A Highly-Sensitive Picric Acid Chemical Sensor Based on ZnO Nanopeanuts
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Synthesis of ZnO Nanopeanuts
2.3. Characterizations of ZnO Nanopeanuts
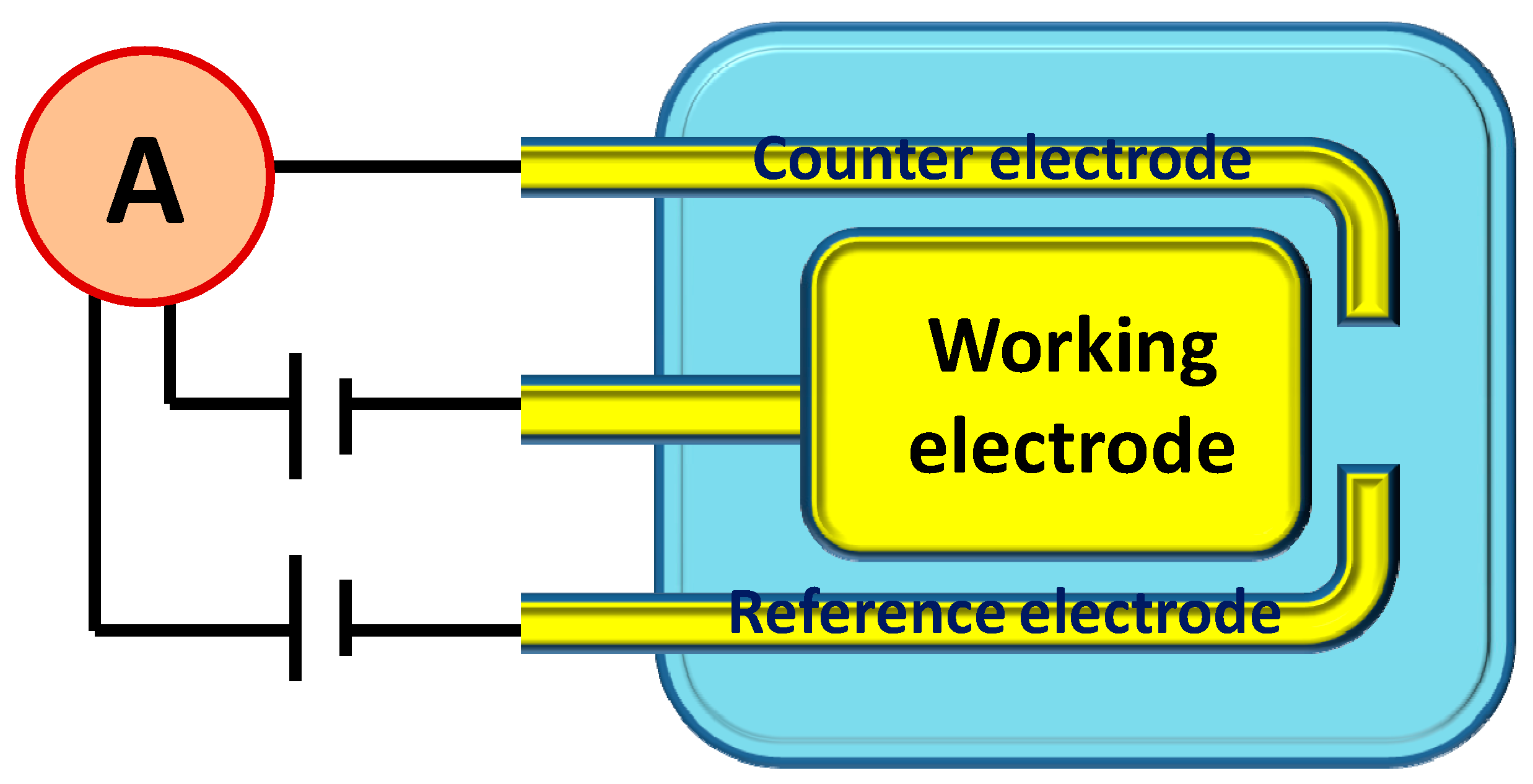
2.4. Fabrication of Electrochemical Sensor Based on Screen Printed Electrode (SPE)
2.5. Fabrication of Picric Acid Sensor Based on Current-Voltage Technique Measurements
3. Results and Discussion
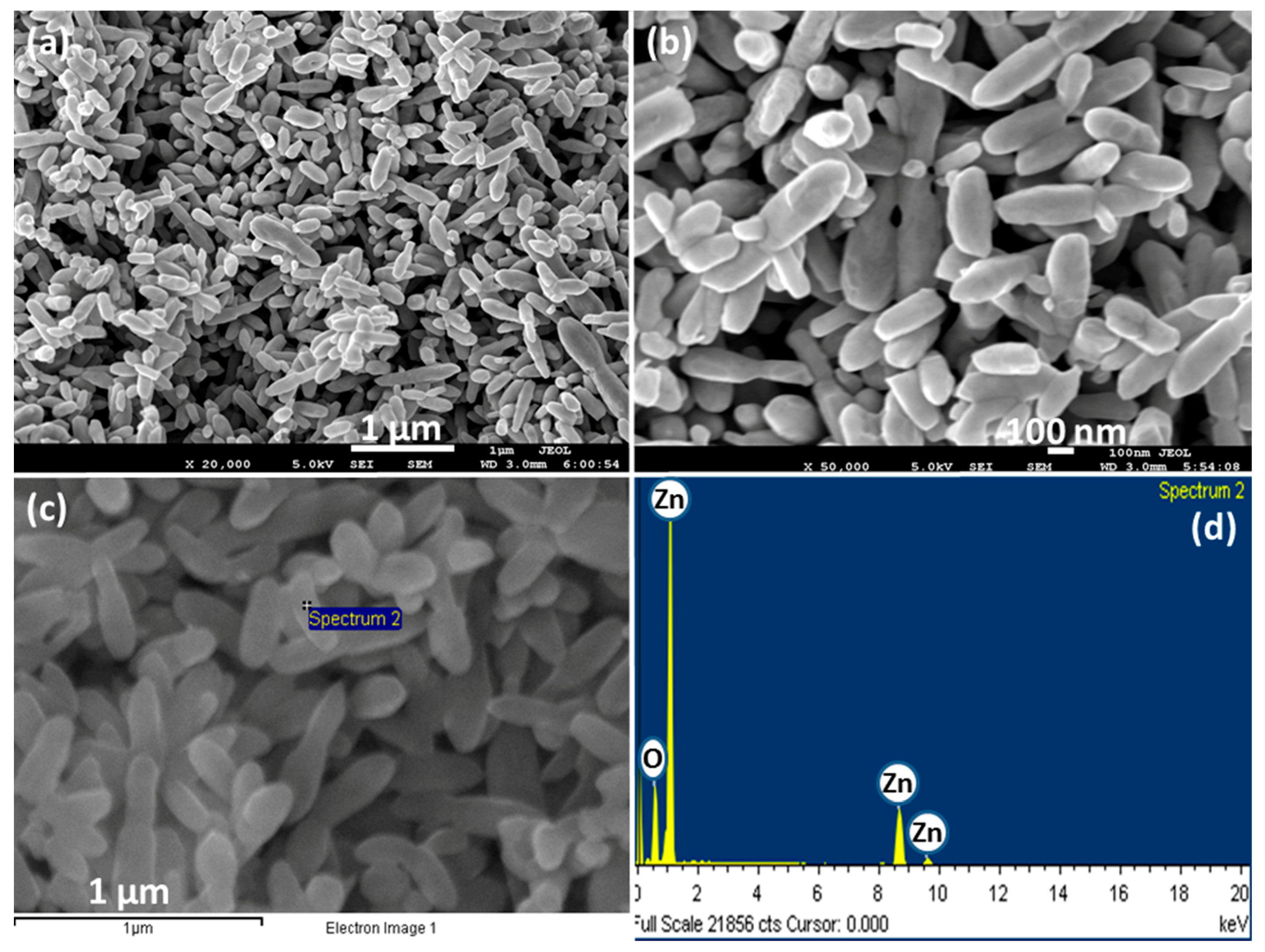
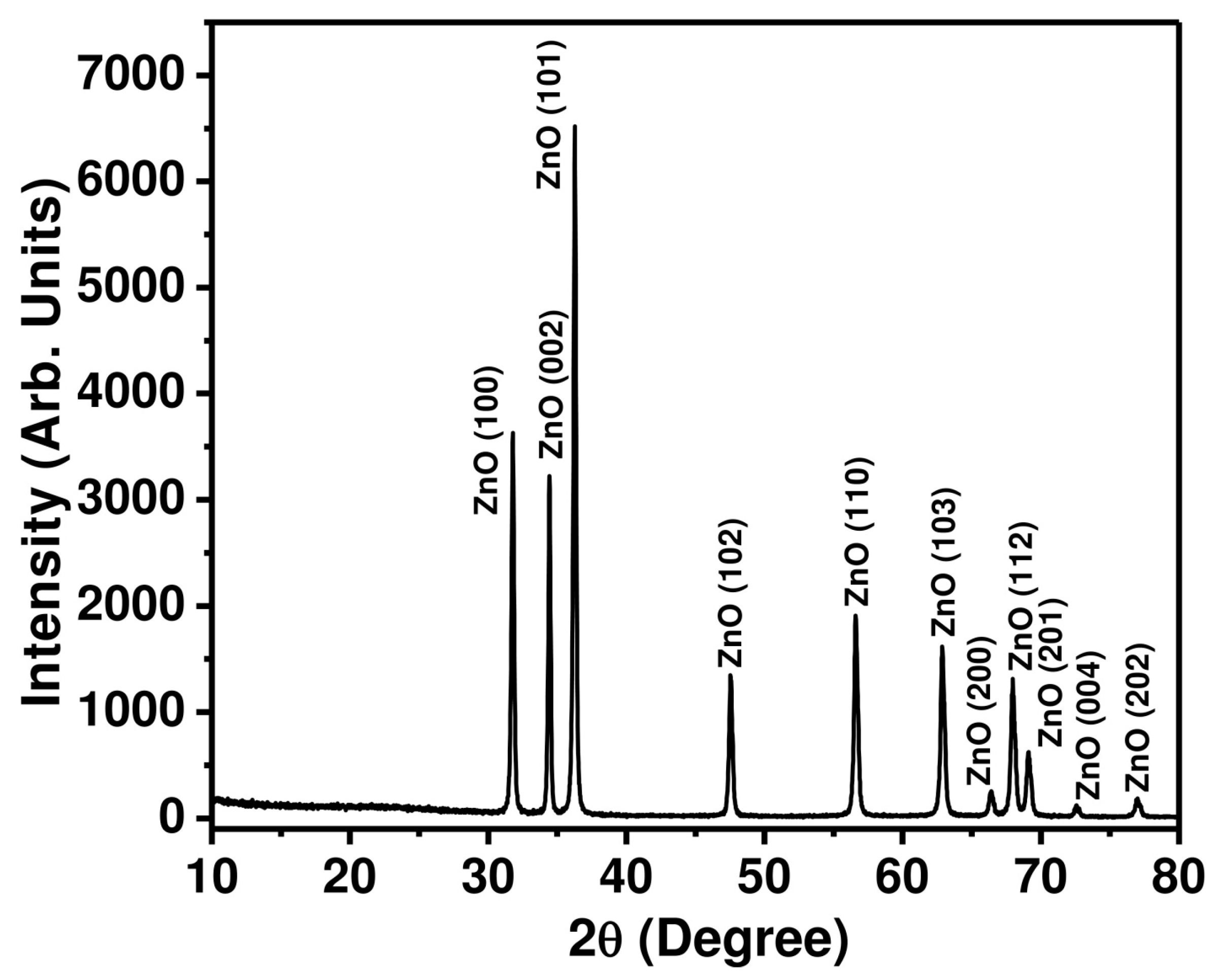
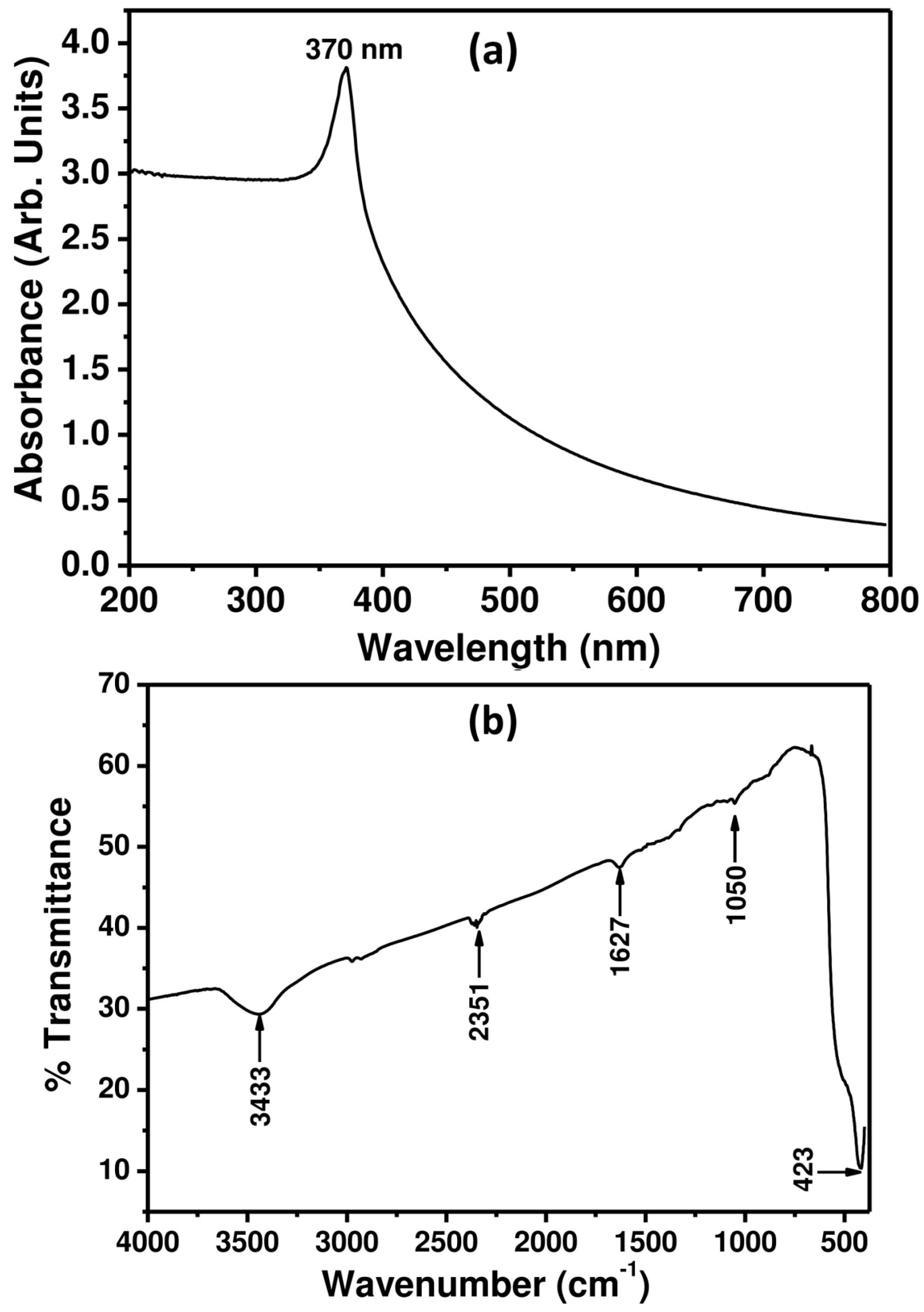
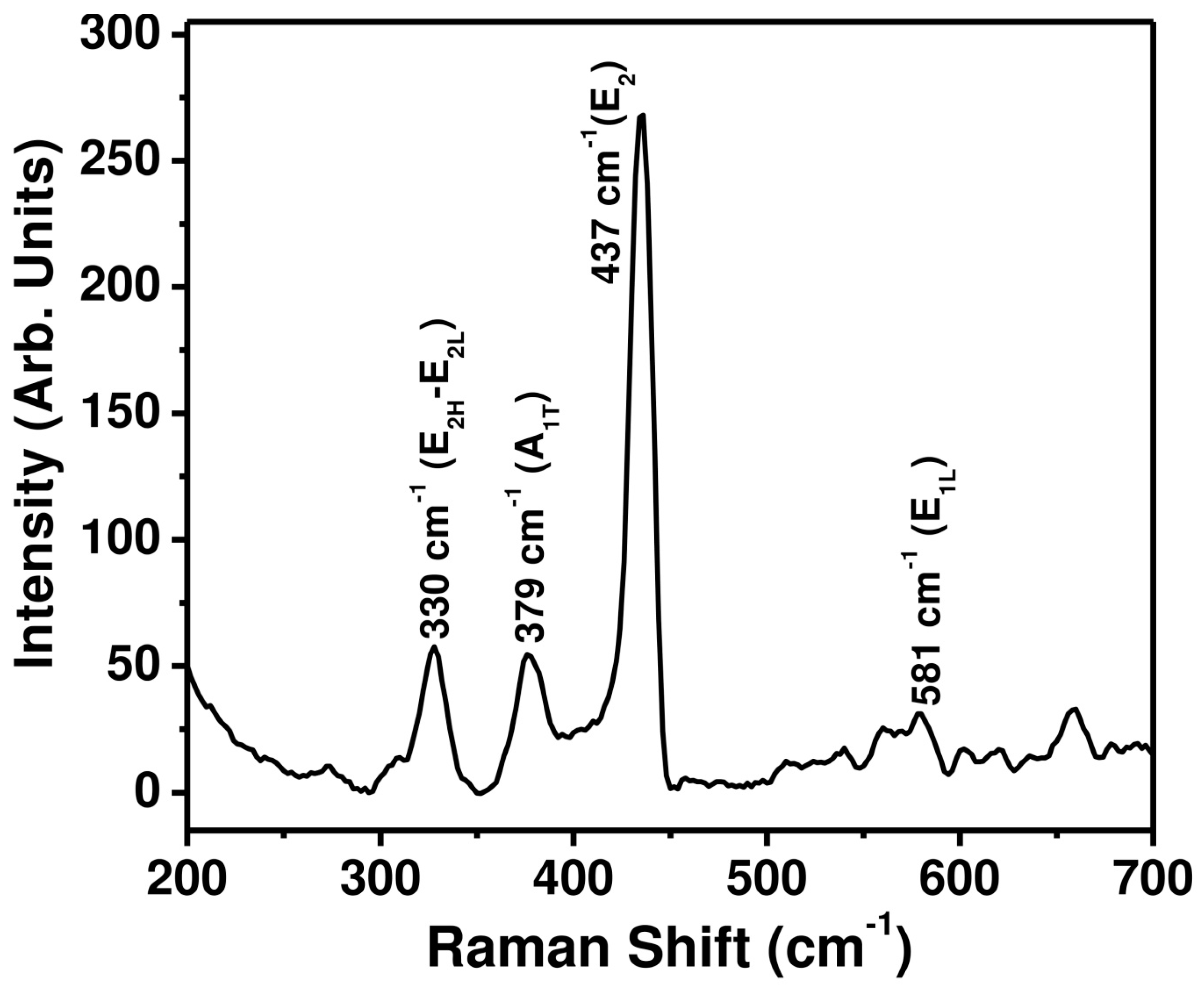
3.1. Characterizations and Properties of ZnO Nanopeanuts
3.2. Performance of Fabricated Picric Acid Sensors Based on ZnO Nanopeanuts
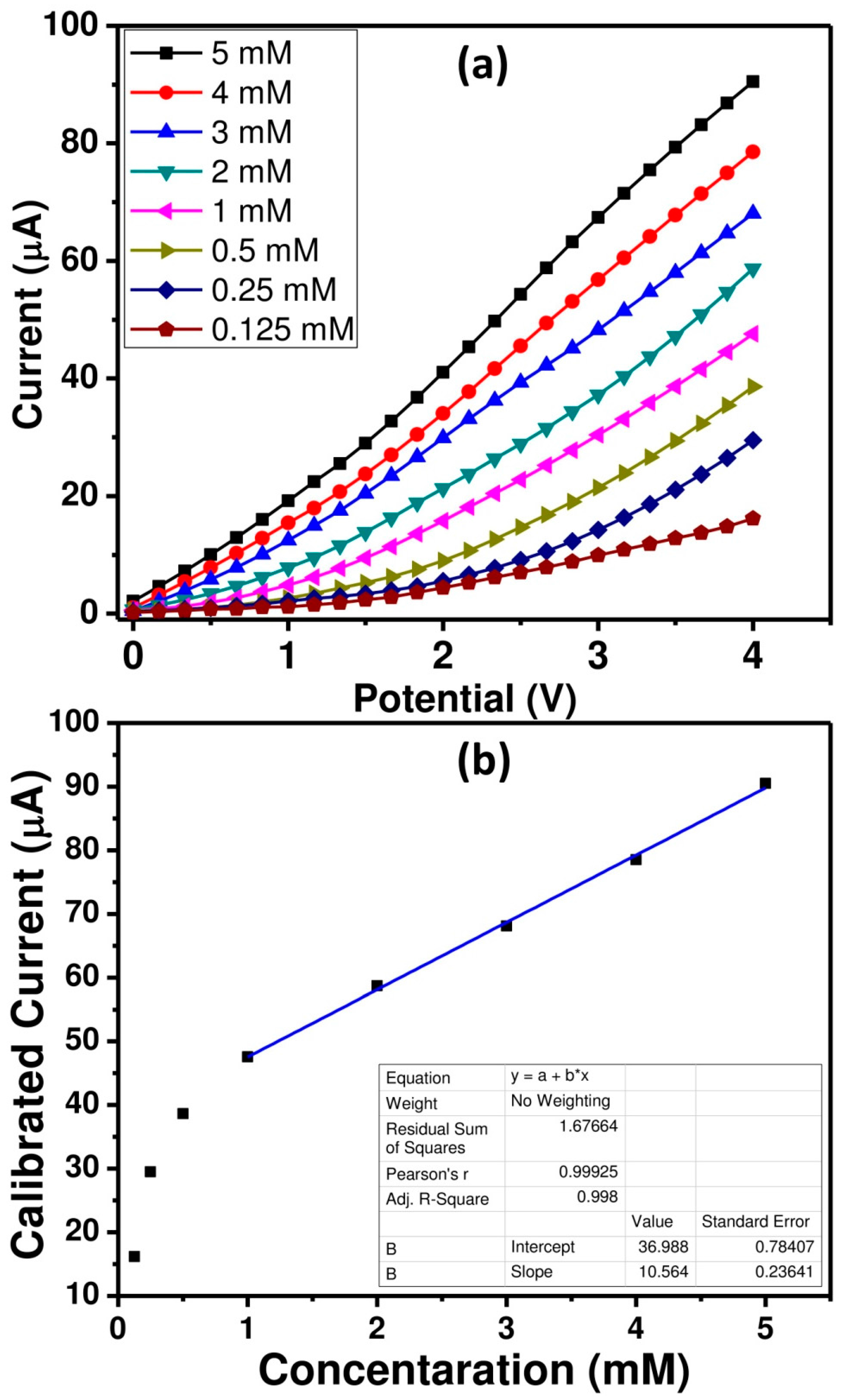
3.2.1. Sensing Properties of Picric Acid Sensor Based on ZnO Nanopeanuts Coated SPE
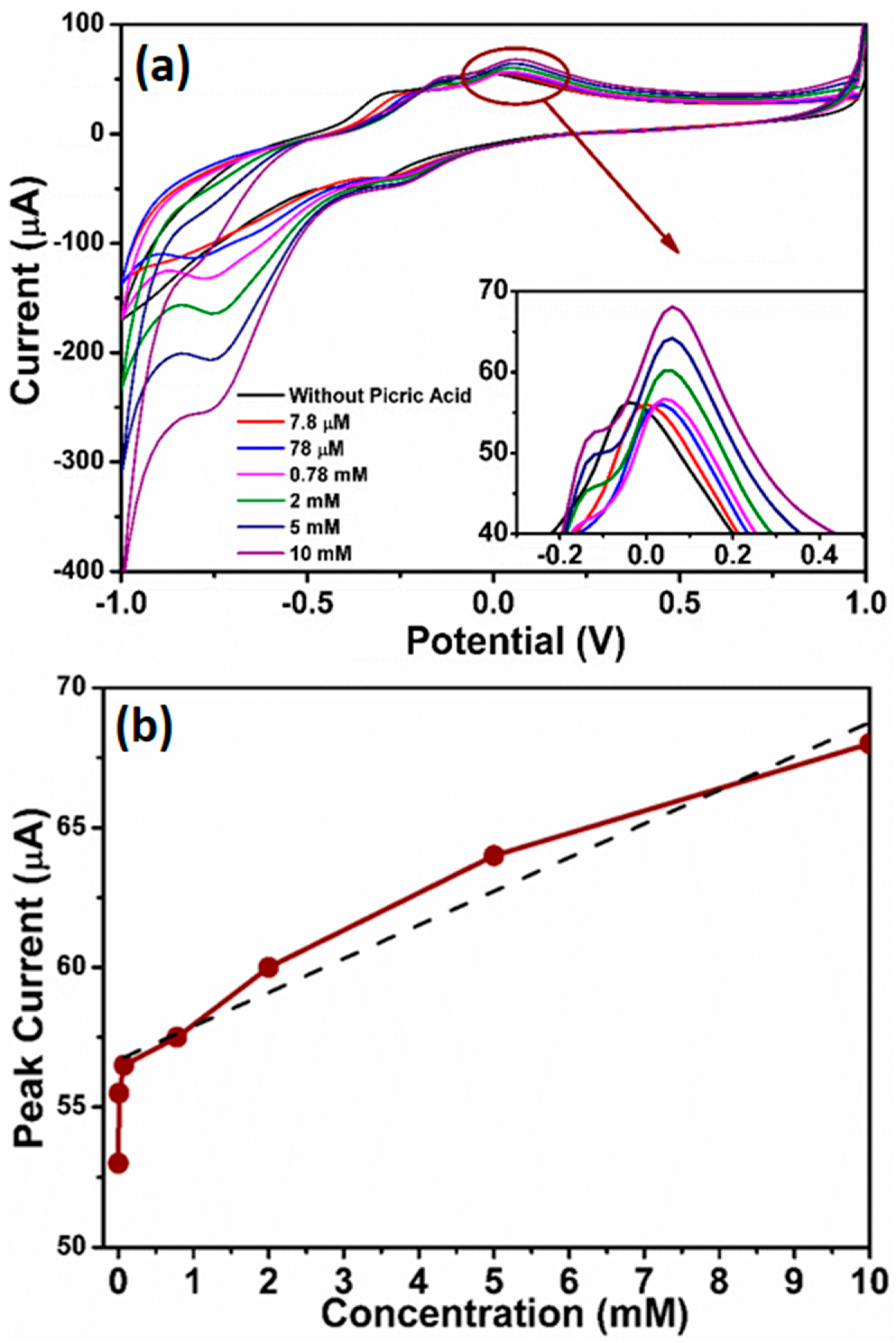
3.2.2. Sensing Properties of Picric Acid Sensor Based on ZnO Nanopeanuts Coated on AgE
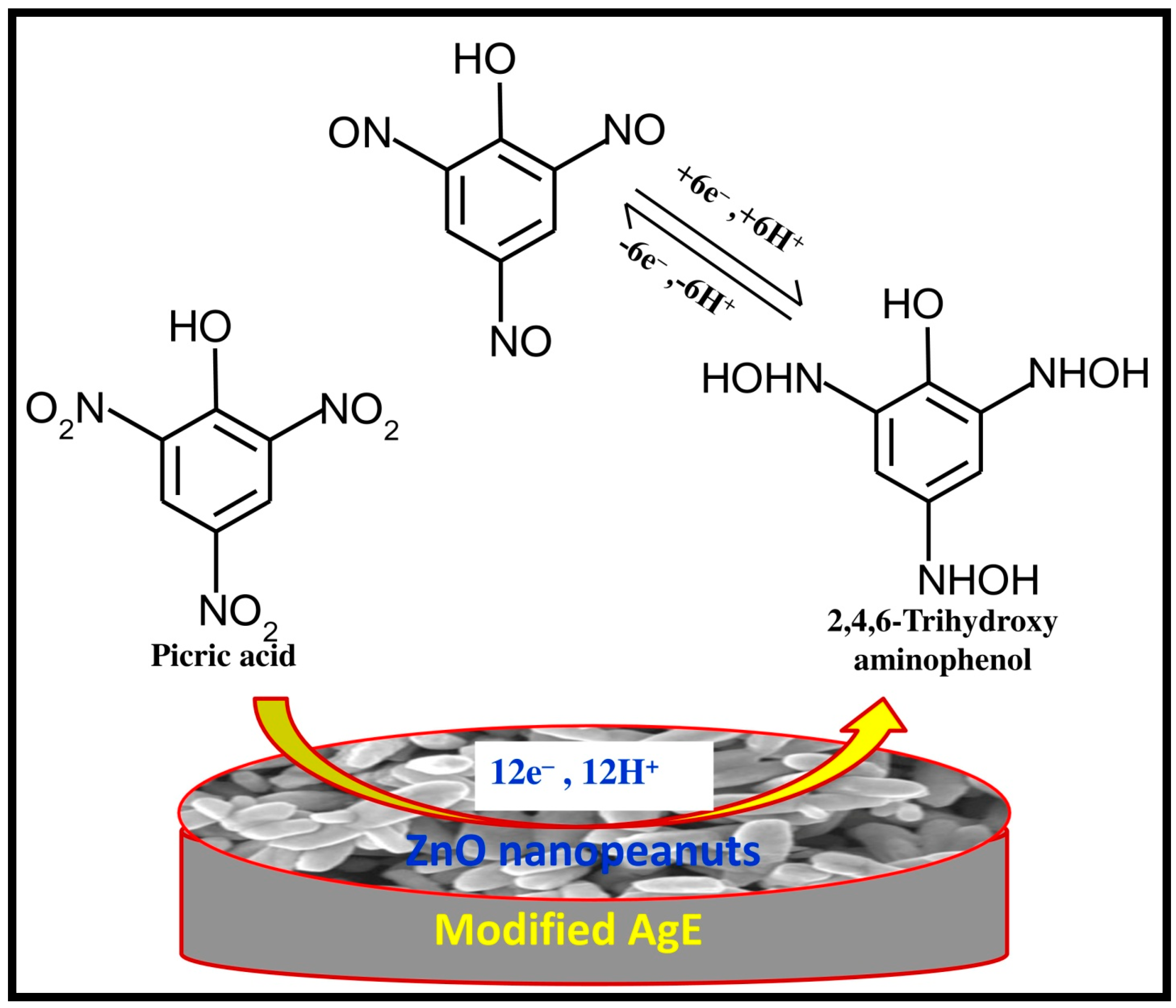
4. Proposed Sensing Mechanism
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, A.; Chatterjee, S. Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev. 2013, 42, 5425–5438. [Google Scholar] [CrossRef] [PubMed]
- Windmiller, J.R.; Wang, J. Wearable electrochemical sensors and biosensors: A Review. Electroanalysis 2013, 25, 29–46. [Google Scholar] [CrossRef]
- Wang, F.; Hu, S. Electrochemical sensors based on metal and semiconductor nanoparticles. Microchim. Acta 2009, 165, 1–22. [Google Scholar] [CrossRef]
- Park, G.C.; Lee, S.M.; Jeong, S.H.; Choi, J.H.; Lee, C.M.; Seo, T.Y.; Jung, S.-B.; Lim, J.H.; Joo, J. Enhanced photocatalytic activity of ZnO nanorods with tubular facet synthesized by hydrothermal method. J. Nanosci. Nanotechnol. 2016, 16, 11164–11168. [Google Scholar] [CrossRef]
- Wei, A.; Pan, L.; Huang, W. Recent progress in the ZnO nanostructure-based sensors. Mater. Sci. Eng. Solid-State Mater. Adv. Technol. 2011, 176, 1409–1421. [Google Scholar] [CrossRef]
