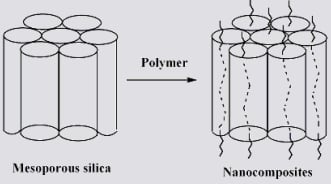
Synthesis of Polymer—Mesoporous Silica Nanocomposites
Abstract
:1. Introduction
2. Mesoporous Silica and Surface Functionalization
| Name | Structure |
|---|---|
| 3-aminopropyltriethoxysilane | H2N(CH2)3Si(OC2H5)3 |
| 3-aminopropyltrimethoxysilane | H2N(CH2)3Si(OCH3)3 |
| Vinyltriethoxysilane | CH2=CHSi(OC2H5)3 |
| Vinyltrimethoxysilane | CH2=CHSi(OCH3)3 |
| 3-isocyanatopropyltriethoxysilane | OCN(CH2)3Si(OC2H5)3 |
| methacryloxymethyltriethoxysilane | CH2=C(CH3)COO(CH2)3Si(OC2H5)3 |
| 3-methacryloxypropyltrimethoxysilane | CH2=C(CH3)COO(CH2)3Si(OCH3)3 |
| mercaptopropyl triethoxysilane | SH(CH2)3Si(OC2H5)3 |
| methyltriethoxysilane | CH3Si(OC2H5)3 |
| phenyltrimethoxysilane | PhSi(OCH3)3 |
| bis(triethoxysilylpropyl)tetrasulfane | (C2H5O)3Si(CH2)3S4(CH2)3Si(OC2H5)3 |
