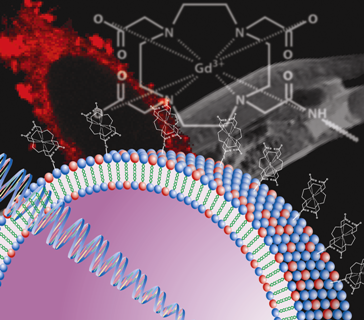
Paramagnetic Liposome Nanoparticles for Cellular and Tumour Imaging
Abstract
:1. Introduction
2. MRI Contrast Agents
3. Cellular Labelling and MRI
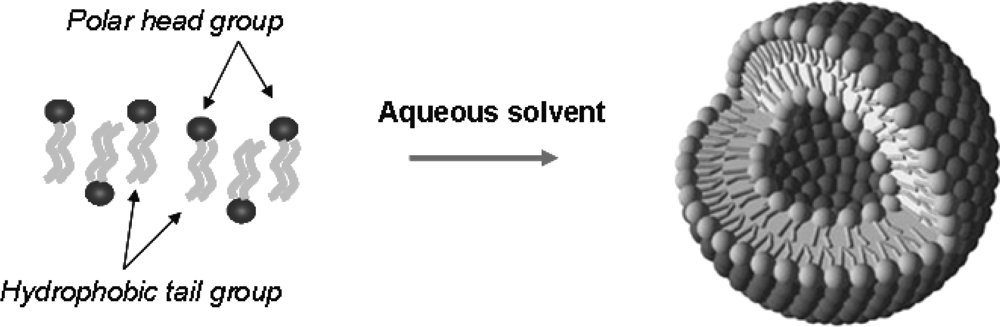
4. Liposome Carriers for Imaging Agents
4.1. Paramagnetic Liposomes by Contrast Agent Encapsulation
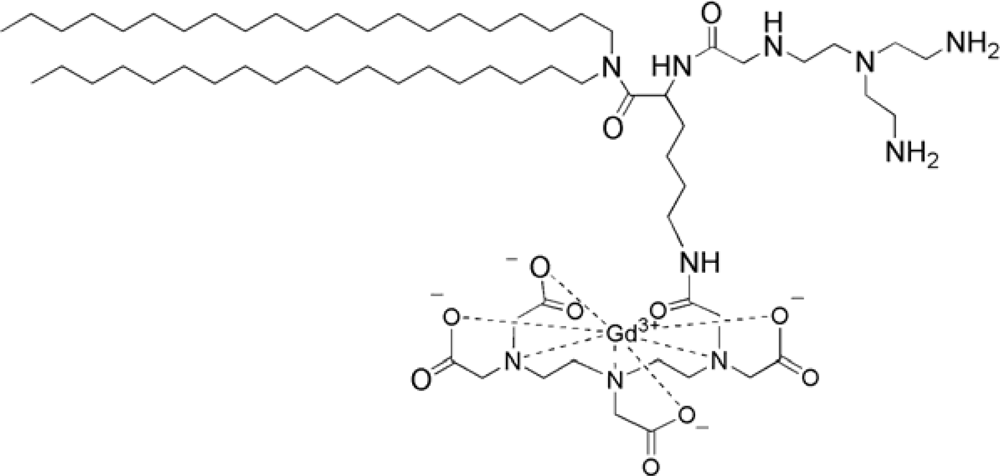
4.2. Paramagnetic Liposomes Involving Gd Lipid Incorporation
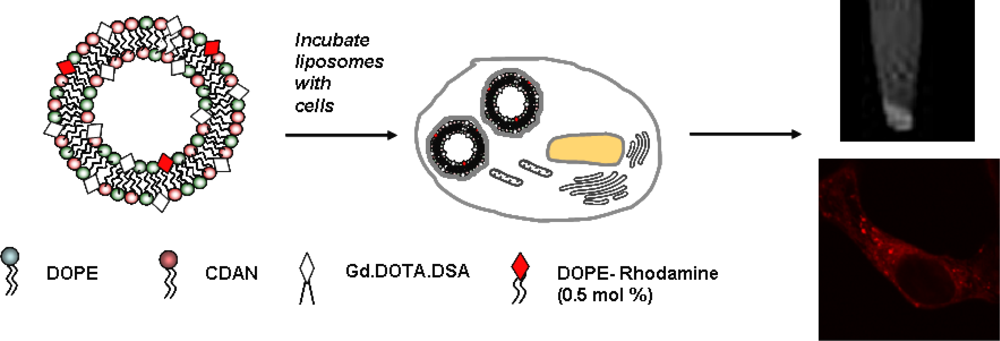
4.3. Effects of Cellular Compartmentalization of Gd Liposomes on MR Relaxivity
5. In Vitro Evaluation of Paramagnetic Liposomes
6. Paramagnetic Liposomes for in Vivo Applications
7. Conclusions
Acknowledgments
References and Notes
- Modo, MMJ; Bulte, JWM. Molecular and Cellular MR Imaging, 1st ed; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Modo, M; Hoehn, M; Bulte, JWM. Cellular MR imaging. Mol. Imaging 2005, 4, 143–164. [Google Scholar]
- Caravan, P; Ellison, JJ; McMurry, TJ; Lauffer, RB. Gadolinium(III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chem. Rev 1999, 99, 2293–2352. [Google Scholar]
- Parac-Vogt, TN; Kimpe, K; Laurent, S; Pierart, C; Elst, LV; Muller, RN; Binemans, K. Gadolinium DTPA-monoamide complexes incorporated into mixed micelles as possible MRI contrast agents. Eur J Inorg Chem 2004, 3538–3543. [Google Scholar]
- Weinmann, HJ; Ebert, W; Misselwitz, B; Schmitt-Willich, H. Tissue-specific MR contrast agents. Eur. J. Radiol 2003, 46, 33–44. [Google Scholar]
- Bellin, MF. MR contrast agents, the old and the new. Eur. J. Radiol 2006, 60, 314–323. [Google Scholar]
- Swanson, SD; Kukowska-Latallo, JF; Patri, AK; Chen, C; Ge, S; Cao, Z; Kotlyar, A; East, AT; Baker, JR. Targeted gadolinium-loaded dendrimer nanoparticles for tumor-specific magnetic resonance contrast enhancement. Int. J. Nanomed 2008, 3, 201–210. [Google Scholar]
- Ke, T; Jeong, EK; Wang, X; Feng, Y; Parker, DL; Lu, ZR. RGD targeted poly(L-glutamic acid)-cystamine-(Gd-DO3A) conjugate for detecting angiogenesis biomarker αvβ3 integrin with MR T1 mapping. Exp. Biol. Med 2007, 232, 1081–1089. [Google Scholar]
- Xu, R; Wang, Y; Wang, X; Jeong, EK; Parker, DL; Lu, ZR. In vivo evaluation of a PAMAM-cystamine-(Gd-DO3A) conjugate as a biodegradable macromolecular MRI contrast agent. Exp. Biol. Med 2007, 232, 1081–1089. [Google Scholar]
- Lei, XG; Jockusch, S; Turro, NJ; Tomalia, DA; Ottaviani, MF. EPR characterization of gadolinium(III)-containing-PAMAM-dendrimers in the absence and in the presence of paramagnetic probes. J. Colloid. Interface Sci 2008, 322, 457–464. [Google Scholar]
- Wang, W; Xiong, W; Zhu, Y; Xu, H; Yang, X. Protective effect of PEGylation against poly(amidoamine) dendrimer-induced hemolysis of human red blood cells. J. Biomed. Mater. Res. B.: Appl. Biomater 2010, 93, 59–64. [Google Scholar]
