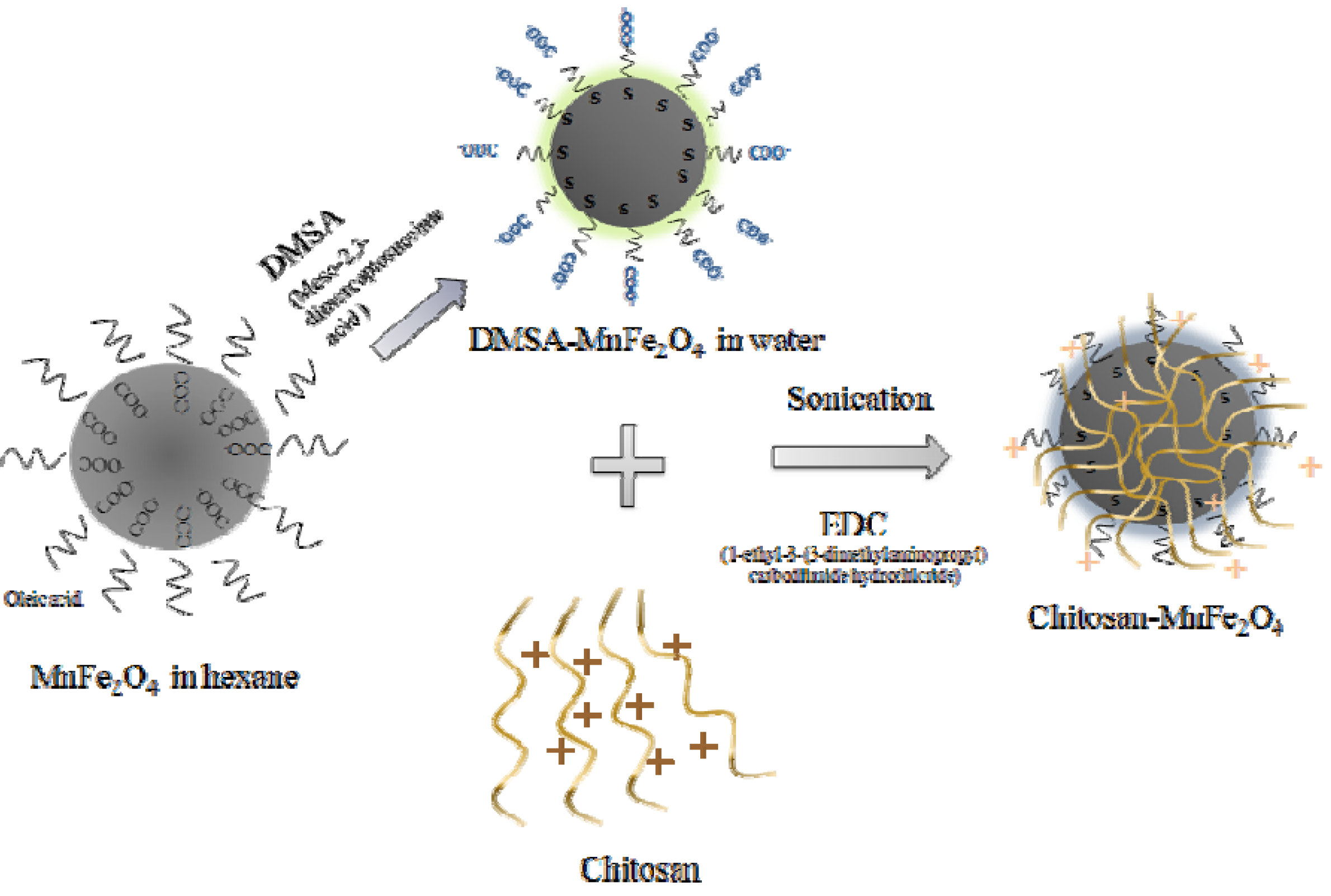
1. Introduction
Recently, interest in the use of magnetic nanoparticles (MNPs) for biomedical applications has increased due to their unique multifunctional properties. When the size of the magnetic particles decreases to below a critical size, generally less than 15 nm [
1], each nanoparticle becomes a single magnetic domain and exhibits remarkable superparamagnetic behavior similar to paramagnetism. Each individual nanoparticle has a large constant magnetic moment and behaves like a giant paramagnetic atom with a fast response to applied magnetic fields with negligible remanence and coercivity. These features make superparamagnetic nanoparticles attractive for a wide range of biomedical applications because the magnetization can be controlled by an external magnetic field without the risk of magnetic attraction at room temperature. By coating MNPs with a polymer, drug molecules can also be sequestered in the same device. Therefore, MNPs have been used for magnetic hyperthermia, triggered or localized drug delivery, magnetic resonance imaging and biosensors [
2,
3,
4,
5].
MNPs can be designed to heat when a high frequency magnetic field is applied. The heating, which has found uses in hyperthermia cancer treatment [
6,
7], is related to losses during the process of magnetization reversal within the particles. However, good control and optimization of the heating is needed to reach and maintain temperatures around 42 °C. By combining MNPs with targeting moieties, heating to thermal ablation temperatures may also be feasible. The design of MNPs with efficient heating profiles can minimize the quantity needed to reach these effective temperatures. Furthermore, including chemotherapy drugs with targeted MNPs may provide effective localized combination therapy, particularly for polymer membranes made of LCST polymers with imbedded magnetite which have been shown to deliver fluorescein in a pulsatile fashion [
8] or block copolymer micelles coated around gold or magnetic nanoparticles which show a squeezing effect in their hydrodynamic size with an increase in temperature [
9].
