2nd; Bialek, P.; Ahn, J.D.; Starbuck, M.; Patel, M.S.; Clevers, H.; Taketo, M.M.; Long, F.; McMahon, A.P.; Lang, R.A.; et al. Canonical wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell 2005, 8, 751–764. [Google Scholar] [CrossRef]
- Kaneko, H.; Arakawa, T.; Mano, H.; Kaneda, T.; Ogasawara, A.; Nakagawa, M.; Toyama, Y.; Yabe, Y.; Kumegawa, M.; Hakeda, Y. Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of bmp receptors in mature osteoclasts. Bone 2000, 27, 479–486. [Google Scholar] [CrossRef]
- Itoh, K.; Udagawa, N.; Katagiri, T.; Iemura, S.; Ueno, N.; Yasuda, H.; Higashio, K.; Quinn, J.M.; Gillespie, M.T.; Martin, T.J.; et al. Bone morphogenetic protein 2 stimulates osteoclast differentiation and survival supported by receptor activator of nuclear factor-kappab ligand. Endocrinology 2001, 142, 3656–3662. [Google Scholar]
- Kim, R.Y.; Oh, J.H.; Lee, B.S.; Seo, Y.K.; Hwang, S.J.; Kim, I.S. The effect of dose on rhBMP-2 signaling, delivered via collagen sponge, on osteoclast activation and in vivo bone resorption. Biomaterials 2014, 35, 1869–1881. [Google Scholar] [CrossRef]
- Robert, L.; Jilka, T.B.; Almeid, M.; Plotkin, L.I.; O’Brien, C.A.; Manolagas, R.S.W.A.S.C. Chapter 13. Apoptosis of bone cells. In Principles of Bone Biology; Bilezikian, J.P., Raisz, L.G., Martin, T.J., Eds.; Academic Press: Elsevier, Amsterdam, The Netherlands, 2008. [Google Scholar]
- Chen, Z.; Wu, C.; Gu, W.; Klein, T.; Crawford, R.; Xiao, Y. Osteogenic differentiation of bone marrow MSCs by beta-tricalcium phosphate stimulating macrophages via BMP2 signalling pathway. Biomaterials 2013, 35, 1507–1518. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moussa, M.; Carrel, J.-P.; Scherrer, S.; Cattani-Lorente, M.; Wiskott, A.; Durual, S. Medium-Term Function of a 3D Printed TCP/HA Structure as a New Osteoconductive Scaffold for Vertical Bone Augmentation: A Simulation by BMP-2 Activation. Materials 2015, 8, 2174-2190. https://doi.org/10.3390/ma8052174
Moussa M, Carrel J-P, Scherrer S, Cattani-Lorente M, Wiskott A, Durual S. Medium-Term Function of a 3D Printed TCP/HA Structure as a New Osteoconductive Scaffold for Vertical Bone Augmentation: A Simulation by BMP-2 Activation. Materials. 2015; 8(5):2174-2190. https://doi.org/10.3390/ma8052174
Chicago/Turabian StyleMoussa, Mira, Jean-Pierre Carrel, Susanne Scherrer, Maria Cattani-Lorente, Anselm Wiskott, and Stéphane Durual. 2015. "Medium-Term Function of a 3D Printed TCP/HA Structure as a New Osteoconductive Scaffold for Vertical Bone Augmentation: A Simulation by BMP-2 Activation" Materials 8, no. 5: 2174-2190. https://doi.org/10.3390/ma8052174






