The Effect of Exogenous Zinc Concentration on the Responsiveness of MC3T3-E1 Pre-Osteoblasts to Surface Microtopography: Part I (Migration)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
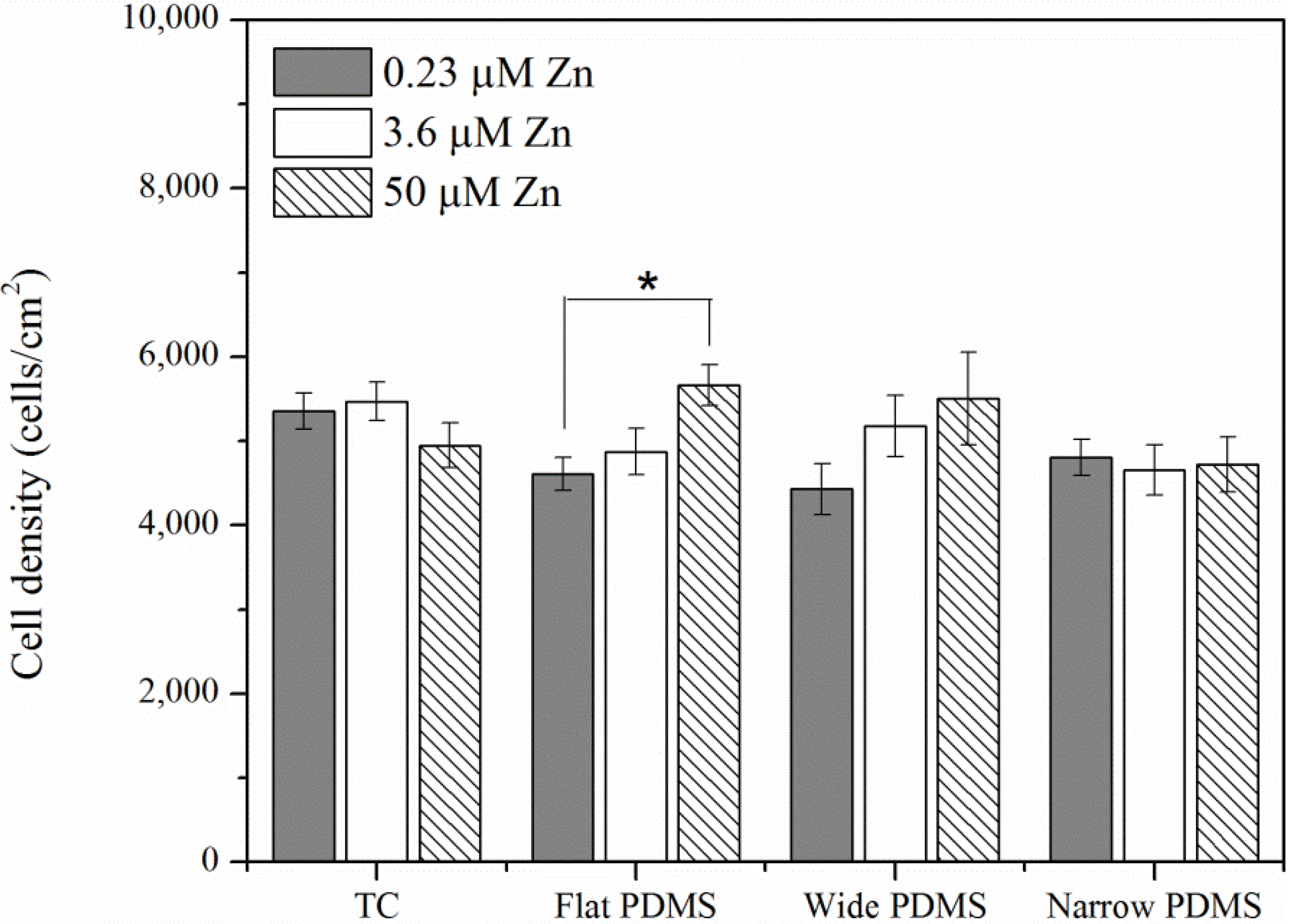
2.1.1. Cell Coverage
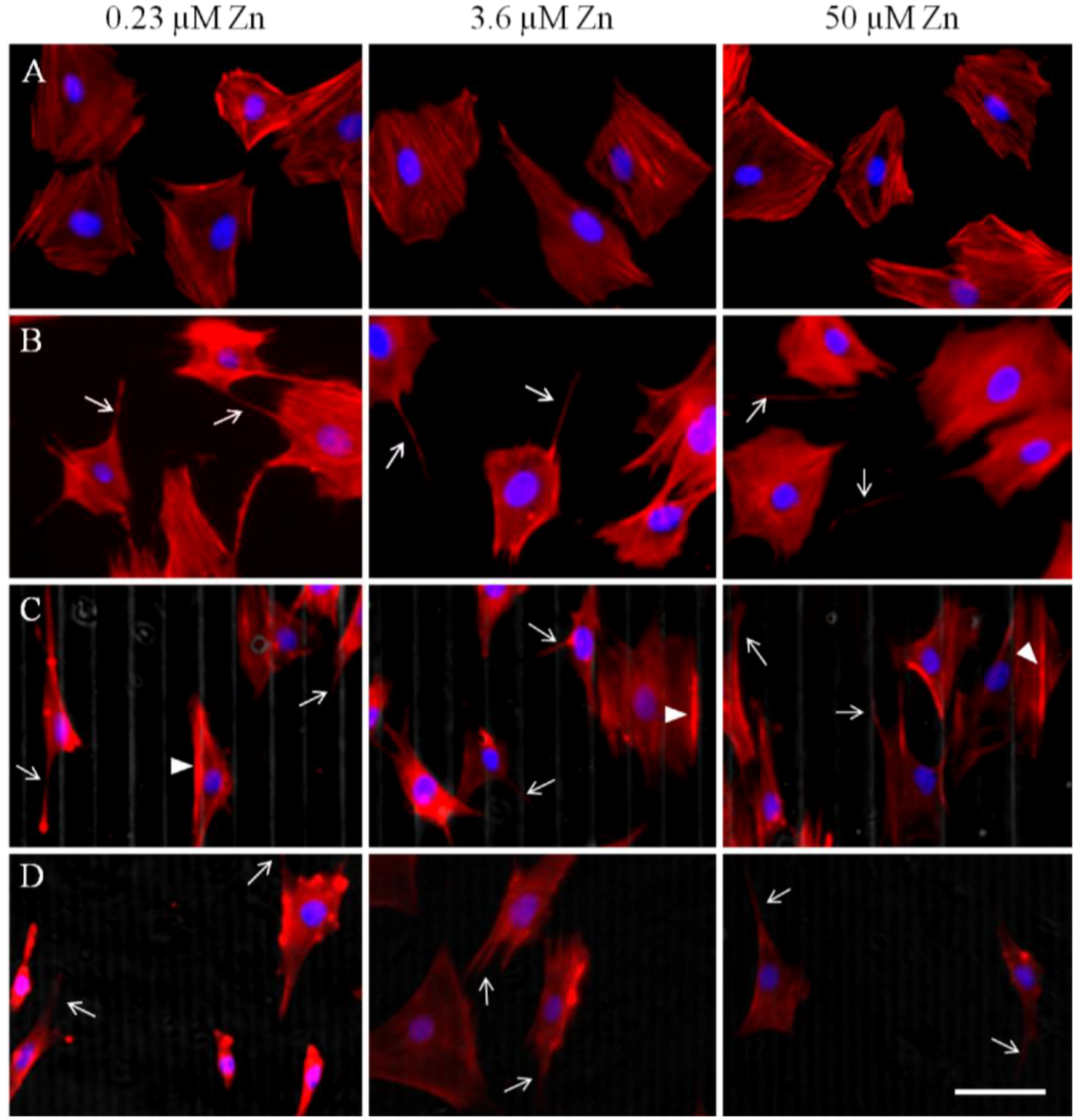
2.1.2. Actin Analysis and Morphology

2.1.3. Cell Migration
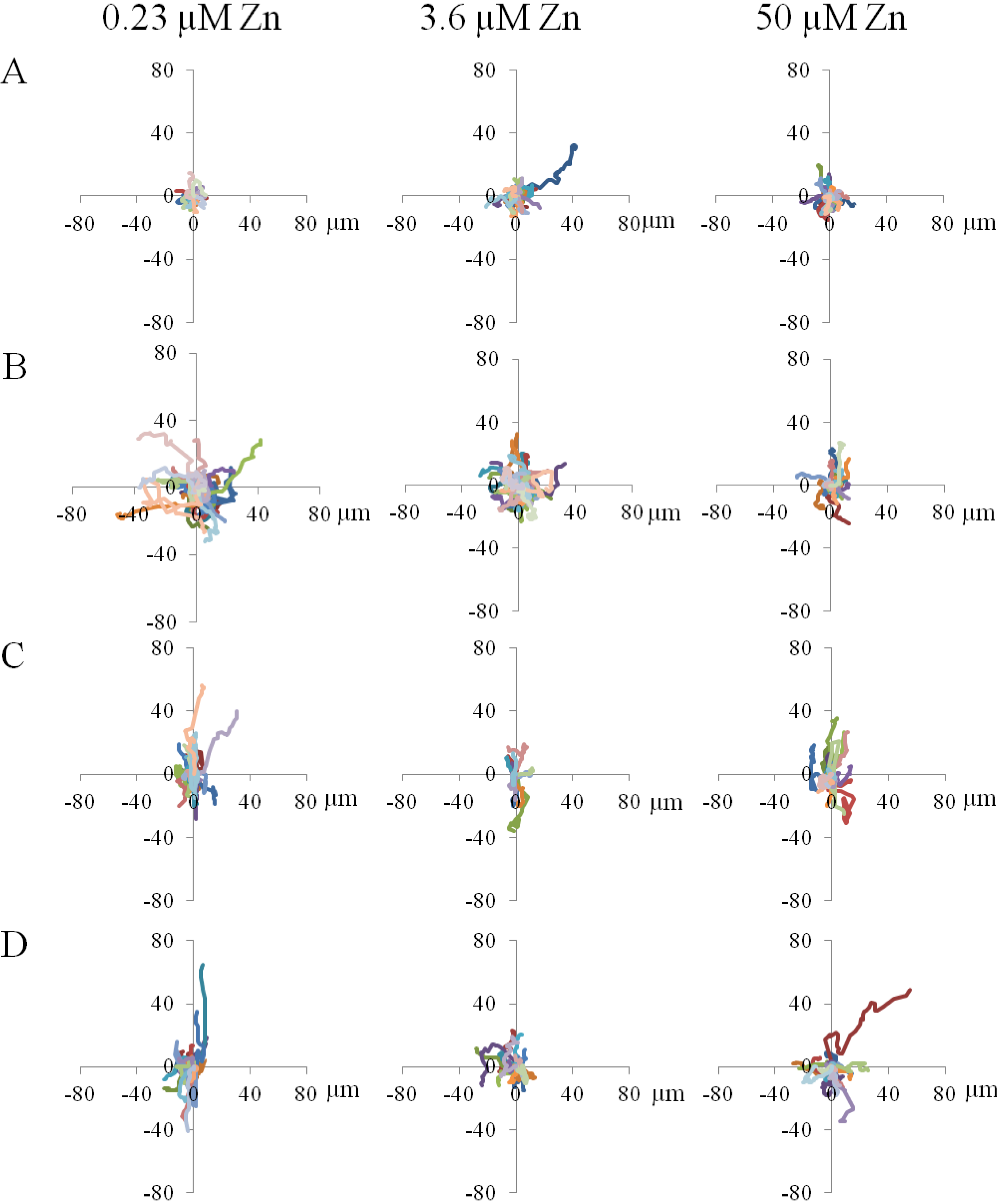
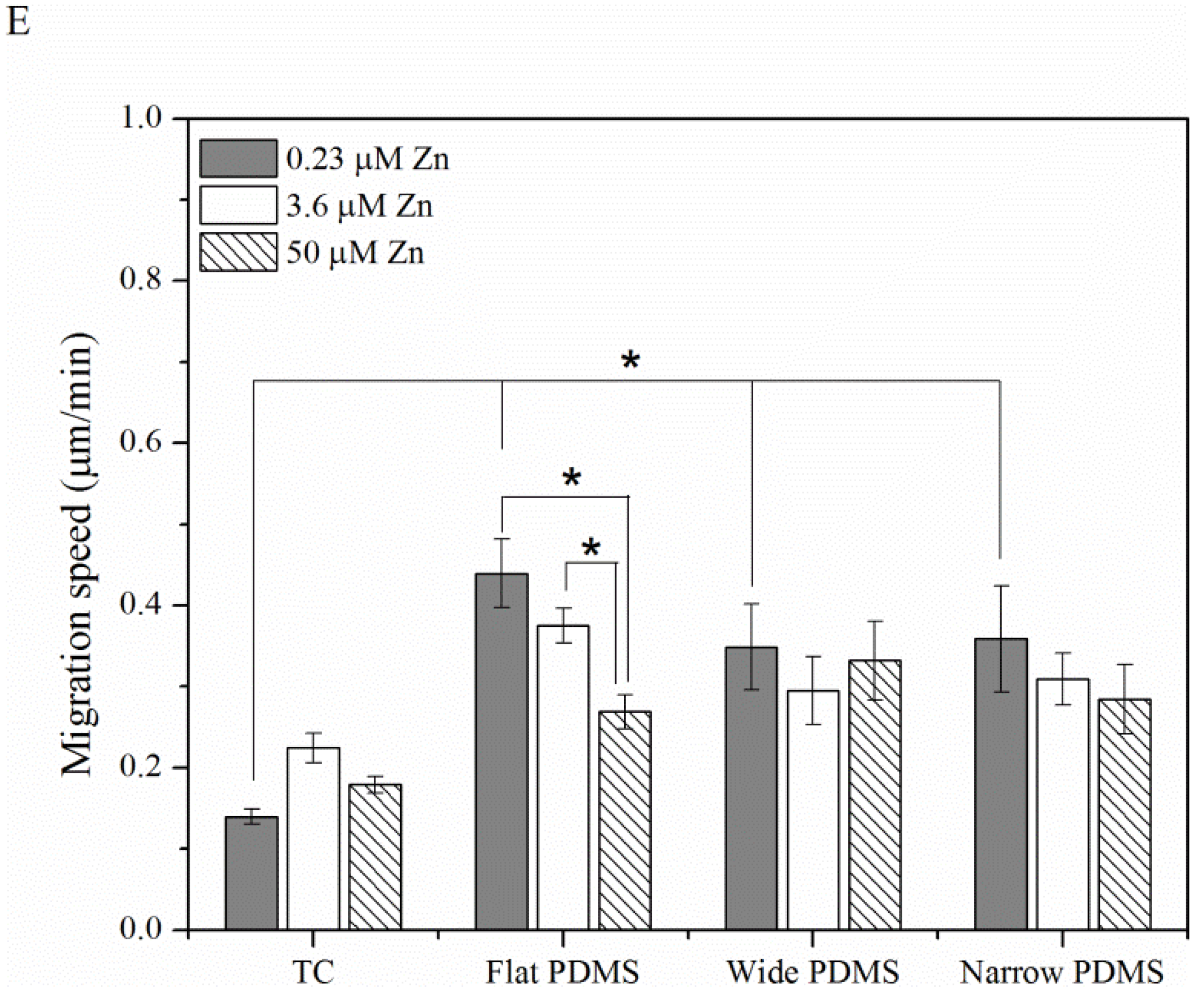
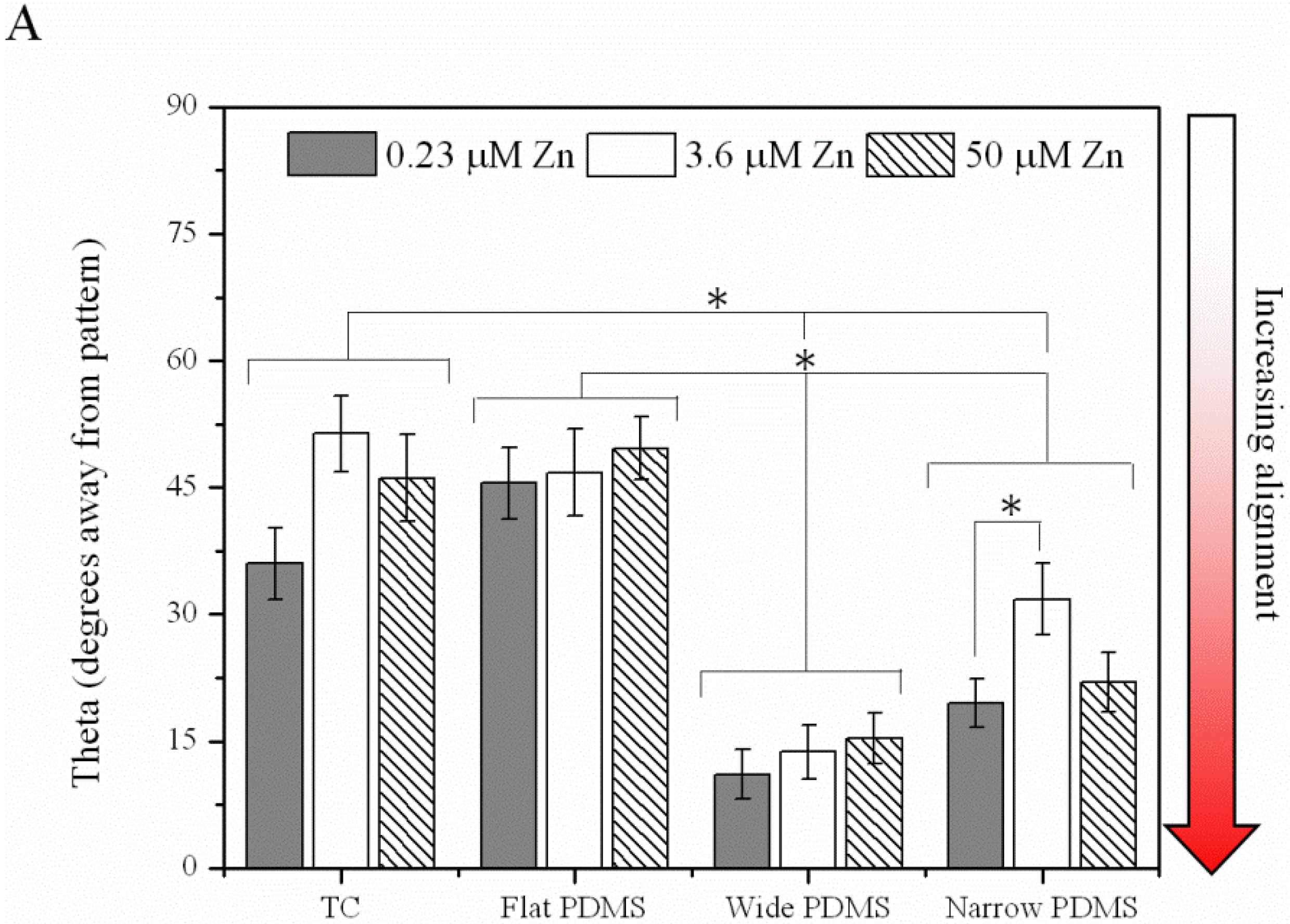
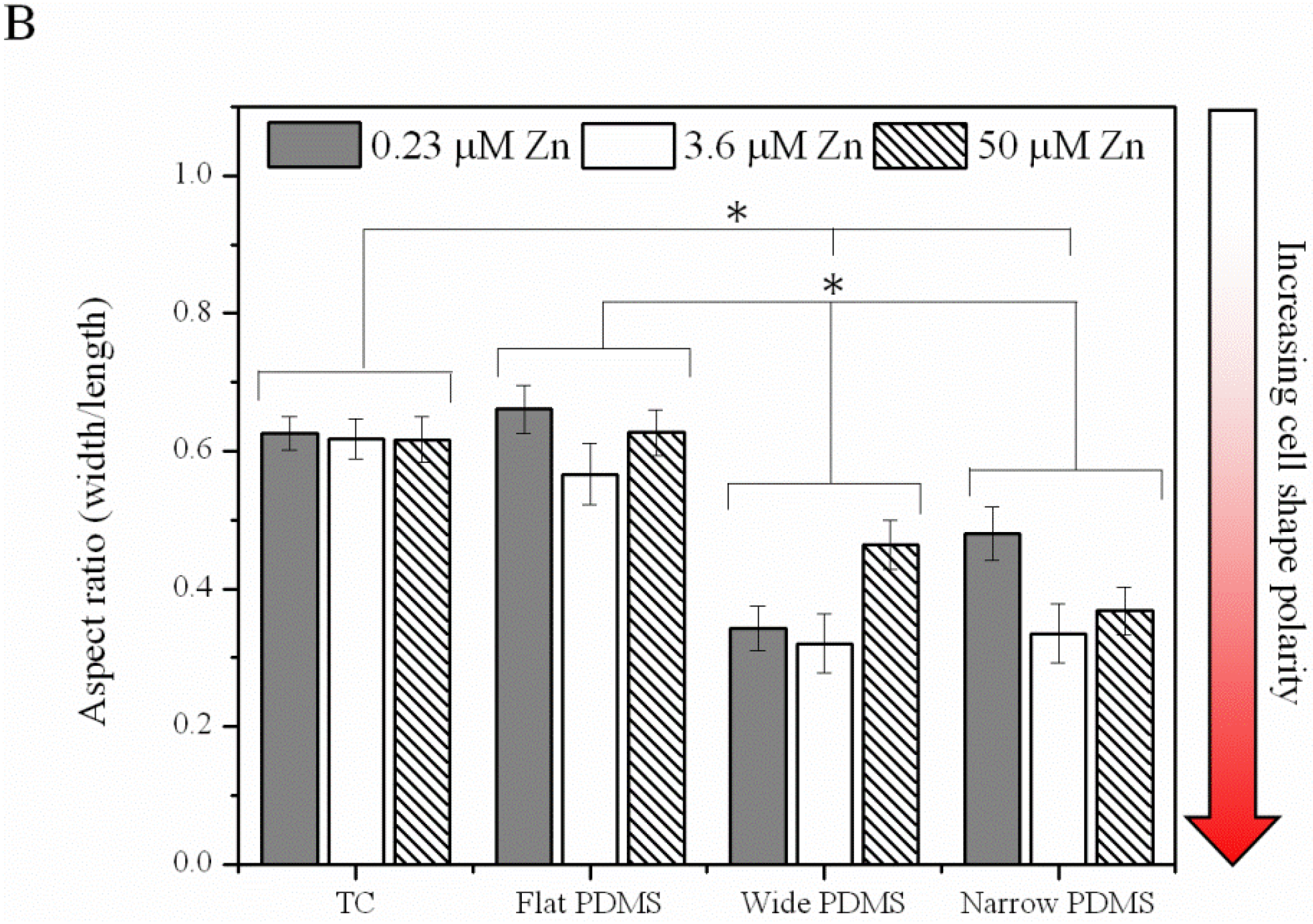
2.1.4. Contact Guidance
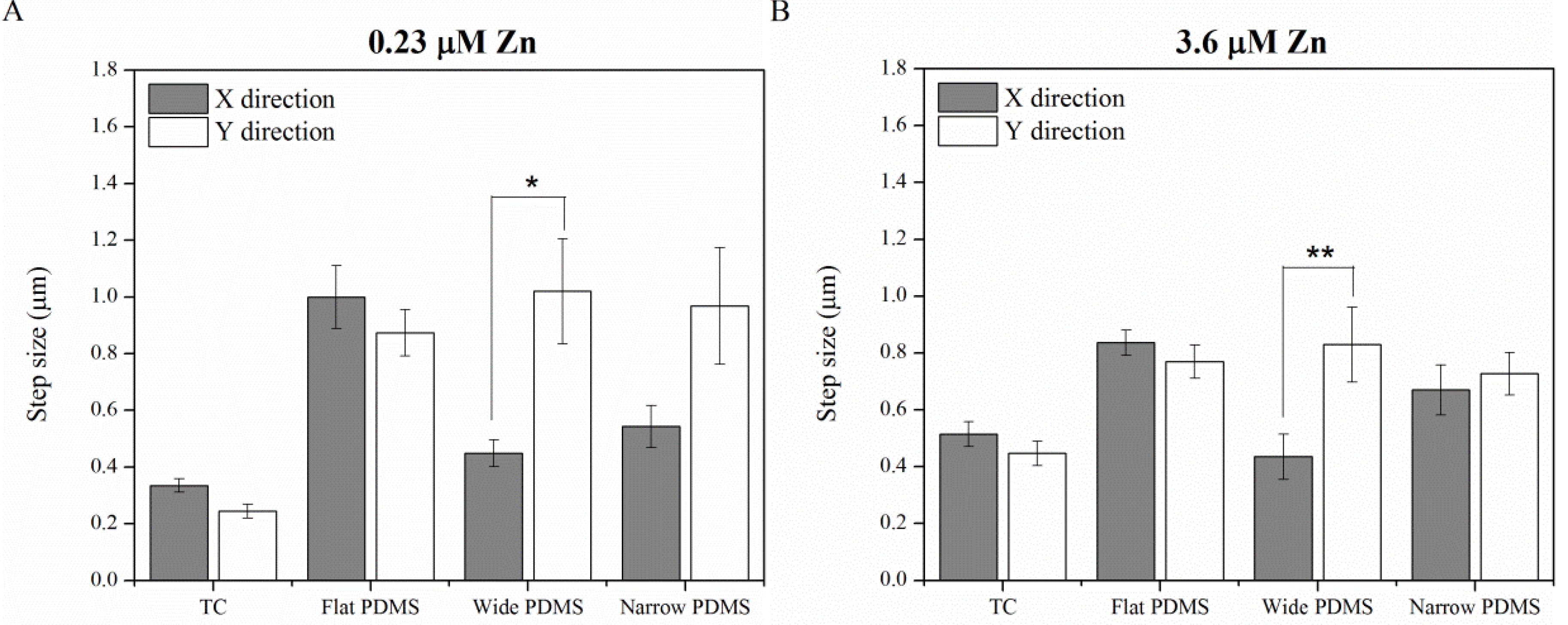

2.2. Discussion
3. Experimental Section
3.1. Microfabrication
3.2. Substrate Preparation
3.3. Cell Culture and Development
3.4. Preparation of Zinc-Deficient and Zinc-Rich Medium
3.5. Cell Coverage
3.6. Actin Analysis
3.7. Cell Migration: Time-Lapse Microscopy
3.8. Numerical Analysis of Migration
3.9. Statistics
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Cao, Y.; Chen, J.; Adeoye, M.O.; Soboyejo, W.O. Investigation of the spreading and adhesion of human osteosarcoma cells on smooth and micro-grooved polydimethylsiloxane surfaces. Mater. Sci. Eng. C 2009, 29, 119–125. [Google Scholar]
- Cavalcanti-Adam, E.A.; Aydin, D.; Hirschfeld-Warneken, V.C.; Spatz, J.P. Cell adhesion and response to synthetic nanopatterned environments by steering receptor clustering and spatial location. HFSP J. 2008, 2, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Wong, P.K.; Park, J.; Levchenko, A.; Sun, Y. Microengineered platforms for cell mechanobiology. Annu. Rev. Biomed. Eng. 2009, 11, 203–233. [Google Scholar] [CrossRef] [PubMed]