| 3-glycidoxypropyltrimethoxysilane | CH2(O)CHCH2O(CH2)3Si(OCH3)3 |
| Dimethyldichlorosilane | (CH3)2SiCl2 |

3. Preparation of Polymer/Mesoporous Silica Nanocomposites
3.1. Blending
3.2. In Situ Polymerization
3.2.1. General Polymerization
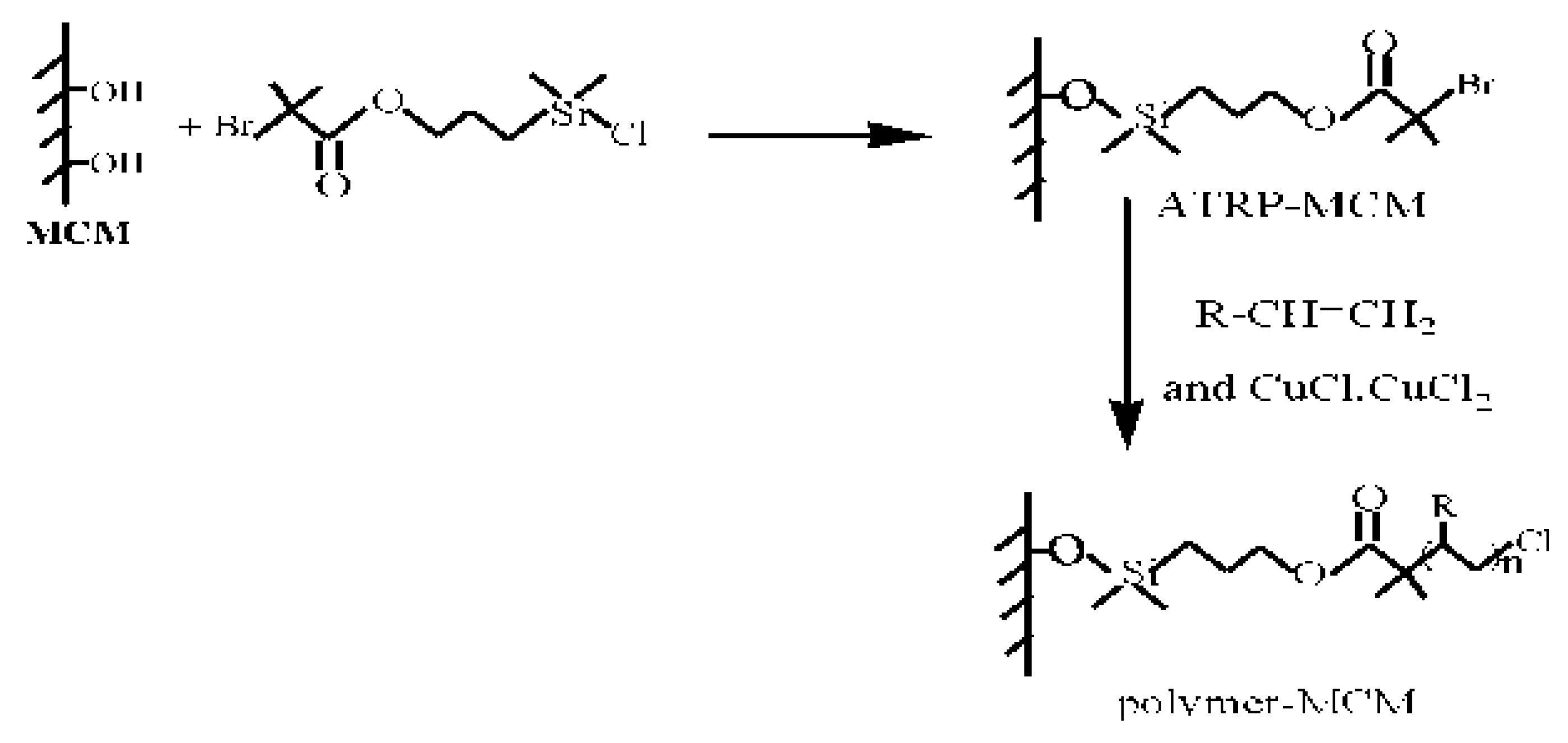
3.3. Surface-Initiated Polymerization

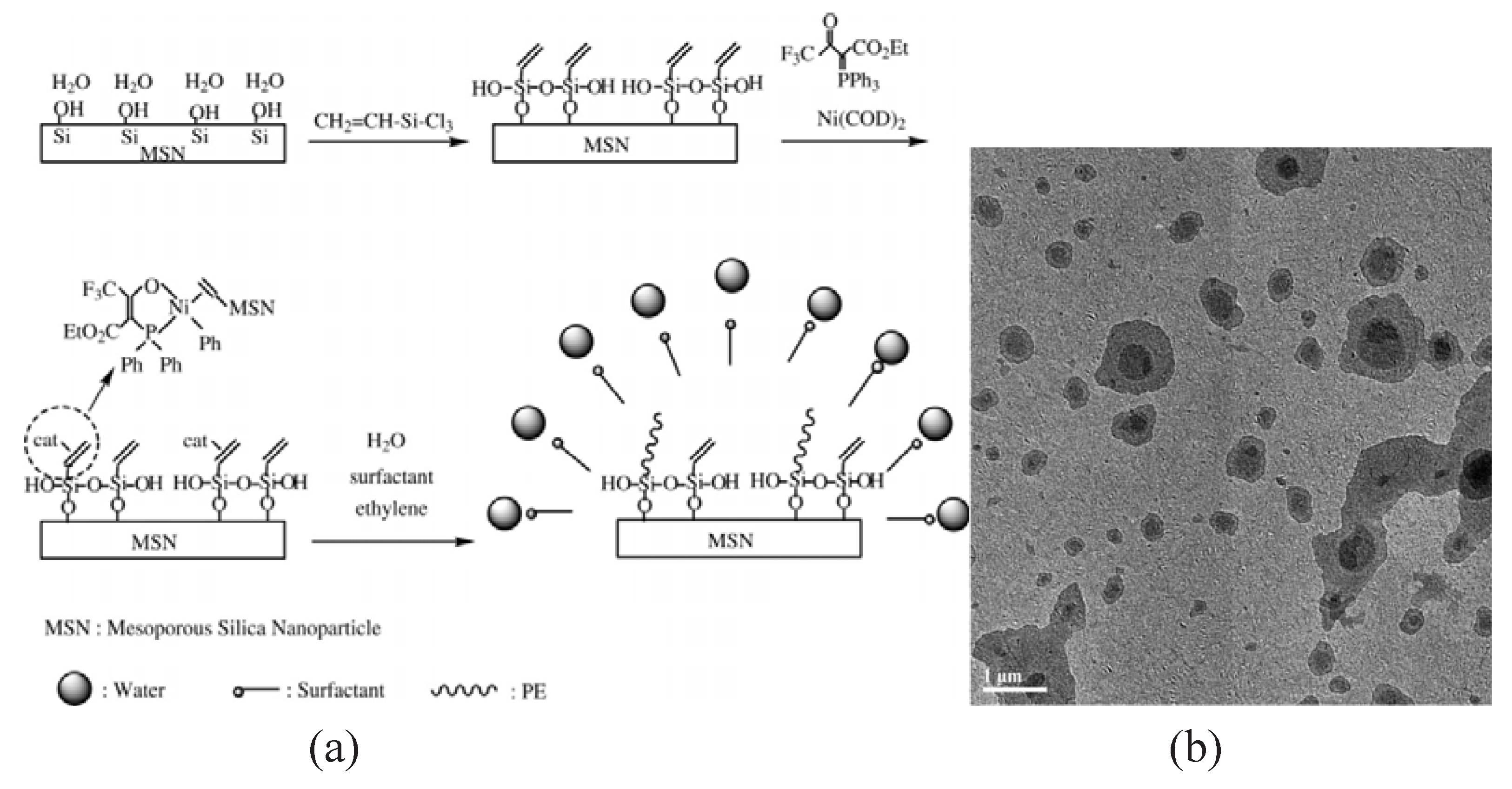
4. Other Methods
5. Summary and Outline
Acknowledgements
References and Notes
- Zou, H.; Wu, S.S.; Shen, J. Polymer/silica nanocomposites: preparation, characterization, properties, and applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef] [PubMed]
- Mark, J.E. Some novel polymeric nanocomposites. Accounts Chem. Res. 2006, 12, 881–888. [Google Scholar] [CrossRef]
- Winey, K.I.; Moniruzzaman, M. Polymer nanocomposites containing carbon nanotubes. Macromolecules 2006, 39, 5194–5205. [Google Scholar] [CrossRef]
- Wei, L.M.; Tang, T.; Huang, B.T. Synthesis and characterization of polyethylene/clay–silica nanocomposites: a montmorillonite/silica- hybrid- supported catalyst and in situ polymerization. J. Polym. Sci. Pol. Chem. 2004, 42, 941–949. [Google Scholar] [CrossRef]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-based mesoporous organic-inorganic hybrid materials. Angew. Chem. Int. Edit. 2006, 45, 3216–3251. [Google Scholar] [CrossRef]
- Giraldo, L.F.; López, B.L.; Pérez, L.; Urrego, S.; Sierra, L.; Mesa, M. Mesoporous silica applications. Macromol. Symp. 2007, 258, 129–141. [Google Scholar] [CrossRef]
- Pérez, L.D.; Giraldo, L.F.; López, B.L.; Hess, M.; de Souza Gomes, A. Reinforcing of elastomers with mesoporous silica. In Proceedings of World Polymer Congress-MACRO, Rio de Janeiro, Brazil, 16-21 July 2006; pp. 628–640.