MnFe
2O
4 MNPs have a greater biocompatibility [
10] and a strong T
2 phase contrast for magnetic resonance imaging [
11,
12] compared with Fe
3O
4, γ-Fe
2O
3, CoFe
2O
4, and NiFe
2O
4 MNPs. Thus, MnFe
2O
4 MNPs can provide the multiple functionalities of imaging, hyperthermia and triggered drug release. The synthesis of monodisperse nanoparticles of Fe
3O
4, γ-Fe
2O
3 and MFe
2O
4 (where M=Co, Ni or Mn) developed by high temperature organic solution phase methods for have been produced and dispersed in various organic solvents [
13]. The magnetic susceptibility of MnFe
2O
4 nanoparticles is higher than for other ferrite nanoparticles such as Fe
3O
4, CoFe
2O
4 and NiFe
2O
4 with magnetic spins of 5 μ
b [
11]. However, a ligand exchange step is required to develop stable aqueous dispersions of MNPs for biomedical applications. These ligands may also facilitate conjugation with targeting biomolecules, drugs or functional polymers.
Coating MNPs with a biopolymer allows sequestration of drug molecules, improves biocompatibility and can offer chemistries to allow attachment of targeting moieties. Chitosan, a naturally-occurring polysaccharide, is used frequently in drug delivery and biomaterials applications. The primary amine groups in chitosan render special attributes for applications in biomedicine. Chitosan has a net positive charge and is mucoadhesive [
14], and has been found to be biocompatible with living tissue since it does not cause allergic reactions and rejection [
15], and has been proven safe in rats when given at up to 10% in the diet [
16]. Chitosan can also be easily crosslinked and modified into gels which can carry biologically-active molecules. Thus, chitosan has been used in conjunction with magnetic particles for magnetic drug targeting [
17,
18,
19,
20,
21]. This technology allows localized delivery as the coated MNPs concentrate drugs in the region where a constant, externally-applied magnetic field is applied. The synthesis of chitosan-coated MNPs has been shown using ionic gelation or using a crosslinking agent (glutaraldehyde) after making MNPs by coprecipitation [
22,
23]. However, these techniques usually yield micron-sized chitosan-coated MNPs.
Magnetic heating can be effectively achieved using an AC magnetic field. Heating is due to two different loss processes (Néel and Brownian relaxation). For superparamagnetic nanoparticles, the power loss is calculated from [
24]:
where m is the MNP magnetic moment, ω the field frequency, k is the Boltzmann constant, T is absolute temperature, H is the AC magnetic field amplitude, V is the nanoparticle volume and τ
eff is the effective relaxation time. When an AC magnetic field is applied to MNPs, their magnetic orientations attempt to align in the direction of the magnetic field. After the magnetic field is switched off, the total magnetization disappears due to the statistical random reorientation of the nanoparticles with an effective relaxation time (τ
eff ) that is a combination of Brownian relaxation (τ
B) and Néel relaxation (τ
N) [
25]:
The Brownian relaxation time constant can be found by:
where η is the carrier fluid viscosity, and V
H is the hydrodynamic volume of the MNP. Néel relaxation is given by:
where τ
0 is on the order of 10
-9 s, K is the anisotropy constant of the MNP, and V is the its volume.