- Chiquet, M. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biol. 1999, 18, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Bruckner-Tuderman, L.; von der Mark, K.; Pihlajaniemi, T.; Unsicker, K. Cell interactions with the extracellular matrix. Cell Tissue Res. 2010, 339, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Malizos, K.N.; Papatheodorou, L.K. The healing potential of the periosteum: Molecular aspects. Injury 2005, 36, S13–S19. [Google Scholar] [CrossRef] [PubMed]
- Baksh, D.; Song, L.; Tuan, R.S. Adult mesenchymal stem cells: Characterization, differentiation, and application in cell and gene therapy. J. Cell. Mol. Med. 2004, 8, 301–316. [Google Scholar] [CrossRef]
- Eghbali-Fatourechi, G.Z.; Lamsam, J.; Fraser, D.; Nagel, D.; Riggs, B.L.; Khosla, S. Circulating osteoblast-lineage cells in humans. N. Engl. J. Med. 2005, 352, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Rumi, M.N.; Deol, G.S.; Singapuri, K.P.; Pellegrini, V.D., Jr. The origin of osteoprogenitor cells responsible for heterotopic ossification following hip surgery: An animal model in the rabbit. J. Orthop. Res. 2005, 23, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Ichida, M.; Yui, Y.; Yoshioka, K.; Tanaka, T.; Wakamatsu, T.; Yoshikawa, H.; Itoh, K. Changes in cell migration of mesenchymal cells during osteogenic differentiation. FEBS Lett. 2011, 585, 4018–4024. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.; Engel, E.; Lopez-Iglesias, C.; Mills, C.A.; Planell, J.A.; Samitier, J. Focused ion beam/scanning electron microscopy characterization of cell behavior on polymer micro-/nanopatterned substrates: A study of cell-substrate