- Pérez, L.D.; Giraldo, L.F.; López, B.L.; Hess, M. Reinforcing of elastomers with mesoporous silica. Macromol. Symp. 2006, 245, 628–640. [Google Scholar]
- López, B.L.; Pérez, L.D.; Mesa, M.; Sierra, L.; Devaux, E.; Camargo, M.; Campagne, C.; Giraud, S. Use of mesoporous silica as a reinforcing agent in rubber compounds. E-Polymer 2005, 018, 1–13. [Google Scholar]
- Wang, N.; Zhao, C.; Shia, Z.; Shao, Y.; Li, H.; Gao, N. Co-incorporation of MMT and MCM-41 nanomaterials used as fillers. Mater. Sci. Eng. B 2009, 157, 44–47. [Google Scholar] [CrossRef]
- Wang, X.L.; Mei, A.; Li, M.; Lin, Y.H.; Nan, C.W. Effect of silane-functionalized mesoporous silica SBA-15 on performance of PEO-based composite polymer electrolytes. Solid State Ionics 2006, 177, 1287–1291. [Google Scholar] [CrossRef]
- Xi, J.Y.; Qiu, X.P.; Zhu, W.T.; Tang, X.Z. Enhanced electrochemical properties of poly (ethylene oxide)-based composite polymer electrolyte with ordered mesoporous materials for lithium polymer battery. Micropor. Mesopor. Mat. 2006, 88, 1–7. [Google Scholar] [CrossRef]
- Tominaga, Y.; Igawa, S.; Asai, S.; Sumita, M. Ion-conductive properties of mesoporous silica-filled composite polymer electrolytes. Electrochim. Acta 2005, 50, 3949–3954. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, E.J.; Park, S.J. An experimental study on the effect of mesoporous silica addition on ion conductivity of poly (ethylene oxide) electrolytes. Curr. Appl. Phys. 2008, 8, 729–731. [Google Scholar] [CrossRef]
- Kao, H.M.; Tsai, Y.Y.; Chao, S.W. Functionalized mesoporous silica MCM-41 in poly(ethylene oxide)-based polymer electrolytes: NMR and conductivity study. Solid State Ionics 2005, 176, 1261–1270. [Google Scholar] [CrossRef]
- Xia, J.Y.; Qiua, X.P.; Ma, X.M.; Cui, M.Z.; Yang, J.; Tang, X.Z.; Zhu, W.T; Chen, L.Q. Composite polymer electrolyte doped with mesoporous silica SBA-15 for lithium polymer battery. Solid State Ionics 2005, 176, 1249–1260. [Google Scholar] [CrossRef]
- Xi, J.Y.; Tang, X.J. Enhanced lithium ion transference number and ionic conductivity of composite polymer electrolyte doped with organic–inorganic hybrid P123@SBA-15. Chem. Phys. Lett. 2004, 400, 68–73. [Google Scholar] [CrossRef]
- Tominaga, Y.; Hong, I.C.; Asai, S.; Sumita, M. Proton conduction in Nafion composite membranes filled with mesoporous silica. J. Power Sources 2007, 171, 530–534. [Google Scholar] [CrossRef]
- Jin, Y.G.; Qiao, S.Z.; Zhang, L.; Xua, Z.P.; Smarta, S; da Costa, J.C.D.; Lu, G.Q. Novel Nafion composite membranes with mesoporous silica nanospheres as inorganic fillers. J. Power Sources 2008, 185, 664–669. [Google Scholar] [CrossRef]
- Cardin, D.J. Encapsulated conducting polymer. Adv. Mater. 2002, 14, 553. [Google Scholar] [CrossRef]
- Xi, H.A.; Wang, B.H.; Zhang, Y.B.; Qian, X.F.; Yin, J.; Zhu, Z.K. Spectroscopic studies on conjugated polymers in mesoporous channels: influence of polymer side-chain length. J. Phys. Chem. Solids 2003, 64, 2451–2455. [Google Scholar] [CrossRef]
- Posudievsky, Y.O.; Telbiza, G.M.; Rossokhaty, V.K. Effect of solvent nature on liquid-phase self-assembly of MEH-PPV/ MCM-41 guest–host composites. J. Mater. Chem. 2006, 16, 2485–2489. [Google Scholar] [CrossRef]