The power loss equation (1) shows that magnetic field heating is a function of the material, MNP size, and the field characteristics. From equation (3), the effect of chitosan coating in MnFe
2O
4 MNPs on the heat generation could be described from the Brownian relaxation which depends on viscosity and hydrodynamic size. If the size of polymer coated MNPs increased above a critical size, Brownian relaxation dominates the effective relaxation time. However, for cases such as this experimental investigation, where the nanoparticles are quite small (12 nm) and they are constrained by the presence of a polymeric surface coating, heat generation in chitosan-MnFe
2O
4 MNPs is expected to depend on the Néel relaxation time. A recent study by Fortin
et al. [
26] has investigated the contributions of Brownian and Néel relaxation to the heating of maghemite (primarily Néel) and cobalt ferrite (primarily Brownian) MNPs, so the exact contributions to heating can vary, depending on the viscosity of the NP microenvironment.
In this study, multifunctional chitosan-MnFe2O4 MNPs were synthesized and characterized for hyperthermia and drug release behavior.
3. Results and Discussion
MnFe
2O
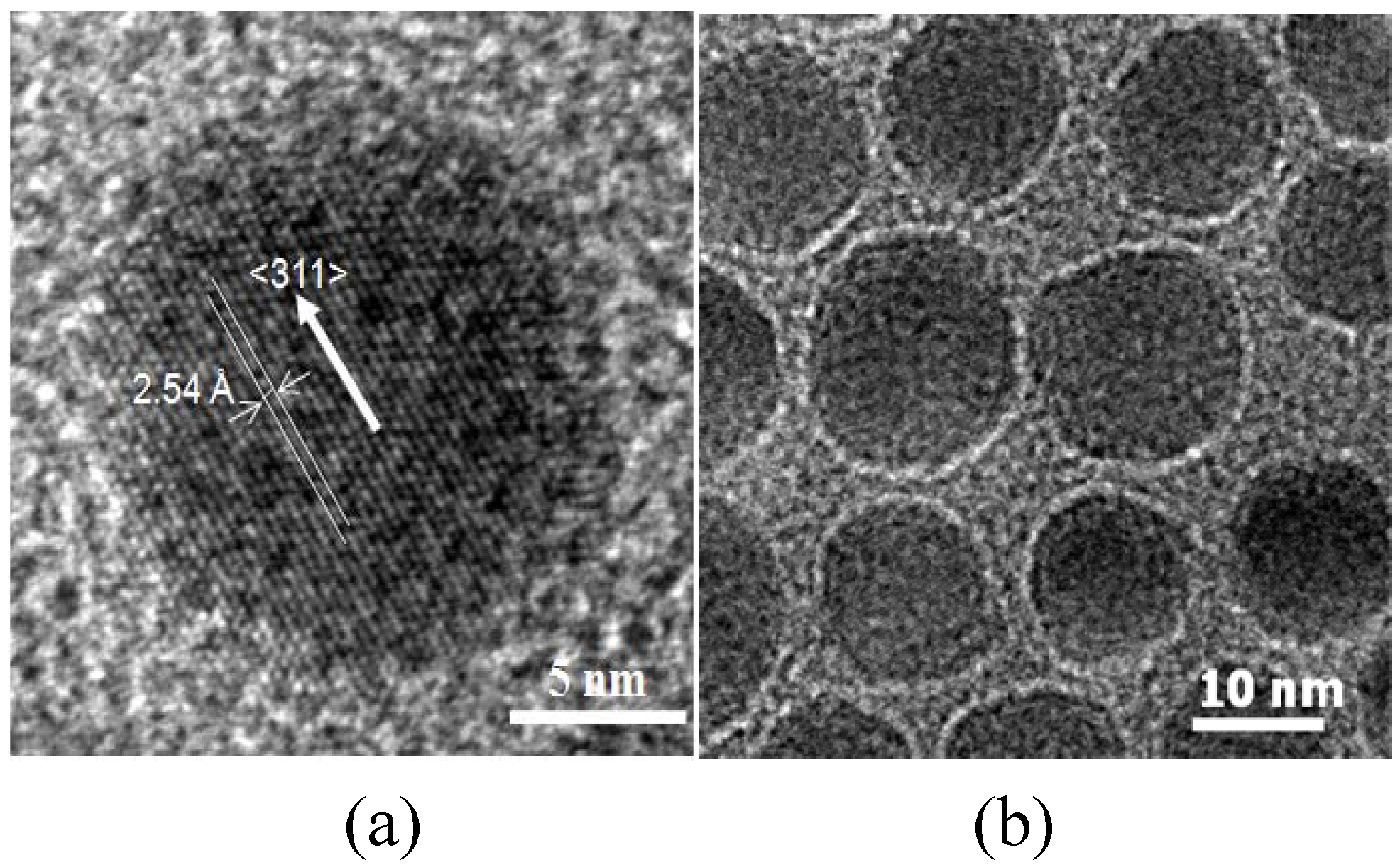
4 MNPs were synthesized by seed-mediated growth. The MNPs had a spinel crystal structure as ascertained by XRD. The MNPs were nearly spherical with an average particle size of 12.1 nm (±0.4 nm) (
Figure 2a). The HRTEM image (
Figure 2a) of MnFe
2O
4 nanoparticles shows lattice fringes with the interfringe distance measured to be 2.54 Å, close to the lattice spacing of the <311> planes at 2.56 Å in the cubic spinel structure of MnFe
2O
4. The MNPs were superparamagnetic with a saturation magnetization, M
s, of 44.1 emu/g. The chitosan coating on MNPs was characterized by TEM, FTIR, TGA and VSM. A layer of chitosan surrounding the core MnFe
2O
4 nanoparticles was observed in TEM images (
Figure 2b), indicating, that while the acetic acid present during the reaction could have also been coupled to chitosan through EDC, the chitosan has been successfully attached to the surface of the MnFe
2O
4 NPs, forming a thin layer of approximately 1 nm. The chitosan-coated MNPs had characteristic FTIR peaks at 1650 and 1560 cm
-1, corresponding to amide I and amide II bands, respectively (
Figure 3) [
29]. The height of these bands increased as the EDC used increased from 0.3 to 0.9 wt % but at 1.2 wt % EDC, these bands decreased to a level similar to 0.6 wt % EDC.
Figure 2.
TEM images of the synthesized (a) MnFe2O4 MNPs in high-resolution and (b) chitosan-MnFe2O4 MNPs.
Figure 2.
TEM images of the synthesized (a) MnFe2O4 MNPs in high-resolution and (b) chitosan-MnFe2O4 MNPs.
The DMSA-coated MNPs were stable in the pH range of 6-11. FTIR spectra confirm the bond of DMSA to MnFe
2O
4 MNPs, as bands at 1600 cm
-1 and 1383 cm
-1 were observed (
Figure 3a), characteristic of the carboxylic acid-iron bond expected. DMSA is a dithiol (containing two sulfhydryl, or S-H, groups) and an analogue of dimercaprol. This is a water-soluble, non-toxic, metal chelator which has been administered orally as an antidote to heavy metal toxicity since the 1950s [
30,
31]. Therefore, MnFe
2O
4 MNPs dispersed in water using DMSA have great potential for use
in vivo.