- Katwal, G.; Paulose, M.; Rusakova, I.A.; Martinez, J.E.; Varghese, O.K. Rapid growth of zinc oxide nanotube-nanowire hybrid architectures and their use in breast cancer related volatile organics detection. Nano Lett. 2016, 16, 3014–3021. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Park, G.C.; Choi, J.H.; Lee, C.M.; Lee, S.M.; Seo, T.Y.; Choi, D.H.; Jung, S.B.; Lim, J.H.; Joo, J. Effect of Al incorporation on morphology and electrical conductivity of ZnO nanorods prepared using hydrothermal method. J. Nanosci. Nanotechnol. 2016, 16, 11272–11276. [Google Scholar] [CrossRef]
- Caglar, M.; Gorgun, K. Characterization and heterojunction application of nanorod structure ZnO films prepared by microwave assisted chemical bath deposition method without any template. J. Nanoelectron. Optoelectron. 2016, 11, 769–776. [Google Scholar] [CrossRef]
- Kumar, R.; Al-Dossary, O.; Kumar, G.; Umar, A. Zinc oxide nanostructures for NO2 gas-sensor applications: A review. Nano-Micro Lett. 2015, 7, 97–120. [Google Scholar] [CrossRef]
- Singh, K.; Chaudhary, G.R.; Singh, S.; Mehta, S.K. Synthesis of highly luminescent water stable ZnO quantum dots as photoluminescent sensor for picric acid. J. Lumin. 2014, 154, 148–154. [Google Scholar] [CrossRef]
- Ameen, S.; Akhtar, M.S.; Seo, H.K.; Shin, H.S. An electrochemical sensing platform based on hollow mesoporous ZnO nanoglobules modified glassy carbon electrode: Selective detection of piperidine chemical. Chem. Eng. J. 2015, 270, 564–571. [Google Scholar] [CrossRef]
- Katoch, A.; Choi, S.-W.; Sun, G.-J.; Kim, S.S. Low temperature sensing properties of Pt nanoparticle-functionalized networked ZnO nanowires. J. Nanosci. Nanotechnol. 2015, 15, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Mani, G.K.; Rayappan, J.B.B. ZnO nanoarchitectures: Ultrahigh sensitive room temperature acetaldehyde sensor. Sens. Actuators Chem. 2016, 223, 2343–2351. [Google Scholar] [CrossRef]
- Farmanzadeh, D.; Tabari, L. DFT study of adsorption of picric acid molecule on the surface of single-walled ZnO nanotube; as potential new chemical sensor. Appl. Surf. Sci. 2015, 324, 864–870. [Google Scholar] [CrossRef]
- Kumar, R.; Rana, D.; Umar, A.; Sharma, P.; Chauhan, S.; Chauhan, M.S. Ag-doped ZnO nanoellipsoids: Potential scaffold for photocatalytic and sensing applications. Talanta 2015, 137, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Ozden, P.B.; Caglar, Y.; Ilican, S.; Caglar, M. Effect of deposition time of electrodeposited ZnO nanorod films on crystallinity, microstructure and absorption edge. J. Nanoelectron. Optoelectron. 2016, 11, 244–249. [Google Scholar] [CrossRef]