- Cadby, A.J.; Tolbert, S.H. Controlling optical properties and interchain interactions in semiconducting polymers by encapsulation in periodic nanoporous silicas with different Pore Sizes. J. Phys. Chem. B. 2005, 109, 17879–17886. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Gross, A.F.; Tolbert, S.H. Host-guest chemistry using an oriented mesoporous host: alignment and Iisolation of a semiconducting polymer in the nanopores of an ordered silica matrix. J. Phys. Chem. B. 1999, 103, 2374–2384. [Google Scholar] [CrossRef]
- Molenkamp, W.C.; Watanabe, M.; Miyata, H.; Tolbert, S.H. Highly polarized luminescence from optical quality films of a semiconducting polymer aligned within oriented mesoporous silica. J. Am. Chem. Soc. 2004, 126, 4476–4477. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.H.; Xi, H.A.; Yin, J.; Qian, X.F.; Zhu, Z.K. Molecular orbital confinement effect of mesoporous silica of MCM-41 on conjugated polymer. Synt. Met. 2003, 139, 187–190. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, S.; Kim, Y.C.; Park, O.O. Polymer/nanoporous silica nanocomposite blue-light-emitting diodes. Nanotechnology 2005, 16, 1793–1797. [Google Scholar] [CrossRef]
- Reid, B.D.; Alberto Ruiz-Trevino, F.; Musselman, I.H.; Balkus, K.J.; Ferraris, J.P. Gas permeability properties of polysulfone membranes containing the mesoporous molecular sieve MCM-41. Chem. Mater. 2001, 13, 2366. [Google Scholar] [CrossRef]
- Zornoza, B.; Irusta, S.; Tellez, C.; Coronas, J. Mesoporous silica sphere-polysulfone mixed matrix membranes for gas separation. Langmuir 2009, 25, 5903–5909. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Matsuda, H.; Zhou, H.; Honma, I. Ultrasound-triggered smart drug release from a poly(dimethylsiloxane)–mesoporous silica composite. Adv. Mater. 2006, 18, 3083–3088. [Google Scholar] [CrossRef]
- Bose, A.; Gilpin, R.K.; Jaroniec, M. Adsorption and thermogravimetric studies of mesoporous silica coated with siloxane polymer. J. Colloid Interf. Sci. 2001, 240, 224–228. [Google Scholar] [CrossRef]
- Ostapenko, N.; Kotova, N.; Telbiz, G.; Suto, S. Size effect in the fluorescence spectra of polysilanes embedded in mesoporous materials. Low. Temp. Phys. 2004, 30, 494. [Google Scholar] [CrossRef]
- He, J.; Shen, Y.B.; Yang, J.; Evans, D.G.; Duan, X. Nanocomposite structure based on silylated MCM-48 and poly(vinyl acetate). Chem. Mater. 2003, 15, 3894–3902. [Google Scholar]
- Moller, K.; Bein, T.; Fischer, R.X. Entrapment of PMMA polymer strands in micro- and mesoporous materials. Chem. Mater. 1998, 10, 1841–1852. [Google Scholar] [CrossRef]
- Zhang, F.A.; Lee, D.K.; Pinnavaia, T.J. PMMA-mesocellular foam silica nanocomposites prepared through batch emulsion polymerization and compression molding. Polymer 2009, 50, 4768–4774. [Google Scholar] [CrossRef]
- Ji, X.L.; Hampsey, J.E.; Hu, Q.Y.; He, J.B.; Yang, Z.Z.; Lu, Y.F. Mesoporous silica-reinforced polymer nanocomposites. Chem. Mater. 2003, 15, 3656–3662. [Google Scholar] [CrossRef]
- Lin, V.S.Y.; Radu, D.R.; Han, M.K.; Deng, W.; Kuroki, S.; Shanks, B.H.; Pruski, M. Oxidative polymerization of 1, 4-diethynylbenzene into highly conjugated poly (phenylene butadiynylene) within the channels of surface-functionalized mesoporous silica and alumina materials. J. Am. Chem. Soc. 2002, 124, 9040–9041. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Yano, J.; Nakaoka, K.; Ogura, K. Electrodeposition of composite films consisting of polypyrrole and mesoporous silica. Synthetic. Met. 2002, 128, 57–62. [Google Scholar] [CrossRef]