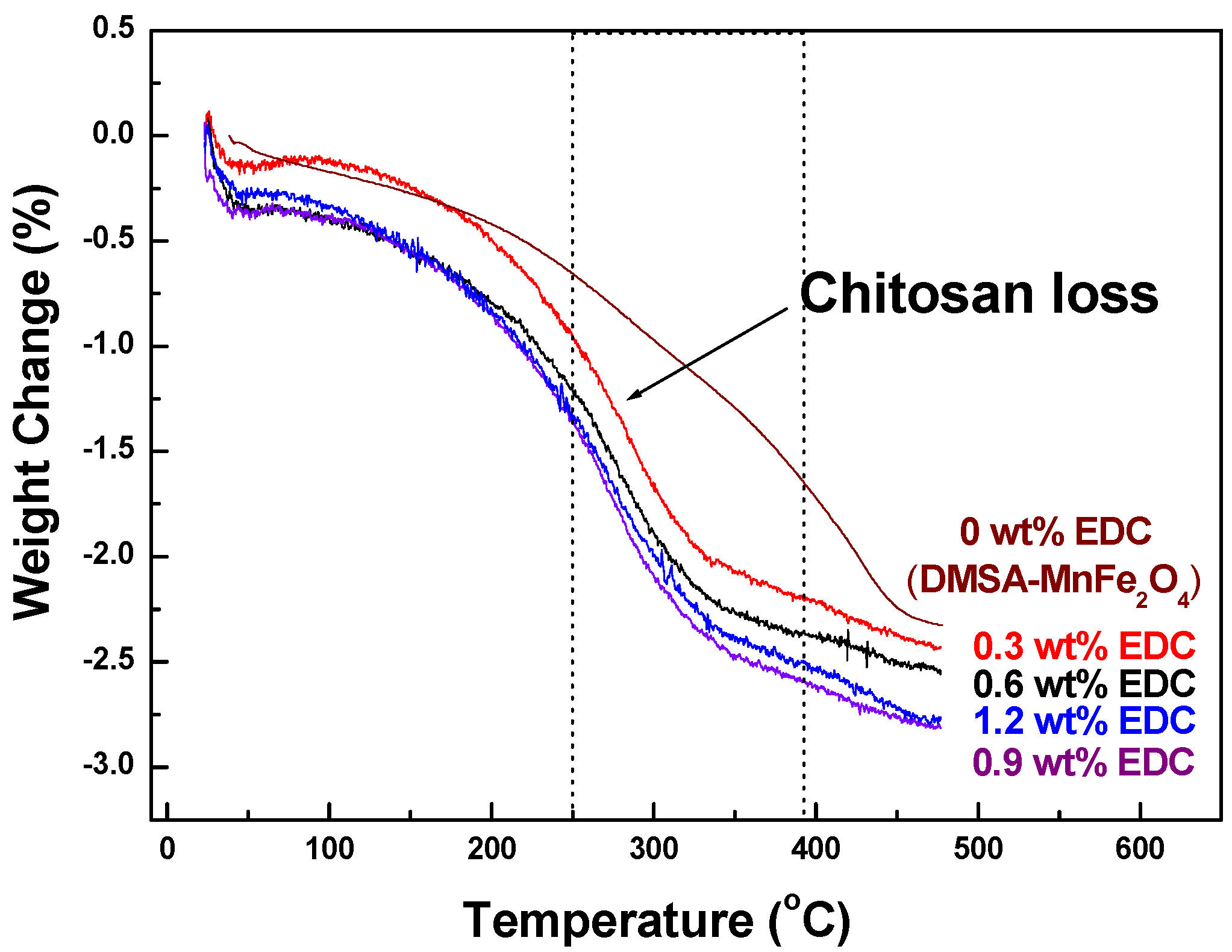
TGA thermograms measured the fraction of organic material (chitosan) on the MNPs. (
Figure 4). For MNPs with no chitosan, the weight of samples gradually decreased between room temperature and 440 °C. However, the characteristic weight loss of chitosan-MnFe
2O
4 MNPs started at 220 °C and continued up to 340 °C, during which there was from 2.03% to 2.39% weight loss due to the degradation of chitosan. The highest mass loss due to chitosan was seen for coated MNPs made with 0.9 wt % EDC, confirming the FTIR data.
Figure 3.
FTIR spectra of (a) DMSA-MnFe2O4 and (b) chitosan-MnFe2O4 MNPs.
Figure 3.
FTIR spectra of (a) DMSA-MnFe2O4 and (b) chitosan-MnFe2O4 MNPs.
Figure 4.
TGA curves of chitosan-MnFe2O4 MNPs synthesized using a range of EDC linker.
Figure 4.
TGA curves of chitosan-MnFe2O4 MNPs synthesized using a range of EDC linker.
The average sizes of chitosan-MnFe
2O
4 MNPs determined by DLS were much larger than observed using TEM, as also shown by Lopez-Cruz
et al. [
32]. The average size of chitosan-MnFe
2O
4 nanoparticles determined by TEM was approximately 18 nm but the diameter of chitosan-MnFe
2O
4 nanoparticles determined by DLS ranged from 94.4 to 104.2 nm, showing aggregation that is common in aqueous nanoparticle systems. In TEM, the chitosan-coated MNPs were spread out in a thin film and dried prior to measurement, while DLS measured particle size in solution. Thus, TEM was capable of distinguishing individually-coated MNPs, while DLS measured the aggregation of MNPs in solution. The hydrodynamic size of chitosan-MnFe
2O
4 was strongly dependent on the quantity of EDC used to attach chitosan (
Figure 5a). The hydrodynamic size of the samples increased from 94.4 to 104.2 nm of diameter as the EDC crosslinker was increased from 0.3-0.9 wt %.
Figure 5.
Average hydrodynamic size of chitosan-MnFe2O4 MNPs dependent on (a) the EDC concentration used during chitosan coating in DI water and (b) pH-dependent average hydrodynamic size of chitosan-MnFe2O4 MNPs at 0.9 wt % EDC. Error bars represent the standard deviation for three replicates. pH buffers (Metrepak pHydrion buffers, Brooklyn, NY) used include: pH 3 and 4: 0.05 M potassium biphthalate pH 7: 0.05 M phosphate; pH 9: 0.04 M sodium bicarbonate/sodium carbonate and pH 11: 0.03 M sodium bicarbonate/sodium carbonate.
Figure 5.
Average hydrodynamic size of chitosan-MnFe2O4 MNPs dependent on (a) the EDC concentration used during chitosan coating in DI water and (b) pH-dependent average hydrodynamic size of chitosan-MnFe2O4 MNPs at 0.9 wt % EDC. Error bars represent the standard deviation for three replicates. pH buffers (Metrepak pHydrion buffers, Brooklyn, NY) used include: pH 3 and 4: 0.05 M potassium biphthalate pH 7: 0.05 M phosphate; pH 9: 0.04 M sodium bicarbonate/sodium carbonate and pH 11: 0.03 M sodium bicarbonate/sodium carbonate.