- Zhu, L.-P.; Jiao, Y.-H.; Bing, N.-C.; Wang, L.-L.; Ye, Y.-K.; Wang, L.-J. Influences of Ni Doping on the morphology, optical and magnetic properties of ZnO nanostructures synthesized by solvothermal process. J. Nanosci. Nanotechnol. 2015, 15, 3234–3238. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Lee, Y.-M.; Yu, J.-H.; Hwang, K.-H.; Boo, J.-H. Patterned well-aligned zno nanorods assisted with polystyrene monolayer by oxygen plasma treatment. Materials (Basel) 2016, 9, 656. [Google Scholar] [CrossRef]
- Molaakbari, E.; Mostafavi, A.; Beitollahi, H.; Alizadeh, R. Synthesis of ZnO nanorods and their application in the construction of a nanostructure-based electrochemical sensor for determination of levodopa in the presence of carbidopa. Analyst 2014, 139, 4356–4364. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Roy, E.; Madhuri, R.; Sharma, P.K. Imprinted ZnO nanostructure-based electrochemical sensing of calcitonin: A clinical marker for medullary thyroid carcinoma. Anal. Chim. Acta 2015, 853, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Dar, G.N.; Umar, A.; Zaidi, S.A.; Baskoutas, S.; Hwang, S.W.; Abaker, M.; Al-Hajry, A.; Al-Sayari, S.A. Ultra-high sensitive ammonia chemical sensor based on ZnO nanopencils. Talanta 2012, 89, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, S.; Fawad, U.; Mujahid, M.; Khasim, S.; Hamdalla, T.; Kim, H.J. Three dimensional spherically evolved nanostructures of ZnO comprised of nanowires and nanorods for optoelectronic devices. J. Nanoelectron. Optoelectron. 2015, 10, 700–704. [Google Scholar] [CrossRef]
- Mehta, S.K.; Singh, K.; Umar, A.; Chaudhary, G.R.; Singh, S. Ultra-high sensitive hydrazine chemical sensor based on low-temperature grown ZnO nanoparticles. Electrochim. Acta 2012, 69, 128–133. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Jiang, J.; Huang, X. C@ZnO nanorod array-based hydrazine electrochemical sensor with improved sensitivity and stability. Dalton Trans. 2010, 39, 8693–8697. [Google Scholar] [CrossRef] [PubMed]