- Toth, E; Helm, L; Kellar, KE; Merbach, AE. Gd(DTPA-bisamide)alkyl copolymers: A hint for the formation of MRI contrast agents with very high relaxivity. Chem. Eur. J 1999, 5, 1202–1211. [Google Scholar]
- Bryant, LH; Jordan, EK; Bulte, JWM; Herynek, V; Frank, JA. Pharmacokinetics of a high-generation dendrimer-Gd-DOTA. Acad. Radiol 2002, 9, S29–S33. [Google Scholar]
- Frangioni, JV; Hajjar, RJ. In vivo tracking of stem cells for clinical trials in cardiovascular disease. Circulation 2004, 110, 3378–3384. [Google Scholar]
- Rogers, WJ; Meyer, CH; Kramer, CM. Technology Insight: In vivo cell tracking by use of MRI. Nature Clin. Prac. Cardiovasc. Med 2006, 3, 554–562. [Google Scholar]
- Mukherjee, S; Ghosh, RN; Maxfield, FR. Endocytosis. Physiol. Rev 1997, 77, 759–804. [Google Scholar]
- Hermann, P; Kotek, J; Kubicek, VC; Lukes, I. Gadolinium(III) complexes as MRI contrast agents: Ligand design and properties of the complexes. J. Chem. Soc., Dalton Trans 2008, 2004, 3027–3047. [Google Scholar]
- Geraldes, CFGC; Laurent, S. Classification and basic properties of contrast agents for magnetic resonance imaging. Contrast Media Mol. Imaging 2009, 4, 1–23. [Google Scholar]
- Davis, SS. Coming of age of lipid-based drug delivery systems. Adv. Drug Del. Rev 2004, 56, 1241–1242. [Google Scholar]
- Lian, T; Ho, RJY. Trends and developments in liposome drug delivery systems. J. Pharm. Sci 2001, 90, 667–680. [Google Scholar]
- Lopes de Menezes, DE; Pilarski, LM; Belch, AR; Allen, TM. Selective targeting of immunoliposomal doxorubicin against human multiple myeloma in vitro and ex vivo. Biochim. Biophys. Acta 2000, 1466, 205–220. [Google Scholar]
- Kostarelos, K; Miller, AD. Synthetic, self-assembly ABCD nanoparticles: A structural paradigm for viable synthetic non-viral vectors. Chem. Soc. Rev 2005, 34, 970–994. [Google Scholar]
- Stewart, L; Manvell, M; Hillery, E; Etheridge, CJ; Cooper, RG; Stark, H; van Heel, M; Preuss, M; Alton, E; Miller, A. Physico-chemical analysis of cationic liposome-DNA complexes (lipoplexes) with respect to in vitro and in vivo gene delivery efficiency. J Chem Soc Perkin 2 2001, 624–632. [Google Scholar]
- Tagawa, T; Manvell, M; Brown, N; Keller, M; Perouzel, E; Murray, KD; Harbottle, RP; Tecle, M; Booy, F; Brahimi-Horn, MC; Coutelle, C; Lemoine, NR; Alton, EW; Miller, AD. Characterisation of LMD virus-like nanoparticles self-assembled from cationic liposomes, adenovirus core peptide m (mu) and plasmid DNA. Gene Ther 2002, 9, 564–576. [Google Scholar]
- Spagnou, S; Miller, AD; Keller, M. Lipidic carriers of siRNA: Differences in the formulation, cellular uptake, and delivery with plasmid DNA. Biochemistry 2004, 43, 13348–13356. [Google Scholar]