- Cheng, Q.L.; Pavlinek, V.; Lengalova, A.; Li, C.Z.; Belza, T.; Saha, P. Electrorheological properties of new mesoporous material with conducting polypyrrole in mesoporous silica. Micropor. Mesopor. Mater. 2006, 94, 193–199. [Google Scholar] [CrossRef]
- Cheng, Q.L.; Pavlinek, V.; Li, C.Z.; Lengalova, A.; He, Y.; Saha, P. Synthesis and characterization of new mesoporous material with conducting polypyrrole confined in mesoporous silica. Mater. Chem. Phys. 2006, 98, 504–508. [Google Scholar] [CrossRef]
- Cheng, Q.L.; Pavlinek, V.; Lengalova, A.; Li, C.; He, Y.; Saha, P. Conducting polypyrrole confined in ordered mesoporous silica SBA-15 channels: preparation and its electrorheology. Micropor. Mesopor. Mater. 2006, 93, 263–269. [Google Scholar] [CrossRef]
- Li, N.; Li, X.T.; Geng, W.C.; Zhang, T.; Zuo, Y.; Qiu, S.L. Synthesis and humidity sensitivity of conducting polyaniline in SBA-15. J. Appl. Polym. Sci. 2004, 93, 1597–1601. [Google Scholar] [CrossRef]
- Fang, F.F.; Choi, H.J.; Ahn, W.S. Electroactive response of mesoporous silica and its nanocomposites with conducting polymers. Compos. Sci. Technol. 2009, 69, 2088–2092. [Google Scholar] [CrossRef]
- Showkata, A.M.; Lee, K.P.; Gopalan, A.I.; Kim, M.S.; Choic, S.H.; Kang, H.D. A novel self-assembly approach to form tubular poly(diphenylamine) inside the mesoporous silica. Polymer 2005, 46, 1804–1812. [Google Scholar] [CrossRef]
- Sasidharan, M.; Mal, N.K.; Bhaumik, A. In-situ polymerization of grafted aniline in the channels of mesoporous silica SBA-15. J. Mater. Chem. 2007, 17, 278–283. [Google Scholar] [CrossRef]
- Pattantyus-Abraham, A.G.; Wolf, M.O. A PPV/MCM-41 composite material. Chem. Mater. 2004, 16, 2180–2186. [Google Scholar] [CrossRef]
- Radu, D.R.; Lai, C.Y.; Wiench, J.W.; Pruski, M.; Lin, V.S.Y. Gatekeeping layer effect: A poly(lactic acid)-coated mesoporous silica nanosphere-based fluorescence probe for detection of amino-containing neurotransmitters. J. Am. Chem. Soc. 2004, 126, 1640–1641. [Google Scholar] [CrossRef] [PubMed]
- Praveen, S.; Sun, Z.F.; Xu, J.G.; Patel, A.; Wei, Y.; Ranade, R.; Baran, G. Compression and aging properties of experimental dental composites containing mesoporous silica as fillers. Mol. Cryst. Liq. Crys. 2006, 448, 223–231. [Google Scholar] [CrossRef]
- Jiao, J.; Sun, X.; Pinnavaia, T.J. Reinforcement of a rubbery epoxy polymer by mesostructured silica and organosilica with wormhole framework structures. Adv. Funct. Mater. 2008, 18, 1067–1074. [Google Scholar] [CrossRef]
- Jiao, J.; Sun, X.; Pinnavaia, T.J. Mesostructured silica for the reinforcement and toughening of rubbery and glassy epoxy polymers. Polymer 2009, 50, 983–989. [Google Scholar] [CrossRef]
- Lin, B.P.; Tang, J.; Liu, H.J.; Sun, Y.M.; Yuan, C.W. Structure and infrared emissivity of polyimide/mesoporous silica composite films. J. Solid. State. Chem. 2005, 178, 650. [Google Scholar] [CrossRef]