Because chitosan has multiple amino groups, it is sensitive to the pH of the solution. It can ionize in buffered solutions, and has been shown to attach to DMSA-MNPs through electrostatic interactions [
33]. In our study, EDC was used as an additional linking agent to build a stronger bond between the MNPs and chitosan. In addition, sonication during the linking reaction broke the ionic interactions, to ensure that chitosan was linked to the MNPs through EDC. EDC reacts with a carboxyl to form an amine reactive O-acylisourea intermediate. If the intermediate does not encounter an amine, it will hydrolyze and regenerate the carboxyl group. Therefore, the amount of chitosan coating was limited by the amount of carboxyl groups on the DMSA-MnFe
2O
4 nanoparticles. Thus, 0.9 wt % EDC was optimal for maximizing chitosan attachment (
Figure 5a). As has been shown by Lopez-Cruz
et al. [
32], the presence of chitosan bound to the MNP surface help to stabilize the dispersion when in the presence of buffer ions, particularly phosphates. Here, we investigated the hydrodynamic particle size as a function of pH, as a potential way to trigger drug release. The average hydrodynamic diameter of the chitosan-MNPs was largest at pH 7 (
Figure 5b). A reduced electrostatic repulsion between the chitosan chains around the pK
a of 6.5 [
34] allowed greater agglomerations at neutral pH values despite chitosan being more expanded on a single nanoparticle when in acidic or basic solutions, as expected from swelling experiments on chitosan hydrogels [
35]. This pH-responsive behavior of chitosan makes it potentially useful in a pH-triggered drug delivery system.
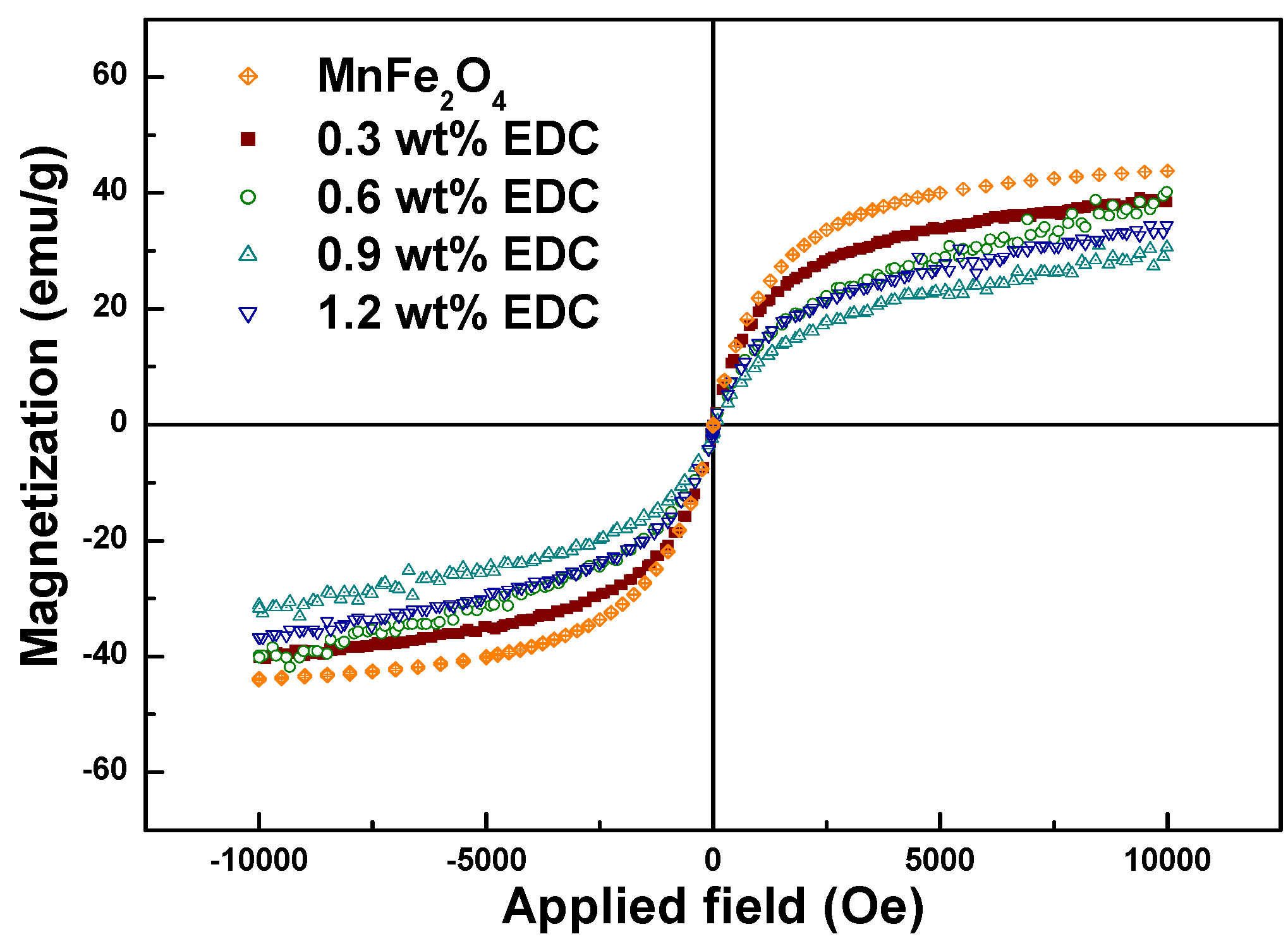
Magnetization values of chitosan-MNPs decreased slightly as the amount of EDC was increased (
Table 1). As shown in the FTIR, TGA and DLS analysis, the amount of EDC used to link chitosan to the MNPs controlled the coating thickness. Since the magnetization was normalized by the total sample weight, it decreased from 40.2 to 31.2 emu/g as the chitosan coating thickness increased (for EDC amounts ranging from 0.3 to 1.2 wt %) with the M
s of chitosan-MnFe
2O
4 nanoparticles synthesized using 0.9 wt % EDC 29.2% lower than that for uncoated MnFe
2O
4 MNPs (
Figure 6). The decrease of total magnetic moment for the chitosan-coated MNPs is likely due to a non-collinear spin structure originating from the pinning of the spins by polymer coating at the MNP surface [
36].