- Lee, Y; Koo, H; Lim, YB; Mo, H; Sang-Park, J. New cationic lipids for gene transfer with high efficiency and low toxicity: T-shape cholesterol ester derivatives. Bioorg. Med. Chem. Lett 2004, 14, 2637–2641. [Google Scholar]
- Kim, HS; Moon, J; Kim, KS; Choi, MM; Lee, JE; Choi; Lee, JE; Heo, Y; Cho, DH; Jang, DO; Park, YS. Gene-transferring efficiencies of novel diamino cationic lipids with varied hydrocarbon chains. Bioconjug. Chem 2004, 15, 1095–1101. [Google Scholar]
- Zhang, S; Xu, Y; Wang, B; Qiao, W; Liu, D; Li, Z. Cationic compounds used in lipoplexes and polyplexes for gene delivery. J. Control. Rel 2004, 100, 165–180. [Google Scholar]
- Bulte, JWM; De Cuyper, M. Magnetoliposomes as contrast agents. Meth Enzymol 2003, 175–197. [Google Scholar]
- Krause, W; Klopp, R; Leike, J; Sachse, A. Liposomes in diagnostic imaging: Comparison of modalities; in vivo visualization of liposomes. J. Liposome Res 1995, 5, 1. [Google Scholar]
- Tilcock, C; Unger, E; Cullis, P; MacDougall, P. Liposomal Gd-DTPA: Preparation and characterization of relaxivity. Radiology 1989, 171, 77–88. [Google Scholar]
- Unger, E; Tilcock, C; Ahkong, QF; Fritz, T. Paramagnetic liposomes as magnetic resonance contrast agents. Invest. Radiol 1990, 25, S65–S66. [Google Scholar]
- Oliver, M; Ahmad, A; Kamaly, N; Perouzel, E; Caussin, A; Keller, M; Herlihy, A; Bell, J; Miller, AD; Jorgensen, MR. MAGfect: A novel liposome formulation for MRI labelling and visualization of cells. Org. Biomol. Chem 2006, 4, 3489–3497. [Google Scholar]
- Caride, VJ; Sostman, HD; Winchell, RJ; Gore, JC. Relaxation enhancement using liposomes carrying paramagnetic species. Magn. Reson. Imaging 1984, 2, 107–112. [Google Scholar]
- Navon, G; Panigel, R; Valensin, G. Liposomes containing paramagnetic macromolecules as MRI contrast agents. Magn. Reson. Med 1986, 3, 876–880. [Google Scholar]
- Magin, RL; Wright, SM; Niesman, MR; Chan, HC; Swartz, HM. Liposome delivery of NMR contrast agents for improved tissue imaging. Magn. Reson. Med 1986, 3, 440–447. [Google Scholar]
- Koenig, SH; Brown, RD; Kurland, R; Ohki, S. Relaxivity and binding of Mn2+ ions in solutions of phosphatidylserine vesicles. Magn. Reson. Med 1988, 7, 133–142. [Google Scholar]
- Devoisselle, JM; Vion-Dury, J; Galons, JP; Confort-Gouny, S; Coustaut, D; Canioni, P; Cozzone, PJ. Entrapment of gadolinium-DTPA in liposomes. Characterization of vesicles by P-31 NMR spectroscopy. Invest. Radiol 1988, 23, 719–724. [Google Scholar]
- Gruender, W; Biesold, M; Wagner, M; Werner, A. Improved nuclear magnetic resonance microscopic visualization of joint cartilage using liposome entrapped contrast agents. Invest. Radiol 1998, 33, 193–202. [Google Scholar]
- Unger, E; Shen, DK; Wu, G; Fritz, T. Liposomes as MR contrast agents: Pros and cons. Magn. Reson. Med 1991, 33, 304–308. [Google Scholar]