- Vabbina, P.K.; Kaushik, A.; Pokhrel, N.; Bhansali, S.; Pala, N. Electrochemical cortisol immunosensors based on sonochemically synthesized zinc oxide 1D nanorods and 2D nanoflakes. Biosens. Bioelectron. 2015, 63, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jo, S.Y.; Sun, G.J.; Katoch, A.; Choi, S.W.; Kim, S.S. Tailoring the surface area of ZnO nanorods for improved performance in glucose sensors. Sens. Actuator Chem. 2014, 192, 216–220. [Google Scholar] [CrossRef]
- Jin, Z.; Park, C.-I.; Hwang, I.-H.; Han, S.-W. Local structural properties and growth mechanism of ZnO nanorods on hetero-interfaces. J. Nanosci. Nanotechnol. 2015, 15, 5306–5309. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Yan, X.; Zhao, J.; Liu, X.; Song, Y.; Luo, N.; Zhang, Y. Improved glucose electrochemical biosensor by appropriate immobilization of nano-ZnO. Colloids Surf. Biointerfaces 2011, 82, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Chiu, Y.S.; Ho, S.C.; Lee, Y.J. Investigation of a photoelectrochemical passivated ZnO-based glucose biosensor. Sensors 2011, 11, 4648–4655. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Tripathy, N.; Hahn, Y.B. Highly stable urea sensor based on ZnO nanorods directly grown on Ag/glass electrodes. Sens. Actuator Chem. 2014, 194, 290–295. [Google Scholar] [CrossRef]
- Ali, S.M.U.; Ibupoto, Z.H.; Salman, S.; Nur, O.; Willander, M.; Danielsson, B. Selective determination of urea using urease immobilized on ZnO nanowires. Sens. Actuator Chem. 2011, 160, 637–643. [Google Scholar] [CrossRef]
- Ali, S.M.U.; Ibupoto, Z.H.; Kashif, M.; Hashim, U.; Willander, M. A potentiometric indirect uric acid sensor based on ZnO nanoflakes and immobilized uricase. Sensors 2012, 12, 2787–2797. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-C.; Chung, C.-C.; Lo, Y.-J.; Wang, C.-C. Microstructure-dependent visible-light driven photoactivity of sputtering-assisted synthesis of sulfide-based visible-light sensitizer onto ZnO nanorods. Materials (Basel) 2016, 9, 1014. [Google Scholar] [CrossRef]
- Gençyilmaz, O.; Atay, F.; Akyüz, D. The effect of Co doping on ZnO films: Structural, morphological characterization and hall effect measurements. J. Nanoelectron. Optoelectron. 2015, 10, 799–805. [Google Scholar] [CrossRef]