Table 1.
Properties of the synthesized MnFe2O4 nanoparticles. Values for SAR are reported for a 7 mg/mL or 0.349 wt % aqueous nanoparticle solution. Reported errors represent the standard deviations for three replicates.
Table 1.
Properties of the synthesized MnFe2O4 nanoparticles. Values for SAR are reported for a 7 mg/mL or 0.349 wt % aqueous nanoparticle solution. Reported errors represent the standard deviations for three replicates.
| Sample | EDC
Linker
Concen-
tration | TEM Particle
Diameter
(nm) | DLS Particle
Diameter
(nm) | Magnetization
at 10 kOe
(emu/g) | SAR (W/g) at 653 Oe, 266 kHz |
DMSA-
MnFe2O4 | - | 12.1 ± 0.4 | 34 ± 1.2 | 44.1 | 1.45 |
Chitosan-
MnFe2O4 | 0.3 wt % | 17.2 ± 1.3 | 94.4 ± 2.6 | 40.2 | 1.20 |
| 0.6 wt % | 17.7 ± 2.1 | 98.2 ± 3.2 | 39.8 | 1.17 |
| 0.9 wt % | 18.3 ± 1.6 | 104.2 ± 2.4 | 31.2 | 1.08 |
| 1.2 wt % | 17.1 ± 1.3 | 96.8 ± 2.2 | 36.7 | 1.15 |
Figure 6.
Magnetization curves for the DMSA-MnFe2O4 and chitosan-MnFe2O4 MNPs formed using different amounts of EDC.
Figure 6.
Magnetization curves for the DMSA-MnFe2O4 and chitosan-MnFe2O4 MNPs formed using different amounts of EDC.
The water-dispersed DMSA-MnFe
2O
4 nanoparticles, both with and without chitosan, were successfully heated using an AC magnetic field. In an earlier study [
35], heating of CoFe
2O
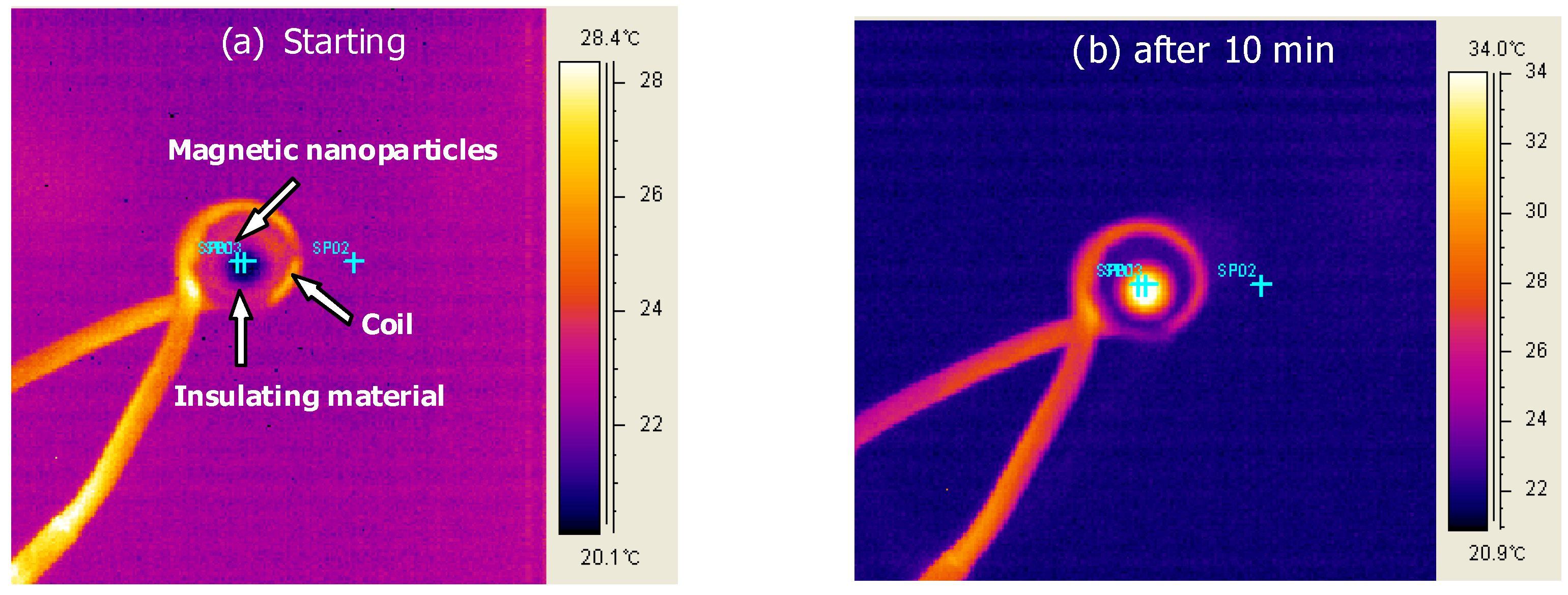
4 MNPs was controlled by the amplitude and frequency of an AC magnetic field. Here, the effect of a polymer coating on the MNPs was investigated. Images collected from the FLIR Thermacam
® show the experimental set-up and were used to measure the magnetically-induced heat generation (
Figure 7).
Figure 7.
FLIR Thermacam® images taken (a) at starting and (b) after 10 minutes for the heating of magnetic nanoparticles under a 653 Oe (266KHz) field.
Figure 7.
FLIR Thermacam® images taken (a) at starting and (b) after 10 minutes for the heating of magnetic nanoparticles under a 653 Oe (266KHz) field.