- Min, C.K.; Wu, T.B.; Yang, W.T.; Chen, C.L. Functionalized mesoporous silica/polyimide nanocomposite thin films with improved mechanical properties and low dielectric constant. Compos. Sci. Technol. 2008, 68, 1570–1578. [Google Scholar] [CrossRef]
- Lin, J.J; Wang, X.D. Novel low-k polyimide/mesoporous silica composite films: Preparation, microstructure, and properties. Polymer 2007, 48, 318–329. [Google Scholar] [CrossRef]
- He, J; Shen, Y.B; Evans, D.G. A nanocomposite structure based on modified MCM-48 and polystyrene. Micropor. Mesopor. Mater. 2008, 109, 73–83. [Google Scholar] [CrossRef]
- Pérez, L.D.; López, J.F.; Orozco, V.H.; Kyu, T.; López, B. L. Effect of the chemical characteristics of mesoporous silica MCM-41 on morphological, thermal, and rheological properties of composites based on polystyrene. J. Appl. Polym. Sci. 2008, 111, 2229–2237. [Google Scholar] [CrossRef]
- Wang, N.; Shi, Z.X.; Zhang, J.; Wang, L. The influence of modification of mesoporous silica with polyethylene via In situ Ziegler–Natta polymerization on PE/MCM-41 nanocomposite. J. Compos. Mater. 2008, 42, 1151. [Google Scholar] [CrossRef]
- Campos, J.M.; Lourenco, J.P.; Perez, E; Cerrada, M.L; Ribeiro, M.R. Self-reinforced hybrid polyethylene/MCM-41 nanocomposites: In-situ polymerisation and effect of MCM-41 content on rigidity. J. Nanosci. Nanotechnol. 2009, 9, 3966–3974. [Google Scholar]
- Fu, Q.; Rama Rao, G.V.; Ward, T.L.; Lu, Y.F.; Lopez, G.P. Thermoresponsive Transport through Ordered mesoporous silica/PNIPAAm copolymer membranes and microspheres. Langmuir 2007, 23, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.S.; Yang, C. Temperature-responsive nanocomposites based on mesoporous SBA-15 silica and PNIPAAm: synthesis and characterization. J. Phys. Chem. C. 2009, 113, 4925–4931. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Kaskel, S.; Ikoma, T.; Hanagata, N. Magnetic SBA-15/poly(N-isopropylacrylamide) composite: Preparation, characterization and temperature-responsive drug release property. Micropor. Mesopor. Mater. 2009, 123, 107–112. [Google Scholar] [CrossRef]
- Liu, C.; Guo, J.; Yang, W.; Hu, J.; Wang, C.; Fu, S. Magnetic mesoporous silica microspheres with thermo-sensitive polymer shell for controlled drug release. J. Mater. Chem. 2009, 19, 4764–4770. [Google Scholar] [CrossRef]
- Gao, Q.; Xu, Y.; Wu, D.; Sun, Y.; Li, X.A. PH-responsive drug release from polymer-coated mesoporous silica spheres. J. Phys. Chem. C. 2009, 113, 12753–12758. [Google Scholar] [CrossRef]
- Chu, H.Q.; Yu, C.; Wan, Y.; Zhao, D.Y. Synthesis of ordered mesoporous bifunctional TiO2-SiO2-polymer nanocomposites. J. Mater. Chem. 2009, 19, 8610–8618. [Google Scholar] [CrossRef]
- Fu, Q.; Rama Rao, G.V.; Ista, L.K.; Wu, Y.; Andrzejewski, B.P.; Sklar, L.A.; Ward, T.L; López, G.P. Control of molecular transport through stimuli-responsive ordered mesoporous materials. Adv. Mater. 2003, 15, 1262. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Zhu, S.M.; Zhang, D. Grafting of thermo-responsive polymer inside mesoporous silica with large pore size using ATRP and investigation of its use in drug release. J. Mater. Chem. 2007, 17, 2428–2433. [Google Scholar] [CrossRef]
- Kruk, M.; Dufour, B.; Celer, E.B.; Kowalewski, T.; Jaroniec, M.; Matyjaszewski, K. Grafting monodisperse polymer chains from concave surfaces of ordered mesoporous silicas. Macromolecules 2008, 41, 8584–8591. [Google Scholar] [CrossRef]