- Unger, EC; Winokur, T; MacDougall, P; Rosenblum, J; Clair, M; Gatenby, R; Tilock, C. Hepatic metastases: Liposomal Gd-DTPA-enhanced MR imaging. Radiology 1989, 171, 81–85. [Google Scholar]
- Alhaique, F; Bertini, I; Fragai, M; Carafa, M; Luchinat, C; Parigi, G. Solvent 1H-NMRD study of biotinylated paramagnetic liposomes containing Gd-bis-SDA-DTPA or Gd-DMPE-DTPA. Inorg. Chim. Acta 2002, 331, 151–157. [Google Scholar]
- Mulder, WJ; Strijkers, GJ; van Tilborg, GA; Griffioen, AW; Nicolay, K. Lipid-based nanoparticles for contrast-enhanced MRI and molecular imaging. NMR Biomed 2006, 19, 142–164. [Google Scholar]
- Strijkers, GJ; Mulder, WJM; van Heeswijk, RB; Frederik, PM; Bomans, P; Magusin, PC; Nicolay, K. Relaxivity of liposomal paramagnetic MRI contrast agents. Mag. Res. Mater.: Phys. Biol. Med 2005, 18, 186–192. [Google Scholar]
- Laurent, S; Elst, LV; Thirifays, C; Muller, RN. Relaxivities of paramagnetic liposomes: On the importance of the chain type and length of the amphiphilic complex. Eur. Biophys.: J. Biophys. Lett 2008, 37, 1007–1014. [Google Scholar]
- Hak, S; Sanders, HMHF; Agrawal, P; Langereis, S; Grull, H; Keizer, HM; Arena, F; Terreno, E; Strijkers, GJ; Nicolay, K. A high relaxivity Gd (II) DOTA-DSPE-based liposomal contrast agent for magnetic resonance imaging. Eur. J. Pharm. Biopharm 2009, 72, 397–404. [Google Scholar]
- Terreno, E; Geninatti, CS; Belfiore, S; Biancone, L; Cabella, C; Esposito, G; Manazza, AD; Aime, S. Effect of the intracellular localization of a Gd-based imaging probe on the relaxation enhancement of water protons. Magn. Reson. Med 2006, 55, 491–497. [Google Scholar]
- Strijkers, GJ; Hak, S; Kok, MB; Springer, CS, Jr; Nocolay, K. Three-compartment T1 relaxation model for intracellular paramagnetic contrast agents. Magn. Reson. Med 2009, 6, 1049–1058. [Google Scholar]
- Kok, MB; Hak, S; Mulder, WJ; van der Schaft, DW; Strijkers, GJ; Nicolay, K. Cellular compartmentalization of internalized paramagnetic liposomes strongly influences both T1 and T2 relaxivity. Magn. Reson. Med 2009, 61, 1022–1032. [Google Scholar]
- Kabalka, GW; Buonocore, E; Hubner, K; Davis, MA; Huang, L. Gadolinium-labeled liposomes containing paramagnetic amphipathic agents: Targeted MRI contrast agents for the Liver. Mag. Res. Imaging 1988, 8, 89–95. [Google Scholar]
- Kabalka, GW; Davis, MA; Holmberg, E; Maruyama, K; Huang, L. Gadolinium-labeled liposomes containing amphiphilic Gd-DTPA derivatives of varying chain length: Targeted MRI contrast enhancement agents for the liver. Mag. Res. Imaging 1991, 9, 373–377. [Google Scholar]
- Jasanada, F; Nepveu, F. Synthesis of bis(hexadecylamido) and bis(octadecylamido) of diethyleneaminepentaacetic acid. Tet Lett 1992, 33, 5745–5748. [Google Scholar]