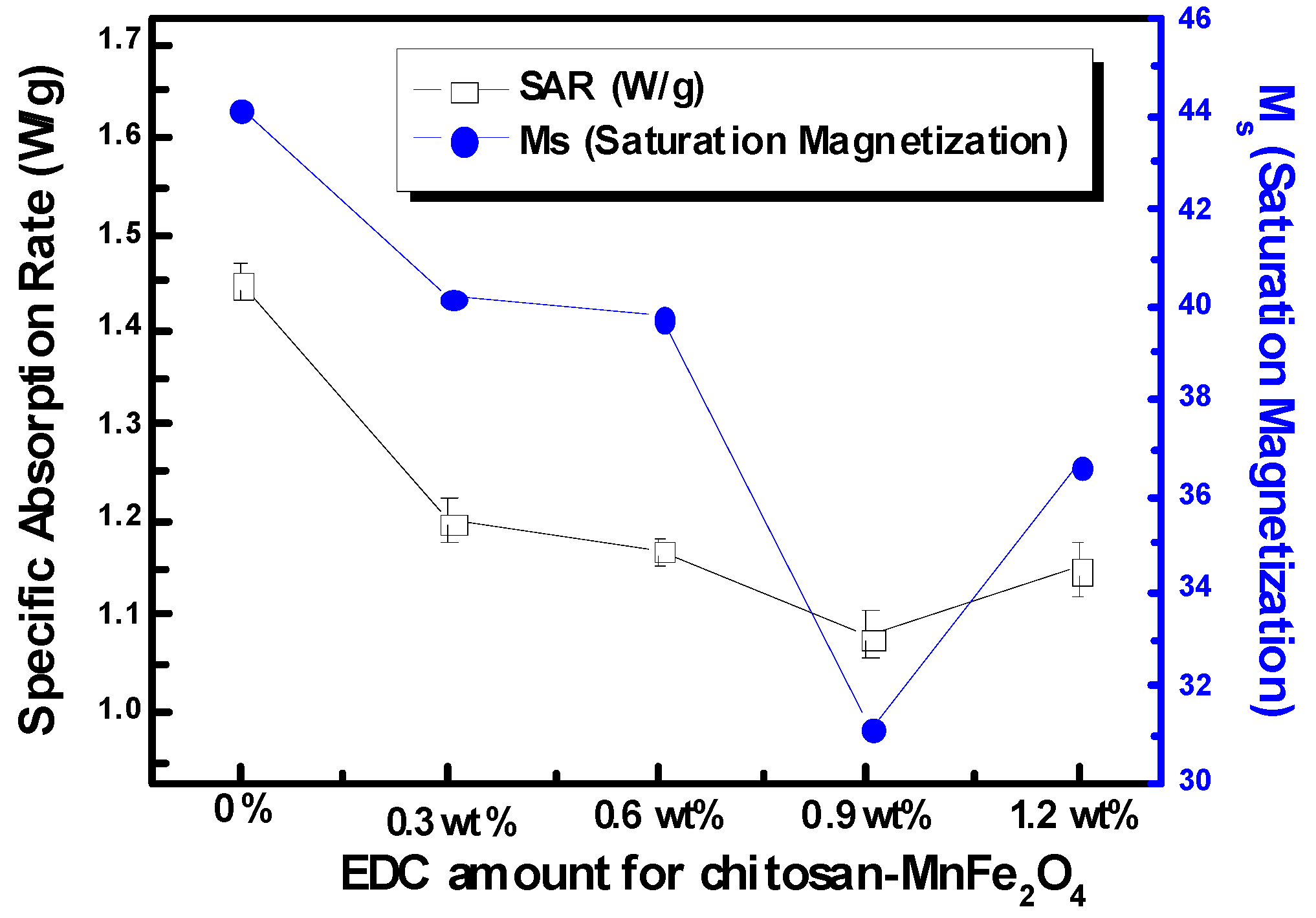
The SAR of the nanoparticle solutions was used as the standard measure of heating efficacy. Using modulated DSC, the heat capacity of MnFe
2O
4 was determined to be 0.857 J/g °C. The heat generated in aqueous solutions of chitosan-MNPs was lower than that for DMSA-MNPs at a concentration of 7 mg/ml (
Figure 8). The solution SARs continued to drop as more EDC was used to coat chitosan onto the MnFe
2O
4 MNPs, largely following the trends observed for saturation magnetization. Although SAR is a standard variable for reporting magnetically-induced heating, it varies with nanoparticle concentration, as well as the applied magnetic field and frequency, making it difficult to compare data from different researchers. To the best of our knowledge, this is the first report of SAR values for MnFe
2O
4 MNPs, as this is a newer material for use in hyperthermia applications. While these values for SAR are considerably lower than for bulk nanoparticles, it should be noted that these values are for the heating of the solution and are not normalized with respect to just the MNP mass. To improve SAR, the concentration of the MNPs can be increased, and other parameters (nanoparticle size, and magnetic field frequency and intensity) can be optimized. Baker
et al. [
28] reported that the SAR of a one wt % Fe
2O
3 (9 nm) nanoparticle concentration in an epoxy resin for localized hyperthermia was 1.25 W/g at 300 kHz and 150 Oe. Here, an aqueous solution of DMSA-MnFe
2O
4 at 0.349 wt % was found to have a SAR of 1.45 W/g at 266 kHz and 653 Oe, which leads to strong possibilities for its use in hyperthermia cancer treatment which can be combined with the usefulness of MnFe
2O
4 as a phase contrast agent for imaging.