- Harraz, F.A.; Ismail, A.A.; Ibrahim, A.A.; Al-Sayari, S.A.; Al-Assiri, M.S. Highly sensitive ethanol chemical sensor based on nanostructured SnO2 doped ZnO modified glassy carbon electrode. Chem. Phys. Lett. 2015, 639, 238–242. [Google Scholar] [CrossRef]
- Jeon, Y.S.; Seo, H.W.; Kim, S.H.; Kim, Y.K. Synthesis of Fe doped ZnO nanowire arrays that detect formaldehyde gas. J. Nanosci. Nanotechnol. 2016, 16, 4814–4819. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z.; Zhang, H.; Luo, L.; Yao, S. Sensitive and selective imprinted electrochemical sensor for p-nitrophenol based on ZnO nanoparticles/carbon nanotubes doped chitosan film. Thin Solid Films 2012, 520, 5314–5321. [Google Scholar] [CrossRef]
- Zhu, D.; He, Q.; Cao, H.; Cheng, J.; Feng, S.; Xu, Y.; Lin, T. Poly(phenylene ethynylene)-coated aligned ZnO nanorod arrays for 2,4,6-trinitrotoluene detection. Appl. Phys. Lett. 2008, 93, 11864. [Google Scholar] [CrossRef]
- Ameen, S.; Akhtar, M.S.; Seo, H.K.; Shin, H.S. Deployment of aligned ZnO nanorod with distinctive porous morphology: Potential scaffold for the detection of p-nitrophenylamine. Appl. Catal. Gen. 2014, 470, 271–277. [Google Scholar] [CrossRef]
- Selvam, N.C.S.; Jesudoss, S.K.; Rajan, P.I.; Kennedy, L.J.; Vijaya, J.J. Comparative investigation on the photocatalytic degradation of 2,4,6-trichlorophenol using pure and m-doped (M = Ba, Ce, Mg) ZnO spherical nanoparticles. J. Nanosci. Nanotechnol. 2015, 15, 5910–5917. [Google Scholar] [CrossRef]
- Ameen, S.; Akhtar, M.S.; Shin, H.S. Low temperature grown ZnO nanotubes as smart sensing electrode for the effective detection of ethanolamine chemical. Mater. Lett. 2013, 106, 254–258. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Nitrophenols, Ambient Water Qualify Criteria; U.S. Environmental Protection Agency: Washington, DC, USA, 1980.
- Kaur, N.; Sharma, S.K.; Kim, D.Y.; Sharma, H.; Singh, N. Synthesis of imine-bearing ZnO nanoparticle thin films and characterization of their structural, morphological and optical properties. J. Nanosci. Nanotechnol. 2015, 15, 8114–8119. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Al-Hajry, A.; Ahmad, R.; Ansari, S.; Al-Assiri, M.S.; Algarni, H. Fabrication and characterization of a highly sensitive hydroquinone chemical sensor based on iron-doped ZnO nanorods. Dalton Trans. 2015, 44, 21081–21087. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Kumar, R.; Umar, A.; Kim, S.H.; Bumajdad, A.; Ansari, Z.A.; Baskoutas, S. Cauliflower-shaped ZnO nanomaterials for electrochemical sensing and photocatalytic applications. Electrochim. Acta 2017, 222, 463–472. [Google Scholar] [CrossRef]
- Ahmed, F.; Arshi, N.; Jeong, Y.S.; Anwar, M.S.; Dwivedi, S.; Alsharaeh, E.; Koo, B.H. Novel biomimatic synthesis of ZnO nanorods using egg white (Albumen) and their antibacterial studies. J. Nanosci. Nanotechnol. 2016, 16, 5959–5965. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, M.; Mayandi, J.; Mariammal, R.N.; Vishnukanthan, V.; Pearce, J.M.; Soundararajan, N.; Ramachandran, K. Peanut shaped ZnO microstructures: Controlled synthesis and nucleation growth toward low-cost dye sensitized solar cells. Mater. Res. Express 2015, 2, 066202. [Google Scholar] [CrossRef]
- Wang, A.J.; Liao, Q.C.; Feng, J.J.; Zhang, P.P.; Li, A.Q.; Wang, J.J. Apple pectin-mediated green synthesis of hollow double-caged peanut-like ZnO hierarchical superstructures and photocatalytic applications. CrystEngComm 2012, 14, 256–263. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, G.; Akhtar, M.S.; Umar, A. Sonophotocatalytic degradation of methyl orange using ZnO nano-aggregates. J. Alloys Compd. 2015, 629, 167–172. [Google Scholar] [CrossRef]
- Lee, C.-H.; Oh, S.-H. Preparation of Ga-doped ZnO thin films by metal-organic chemical vapor deposition with ultrasonic nebulization. J. Nanosci. Nanotechnol. 2016, 16, 11552–11557. [Google Scholar] [CrossRef]
- Umar, A.; Kumar, R.; Kumar, G.; Algarni, H.; Kim, S.H. Effect of annealing temperature on the properties and photocatalytic efficiencies of ZnO nanoparticles. J. Alloys Compd. 2015, 648, 46–52. [Google Scholar] [CrossRef]