interactions. Micron 2008, 39, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Su, W.T.; Yang, J.Y.; Lin, C.D.; Chu, I.M. Control cell behavior on physical topographical surface. Jpn. J. Appl. Phys. 2004, 43, 3806–3809. [Google Scholar] [CrossRef]
- Lim, J.Y.; Dreiss, A.D.; Zhou, Z.Y.; Hansen, J.C.; Siedlecki, C.A.; Hengstebeck, R.W.; Cheng, J.; Winograd, N.; Donahue, H.J. The regulation of integrin-mediated osteoblast focal adhesion and focal adhesion kinase expression by nanoscale topography. Biomaterials 2007, 28, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Soboyejo, W.O. Cell/surface interactions of human osteo-sarcoma (HOS) cells and micro-patterned polydimelthylsiloxane (PDMS) surfaces. Mater. Sci. Eng. C 2009, 29, 2011–2018. [Google Scholar] [CrossRef]
- Soboyejo, W.; Nemetski, B.; Allameh, S.; Marcantonio, N.; Mercer, C.; Ricci, J. Interactions between MC3T3-E1 cells and textured Ti6Al4V surfaces. J. Biomed. Mater. Res. 2002, 62, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Zahor, D.; Radko, A.; Vago, R.; Gheber, L.A. Organization of mesenchymal stem cells is controlled by micropatterned silicon substrates. Mater. Sci. Eng. C 2007, 27, 117–121. [Google Scholar] [CrossRef]
- Wilkinson, C.D.W.; Riehle, M.; Wood, M.; Gallagher, J.; Curtis, A.S.G. The use of materials patterned on a nano- and micro-metric scale in cellular engineering. Mater. Sci. Eng. C 2002, 19, 263–269. [Google Scholar] [CrossRef]
- Ohara, P.T.; Buck, R.C. Contact guidance in vitro: A light, transmission, and scanning electron microscopic study. Exp. Cell Res. 1979, 121, 235–249. [Google Scholar] [PubMed]