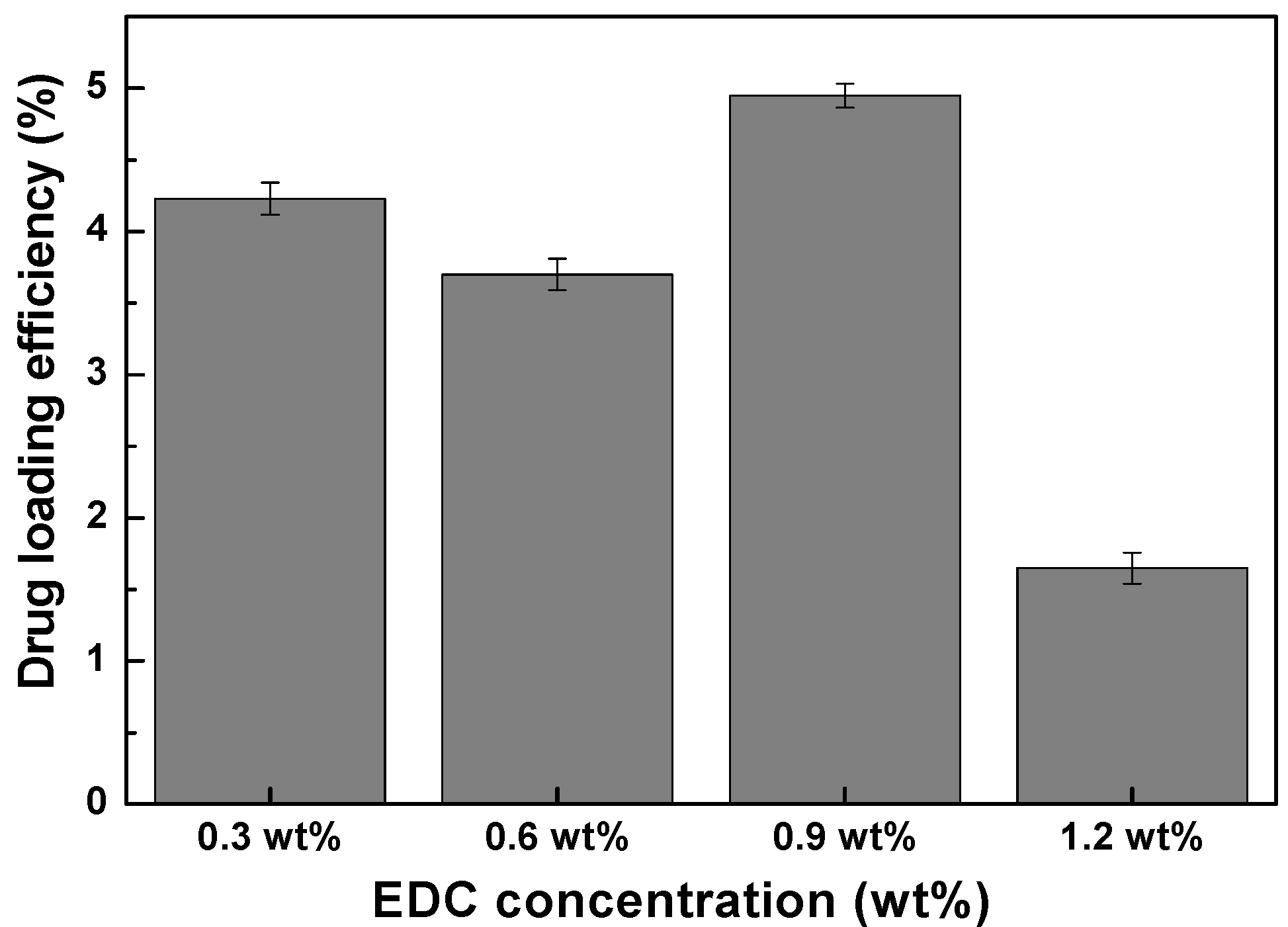
The drug loading efficiency of chitosan-MnFe
2O
4 nanoparticles of different hydrodynamic sizes were measured using theophylline. Theophylline release was achieved and monitored by separating the supernatant theophylline (with molecular diameter 3.8 Å) released from the 18 nm chitosan-MnFe
2O
4 nanoparticles by centrifugal ultrafiltration at 3,500 rpm for 15 minutes. The drug-loading efficiency in chitosan-MnFe
2O
4 nanoparticles was calculated by comparing the amount of drug effectively released with the initial amount of theophylline used in the loading solution (
Figure 9). The loading was shown to be most effective for the chitosan-MnFe
2O
4 nanoparticles synthesized using 0.9 wt % EDC. Thus, the chitosan-MnFe
2O
4 nanoparticles with the thickest chitosan layer were shown to absorb the greatest amount of theophylline. For the chitosan-MNPs linked using 1.2 wt % EDC, even though the chitosan layer was observed to be substantial (
Figure 5), the additional crosslinking may have prevented effective uptake of the model drug theophylline.
Figure 8.
SARs and Ms (saturation magnetization) of the chitosan-MnFe2O4 MNP solutions formed using various EDC amount at the concentrations of 7 mg/ml of the MnFe2O4 nanoparticles under a 653 Oe (266 kHz) field. Error bars for SAR represent the standard deviation for three samples. No error is reported for Ms.
Figure 8.
SARs and Ms (saturation magnetization) of the chitosan-MnFe2O4 MNP solutions formed using various EDC amount at the concentrations of 7 mg/ml of the MnFe2O4 nanoparticles under a 653 Oe (266 kHz) field. Error bars for SAR represent the standard deviation for three samples. No error is reported for Ms.
Figure 9.
Drug-loading efficiency of the chitosan-MnFe2O4 MNP samples, as % of initial drug mixed with the MNPs that were effectively released. Error bars represent the standard deviation for three replicates.
Figure 9.
Drug-loading efficiency of the chitosan-MnFe2O4 MNP samples, as % of initial drug mixed with the MNPs that were effectively released. Error bars represent the standard deviation for three replicates.