- Kimpe, K; Parac-Vogt, TN; Laurent, S; Pierart, C; Elst, LV; Muller, RN; Binnemans, K. Potential MRI contrast agents based on micellar incorporation of amphiphilic bis(alkylamide) derivatives of [(Gd-DTPA)(H2O)]2−. Eur J Inorg Chem 2003, 3021–3027. [Google Scholar]
- Storrs, RW; Tropper, FD; Li, HY; Song, CK. Paramagnetic polymerized liposomes: Synthesis, characterization, and applications for magnetic resonance imaging. J. Am. Chem. Soc 1995, 117, 7301–7306. [Google Scholar]
- Urizzi, P; Souchard, JP; Nepveu, F. EDTA and DTPA analogues of dipalmitoylphosphatidylethanolamine as lipophilic chelating agents for metal labeling of LDL. Tet. Lett 1996, 37, 4685–4688. [Google Scholar]
- Tournier, H; Hyacinthe, R; Schneider, M. Gadolinium-containing mixed micelle formulations: A new class of blood pool MRI/MRA contrast agents. Acad. Radiol 2002, 9, S20–S28. [Google Scholar]
- Lattuada, L; Lux, G. Synthesis of Gd-DTPA-cholesterol: A new lipophilic gadolinium complex as a potential MRI contrast agent. Tet. Lett 2003, 44, 3893–3895. [Google Scholar]
- Parac-Vogt, TN; Kimpe, K; Laurent, S; Pierart, C; Elst, LV; Muller, RN; Binnemans, K. Paramagnetic liposomes containing amphiphilic bisamide derivatives of Gd-DTPA with aromatic side chain groups as possible contrast agents for magnetic resonance imaging. Eur. Biophys. J 2006, 35, 136–144. [Google Scholar]
- Anelli, PL; Lattuada, L; Gabellini, M; Recanati, P. DOTA Tris(phenylmethyl) ester: A new useful synthon for the synthesis of DOTA monoamides containing acid-labile bonds. Bioconjug. Chem 2001, 12, 1081–1084. [Google Scholar]
- Leclercq, F; Cohen-Ohana, M; Mignet, N; Sbarbati, A; Herscovici, J; Scherman, D; Byk, G. Design, synthesis, and evaluation of gadolinium cationic lipids as tools for biodistribution studies of gene delivery complexes. Bioconjug. Chem 2003, 14, 112–119. [Google Scholar]
- Kamaly, N; Kalber, T; Ahmad, A; Oliver, MH; So, PW; Herlihy, AH; Bell, JD; Jorgensen, MR; Miller, AD. Bimodal paramagnetic and fluorescent liposomes for cellular and tumor magnetic resonance imaging. Bioconjug. Chem 2008, 19, 118–129. [Google Scholar]
- Keller, M; Jorgensen, MR; Perouzel, E; Miller, AD. Thermodynamic aspects and biological profile of CDAN/DOPE and DC-Cho1/DOPE lipoplexes. Biochemistry 2003, 42, 6067–6077. [Google Scholar]
- Fletcher, S; Ahmad, A; Perouzel, E; Jorgensen, MR; Miller, AD. A dialkynoyl analogue of DOPE improves gene transfer efficiency of lower-charged, cationic lipoplexes. Org. Biomol. Chem 2006, 4, 196–199. [Google Scholar]
- Fletcher, S; Ahmad, A; Price, WS; Jorgensen, MR; Miller, AD. Biophysical properties of CDAN/DOPE-analogue lipoplexes account for enhanced gene delivery. ChemBioChem 2008, 9, 455–463. [Google Scholar]