- Umar, A.; Kumar, R.; Akhtar, M.S.; Kumar, G.; Kim, S.H. Growth and properties of well-crystalline cerium oxide (CeO2) nanoflakes for environmental and sensor applications. J. Colloid Interface Sci. 2015, 454, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Punnoose, A.; Dodge, K.; Rasmussen, J.W.; Chess, J.; Wingett, D.; Anders, C. Cytotoxicity of ZnO nanoparticles can be tailored by modifying their surface structure: A green chemistry approach for safer nanomaterials. ACS Sustain. Chem. Eng. 2014, 2, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Lee, J.; Kumar, R.; Al-Dossary, O.; Ibrahim, A.; Baskoutas, S. Development of highly sensitive and selective ethanol sensor based on lance-shaped CuO nanostructures. Mater. Des. 2016, 105, 16–24. [Google Scholar] [CrossRef]
- Kim, S.; Umar, A.; Kumar, R.; Algarni, H.; Kumar, G. Facile and rapid synthesis of ZnO nanoparticles for photovoltaic device application. J. Nanosci. Nanotechnol. 2015, 15, 6807–6812. [Google Scholar] [CrossRef] [PubMed]
- Rana, U.H.S.; Kang, M.; Jeong, E.-S.; Kim, H.-S. Transition between ZnO nanorods and ZnO nanotubes with their antithetical properties. J. Nanosci. Nanotechnol. 2016, 16, 10772–10776. [Google Scholar] [CrossRef]
- Kuriakose, S.; Satpati, B.; Mohapatra, S. Enhanced photocatalytic activity of Co doped ZnO nanodisks and nanorods prepared by a facile wet chemical method. Phys. Chem. Chem. Phys. 2014, 16, 12741–12749. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, H.; Li, S.; Li, M.; Yan, L. Synthesis, characterization and optical properties of ZnO nanostructure. J. Nanosci. Nanotechnol. 2016, 16, 8766–8771. [Google Scholar] [CrossRef]
- Chang, W.-C.; Yu, W.-C.; Wu, C.-H.; Wang, C.-Y.; Hong, Z.-S.; Wu, R.-J. Flower-Like ZnO nanostructure for NO sensing at room temperature. J. Nanosci. Nanotechnol. 2016, 16, 9209–9214. [Google Scholar] [CrossRef]
- Takeuchi, E.S.; Murray, R.W. Metalloporphyrin containing carbon paste electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1985, 188, 49–57. [Google Scholar] [CrossRef]
- Singh, K.; Ibrahim, A.A.; Umar, A.; Kumar, A.; Chaudhary, G.R.; Singh, S.; Mehta, S.K. Synthesis of CeO 2–ZnO nanoellipsoids as potential scaffold for the efficient detection of 4-nitrophenol. Sens. Actuator Chem. B 2014, 202, 1044–1050. [Google Scholar] [CrossRef]
- Kim, K.Y.; Cho, C.H.; Le Shim, E. Effect of working pressure during ZnO thin-film layer deposition on transparent resistive random access memory device characteristics. J. Nanosci. Nanotechnol. 2016, 16, 10313–10318. [Google Scholar] [CrossRef]