- Wojciak-Stothard, B.; Madeja, Z.; Korohoda, W.; Curtis, A.; Wilkinson, C. Activation of macrophage-like cells by multiple grooved substrata. Topographical control of cell behavior. Cell Biol. Int. 1995, 19, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, R.B. A generalized transport model for biased cell migration in an anisotropic environment. J. Math. Biol. 2000, 40, 97–135. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell migration: Integrating signals from front to back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef]
- Boyan, B.D.; Batzer, R.; Kieswetter, K.; Liu, Y.; Cochran, D.L.; Szmuckler-Moncler, S.; Dean, D.D.; Schwartz, Z. Titanium surface roughness alters responsiveness of MG63 osteoblast-like cells to 1 alpha,25-(OH)2D3. J. Biomed. Mater. Res. 1998, 39, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Aaseth, J.; Boivin, G.; Andersen, O. Osteoporosis and trace elements—An overview. J. Trace Elem. Med. Biol. 2012, 26, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Fukada, T.; Yamasaki, S.; Nishida, K.; Murakami, M.; Hirano, T. Zinc homeostasis and signaling in health and diseases: Zinc signaling. J. Biol. Inorg. Chem. 2011, 16, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Fukada, T.; Hojyo, S.; Furuichi, T. Zinc signal: A new player in osteobiology. J. Bone Miner. Metab. 2013, 31, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Nutritional factors and bone homeostasis: Synergistic effect with zinc and genistein in osteogenesis. Mol. Cell. Biochem. 2012, 366, 201–221. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, E.H.; Lomeda, R.A.; Feldmann, J.; Nixon, G.F.; Beattie, J.H.; Kwun, I.S. Zinc deprivation inhibits extracellular matrix calcification through decreased synthesis of matrix proteins in osteoblasts. Mol. Nutr. Food Res. 2011, 55, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Migliaccio, S.; Corsi, A.; Marzia, M.; Bianco, P.; Teti, A.; Gambelli, L.; Cianfarani, S.; Paoletti, F.; Branca, F. Reduced growth and skeletal changes in zinc-deficient growing rats are due to impaired growth plate activity and inanition. J. Nutr. 2001, 131, 1142–1146. [Google Scholar] [PubMed]
- Sariahmetoglu, M.; Crawford, B.D.; Leon, H.; Sawicka, J.; Li, L.; Ballermann, B.J.; Holmes, C.; Berthiaume, L.G.; Holt, A.; Sawicki, G.; et al. Regulation of matrix metalloproteinase-2 (MMP-2) activity by phosphorylation. FASEB J. 2007, 21, 2486–2495. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.L.; Dimai, H.P.; Farley, J.R. Effects of zinc on human skeletal alkaline phosphatase activity in vitro. Calcif. Tissue Int. 1999, 64, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Hiscox, S.; Nicholson, R.I.; Hogstrand, C.; Kille, P. Protein kinase CK2 triggers cytosolic zinc signaling pathways by phosphorylation. Sci. Signal. 2012, 5, ra11. [Google Scholar] [PubMed]
- Taylor, K.M.; Kille, P.; Hogstrand, C. Protein kinase CK2 opens the gate for zinc signaling. Cell Cycle 2012, 11, 1863–1864. [Google Scholar] [CrossRef] [PubMed]
- Houard, X.; Germain, S.; Gervais, M.; Michaud, A.; van den Brule, F.; Foidart, J.M.; Noel, A.; Monnot, C.; Corvol, P. Migration-stimulating factor displays HEXXH-dependent catalytic activity important for promoting tumor cell migration. Int. J. Cancer 2005, 116, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Fukada, T.; Civic, N.; Furuichi, T.; Shimoda, S.; Mishima, K.; Higashiyama, H.; Idaira, Y.; Asada, Y.; Kitamura, H.; Yamasaki, S.; et al. The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; its involvement in BMP/TGF-beta signaling pathways. PLoS One 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Hojyo, S.; Fukada, T.; Shimoda, S.; Ohashi, W.; Bin, B.H.; Koseki, H.; Hirano, T. The zinc transporter SLC39A14/ZIP14 controls G-protein coupled receptor-mediated signaling required for systemic growth. PLoS One 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Matsuda, K.; Itoh, M.; Kawaguchi, H.; Tomoike, H.; Aoyagi, T.; Nagai, R.; Hori, M.; Nakamura, Y.; Tanaka, T. Osteopenia and male-specific sudden cardiac death in mice lacking a zinc transporter gene, Znt5. Hum. Mol. Genet. 2002, 11, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Hie, M.; Iitsuka, N.; Otsuka, T.; Nakanishi, A.; Tsukamoto, I. Zinc deficiency decreases osteoblasts and osteoclasts associated with the reduced expression of Runx2 and RANK. Bone 2011, 49, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Azuma, Y.; Fukuyama, R.; Hattori, Y.; Yoshida, C.; Koida, M.; Ogita, K.; Komori, T. Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. J. Cell Biol. 2004, 166, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, E.; Klvacova, L.; John, S.; Cervinka, M. Zinc alters cytoskeletal integrity and migration in colon cancer cells. Acta Medica 2008, 51, 51–57. [Google Scholar] [PubMed]