- Kamaly, N; Kalber, T; Kenny, G; Bell, J; Jorgensen, M; Miller, AD. A novel bimodal lipidic contrast agent for cellular labelling and tumour MRI. Org. Biomol. Chem 2010, 8, 201–211. [Google Scholar]
- Klibanov, AL; Maruyama, K; Torchilin, VP; Huang, L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett 1990, 268, 235–237. [Google Scholar]
- Drummond, DC; Meyer, O; Hong, K; Kirpotin, DB; Papahadjopoulos, D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol. Rev 1999, 51, 691–743. [Google Scholar]
- Bertini, I; Bianchini, F; Calorini, L; Colagrande, S; Fragai, M; Franch, A; Gallo, O; Gavazzi, C; Luchinat, C. Persistent contrast enhancement by sterically stabilized paramagnetic liposomes in murine melanoma. Magn. Res. Med 2004, 52, 669–672. [Google Scholar]
- Ayyagari, AL; Zhang, X; Ghaghada, KB; Annapragada, A; Hu, X; Bellamkonda, RV. Long-circulating liposomal contrast agents for magnetic resonance imaging. Magn. Res. Med 2006, 55, 1023–1029. [Google Scholar]
- Erdogan, S; Roby, A; Sawant, R; Hurley, J; Torchilin, VP. Gadolinium-loaded polychelating polymer-containing cancer cell-specific immunoliposomes. J. Liposome Res 2006, 16, 45–55. [Google Scholar]
- Mulder, WJM; Strijkers, GJ; Habets, JW; Bleeker, EJW; van der Schaft, DWJ; Storm, G; Koning, GA; Griffioen, AW; Nicolay, K. MR molecular imaging and fluorescent microscopy for identification of activated tumor endothelium using a bimodal lipidic nanoparticle. Faseb J 2005, 19, 2008–2010. [Google Scholar]
- Brandwijk, RJMGE; Mulder, WJM; Nicolay, K; Mayo, KH; Thijssen, VLJL; Griffioen, AW. Anginex-conjugated liposomes for targeting of angiogenic endothelial cells. Bioconjug. Chem 2007, 18, 785–790. [Google Scholar]
- Mulder, WJM; Strijkers, GJ; Griffioen, AW; van Bloois, L; Molema, G; Storm, G; Koning, GA; Nicolay, K. A liposomal system for contrast-enhanced magnetic resonance imaging of molecular targets. Bioconjug. Chem 2004, 15, 799–806. [Google Scholar]
- Kamaly, N; Kalber, T; Thanou, M; Bell, JD; Miller, AD. Folate receptor targeted bimodal liposomes for tumor magnetic resonance imaging. Bioconjug. Chem 2009, 20, 648–655. [Google Scholar]





© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kamaly, N.; Miller, A.D. Paramagnetic Liposome Nanoparticles for Cellular and Tumour Imaging. Int. J. Mol. Sci. 2010, 11, 1759-1776. https://doi.org/10.3390/ijms11041759
Kamaly N, Miller AD. Paramagnetic Liposome Nanoparticles for Cellular and Tumour Imaging. International Journal of Molecular Sciences. 2010; 11(4):1759-1776. https://doi.org/10.3390/ijms11041759
Chicago/Turabian StyleKamaly, Nazila, and Andrew D. Miller. 2010. "Paramagnetic Liposome Nanoparticles for Cellular and Tumour Imaging" International Journal of Molecular Sciences 11, no. 4: 1759-1776. https://doi.org/10.3390/ijms11041759