- Audouin, F.; Blas, H.; Pasetto, P.; Beaunier, P.; Boissière, C.; Sanchez, C.; Save, M.; Charleux, B. Structured hybrid nanoparticles via surface-initiated ATRP of methyl methacrylate from ordered mesoporous silica. Macromol. Rapid Comm. 2008, 29, 914–921. [Google Scholar] [CrossRef]
- Anwander, R.; Nagl, I.; Zapilko, C.; Widenmeyer, M. Methyl methacrylate polymerization at samarium(II)-grafted MCM-41. Tetrahedron 2003, 59, 10567–10574. [Google Scholar] [CrossRef]
- Lunn, J.D.; Shantz, D.F. Peptide brush-ordered mesoporous silica nanocomposite materials. Chem. Mater. 2009, 21, 3638–3648. [Google Scholar] [CrossRef]
- Chung, P.W.; Kumar, R.; Pruski, M.; Lin, V.S.Y. Temperature responsive solution partition of organic–inorganic hybrid poly(N-isopropylacrylamide)-coated mesoporous silica nanospheres. Adv. Funct. Mater. 2008, 18, 1390–1398. [Google Scholar] [CrossRef]
- Hong, C.Y.; Li, X.; Pan, C.Y. Smart core-shell nanostructure with a mesoporous core and a stimuli-responsive nanoshell synthesized via surface reversible addition-fragmentation chain transfer polymerization. J. Phys. Chem. C. 2008, 112, 15320–15324. [Google Scholar] [CrossRef]
- Roohi, F.; Titirici, M.M. Thin thermo-responsive polymer films onto the pore system of chromatographic beads via reversible addition–fragmentation chain transfer polymerization. New J. Chem. 2008, 32, 1409–1414. [Google Scholar] [CrossRef]
- Hong, C.Y.; Li, X.; Pan, C.Y. Grafting polymer nanoshell onto the exterior surface of mesoporous silica nanoparticles via surface reversible addition-fragmentation chain transfer polymerization. Euro. Polym. J. 2007, 43, 4114–4122. [Google Scholar] [CrossRef]
- Wei, L.M.; Zhang, Y.F. Emulsion polymerization of ethylene from mesoporous silica nanoparticles with vinyl functionalized monolayers. J. Polym. Sci. Part A: Polym. Chem. 2009, 47, 1393–1402. [Google Scholar] [CrossRef]
- Fujiwara, M.; Shiokawa, K.; Zhu, Y.C. Preparation of mesoporous silica/polymer sulfonate composite materials. J.Mol. Catal. A: Chem 2007, 264, 153–161. [Google Scholar] [CrossRef]
- Madhugiri, S.; Dalton, A.; Gutierrez, J.; Ferraris, J. P.; Balkus, K.J., Jr. Electrospun MEH-PPV/SBA-15 composite nanofibers using a dual syringe method. J. Am. Chem. Soc. 2003, 125, 14531–14538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. X.; Zhu, M.; Guo, L. M.; Li, L.; Shi, J.L. Bilirubin adsorption property of mesoporous silica and amine-grafted mesoporous silica. Nano-Micro Lett. 2009, 1, 14–18. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wei, L.; Hu, N.; Zhang, Y. Synthesis of Polymer—Mesoporous Silica Nanocomposites. Materials 2010, 3, 4066-4079. https://doi.org/10.3390/ma3074066
Wei L, Hu N, Zhang Y. Synthesis of Polymer—Mesoporous Silica Nanocomposites. Materials. 2010; 3(7):4066-4079. https://doi.org/10.3390/ma3074066
Chicago/Turabian StyleWei, Liangming, Nantao Hu, and Yafei Zhang. 2010. "Synthesis of Polymer—Mesoporous Silica Nanocomposites" Materials 3, no. 7: 4066-4079. https://doi.org/10.3390/ma3074066
APA StyleWei, L., Hu, N., & Zhang, Y. (2010). Synthesis of Polymer—Mesoporous Silica Nanocomposites. Materials, 3(7), 4066-4079. https://doi.org/10.3390/ma3074066