- Lymburner, S.; McLeod, S.; Purtzki, M.; Roskelley, C.; Xu, Z. Zinc inhibits magnesium-dependent migration of human breast cancer MDA-MB-231 cells on fibronectin. J. Nutr. Biochem. 2013, 24, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Wojciak-Stothard, B.; Curtis, A.; Monaghan, W.; Macdonald, K.; Wilkinson, C. Guidance and activation of murine macrophages by nanometric scale topography. Exp. Cell Res. 1996, 223, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y. Studies on cell migration, adenylate cyclase and membrane-coating granules in the buccal epithelium of the zinc-deficient rabbit, including the influence of isoproterenol. Arch. Oral Biol. 1988, 33, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, S.; Hunley, S.C.; Pawelec, K.M.; Zhang, L.; Lee, I.; Baek, S.; Chan, C. Cell adhesive behavior on thin polyelectrolyte multilayers: Cells attempt to achieve homeostasis of its adhesion energy. Langmuir 2010, 26, 12794–12802. [Google Scholar] [CrossRef] [PubMed]
- Elineni, K.K.; Gallant, N.D. Regulation of cell adhesion strength by peripheral focal adhesion distribution. Biophys. J. 2011, 101, 2903–2911. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Mechanical control of tissue morphogenesis during embryological development. Int. J. Dev. Biol. 2006, 50, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Graille, M.; Pagano, M.; Rose, T.; Ravaux, M.R.; van Tilbeurgh, H. Zinc induces structural reorganization of gelatin binding domain from human fibronectin and affects collagen binding. Structure 2010, 18, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Depoortere, I. GI functions of GPR39: Novel biology. Curr. Opin. Pharmacol. 