- Picric Acid, Human Health Effects, US Departement of Human & Health Services. Available online: https://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+2040 (accessed on 4 January 2011).
- Huang, J.; Wang, L.; Shi, C.; Dai, Y.; Gu, C.; Liu, J. Selective detection of picric acid using functionalized reduced graphene oxide sensor device. Sens. Actuator Chem. 2014, 196, 567–573. [Google Scholar] [CrossRef]
- Roy, N.; Chowdhury, A.; Paul, T.; Roy, A. Morphological, optical, and raman characteristics of ZnO nanoflowers on ZnO-seeded Si substrates synthesized by chemical method. J. Nanosci. Nanotechnol. 2016, 16, 9738–9745. [Google Scholar] [CrossRef]
- Junqueira, J.R.C.; de Araujo, W.R.; Salles, M.O.; Paixão, T.R.L.C. Flow injection analysis of picric acid explosive using a copper electrode as electrochemical detector. Talanta 2013, 104, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Sadhanala, H.K.; Nanda, K.K. Boron and nitrogen Co-doped carbon nanoparticles as photoluminescent probes for selective and sensitive detection of picric acid. J. Phys. Chem. 2015, 119, 13138–13143. [Google Scholar] [CrossRef]
- Sharma, P.; Rana, D.S.; Umar, A.; Kumar, R.; Chauhan, M.S.; Chauhan, S. Synthesis of cadmium sulfide nanosheets for smart photocatalytic and sensing applications. Ceram. Int. 2016, 42, 6601–6609. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, A.A.; Tiwari, P.; Al-Assiri, M.S.; Al-Salami, A.E.; Umar, A.; Kumar, R.; Kim, S.H.; Ansari, Z.A.; Baskoutas, S. A Highly-Sensitive Picric Acid Chemical Sensor Based on ZnO Nanopeanuts. Materials 2017, 10, 795. https://doi.org/10.3390/ma10070795
Ibrahim AA, Tiwari P, Al-Assiri MS, Al-Salami AE, Umar A, Kumar R, Kim SH, Ansari ZA, Baskoutas S. A Highly-Sensitive Picric Acid Chemical Sensor Based on ZnO Nanopeanuts. Materials. 2017; 10(7):795. https://doi.org/10.3390/ma10070795
Chicago/Turabian StyleIbrahim, Ahmed A., Preeti Tiwari, M. S. Al-Assiri, A. E. Al-Salami, Ahmad Umar, Rajesh Kumar, S. H. Kim, Z. A. Ansari, and S. Baskoutas. 2017. "A Highly-Sensitive Picric Acid Chemical Sensor Based on ZnO Nanopeanuts" Materials 10, no. 7: 795. https://doi.org/10.3390/ma10070795