2012, 12, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.M.; Kim, S.H.; Kim, J.H.; Hwang, H.I. Hydrophilic surface modification of PDMS using atmospheric RF plasma. J. Phys. Conf. Ser. 2006, 34, 656. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc in human health: Effect of zinc on immune cells. Mol. Med. 2008, 14, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.E.; Lomeda, R.A.; Ryu, S.H.; Lee, J.H.; Beattie, J.H.; Kwun, I.S. Cellular Zn depletion by metal ion chelators (TPEN, DTPA and chelex resin) and its application to osteoblastic MC3T3-E1 cells. Nutr. Res. Pract. 2007, 1, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Byrne, H.; Drasdo, D. Individual-based and continuum models of growing cell populations: A comparison. J. Math. Biol. 2009, 58, 657–687. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dorst, K.; Rammelkamp, D.; Hadjiargyrou, M.; Gersappe, D.; Meng, Y. The Effect of Exogenous Zinc Concentration on the Responsiveness of MC3T3-E1 Pre-Osteoblasts to Surface Microtopography: Part I (Migration). Materials 2013, 6, 5517-5532. https://doi.org/10.3390/ma6125517
Dorst K, Rammelkamp D, Hadjiargyrou M, Gersappe D, Meng Y. The Effect of Exogenous Zinc Concentration on the Responsiveness of MC3T3-E1 Pre-Osteoblasts to Surface Microtopography: Part I (Migration). Materials. 2013; 6(12):5517-5532. https://doi.org/10.3390/ma6125517
Chicago/Turabian StyleDorst, Kathryn, Derek Rammelkamp, Michael Hadjiargyrou, Dilip Gersappe, and Yizhi Meng. 2013. "The Effect of Exogenous Zinc Concentration on the Responsiveness of MC3T3-E1 Pre-Osteoblasts to Surface Microtopography: Part I (Migration)" Materials 6, no. 12: 5517-5532. https://doi.org/10.3390/ma6125517





