Effect of Preservative Pretreatment on the Biological Durability of Corn Straw Fiber/HDPE Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Preparation
2.2. Blend Design and Sample Fabrication
2.3. Biological Tests
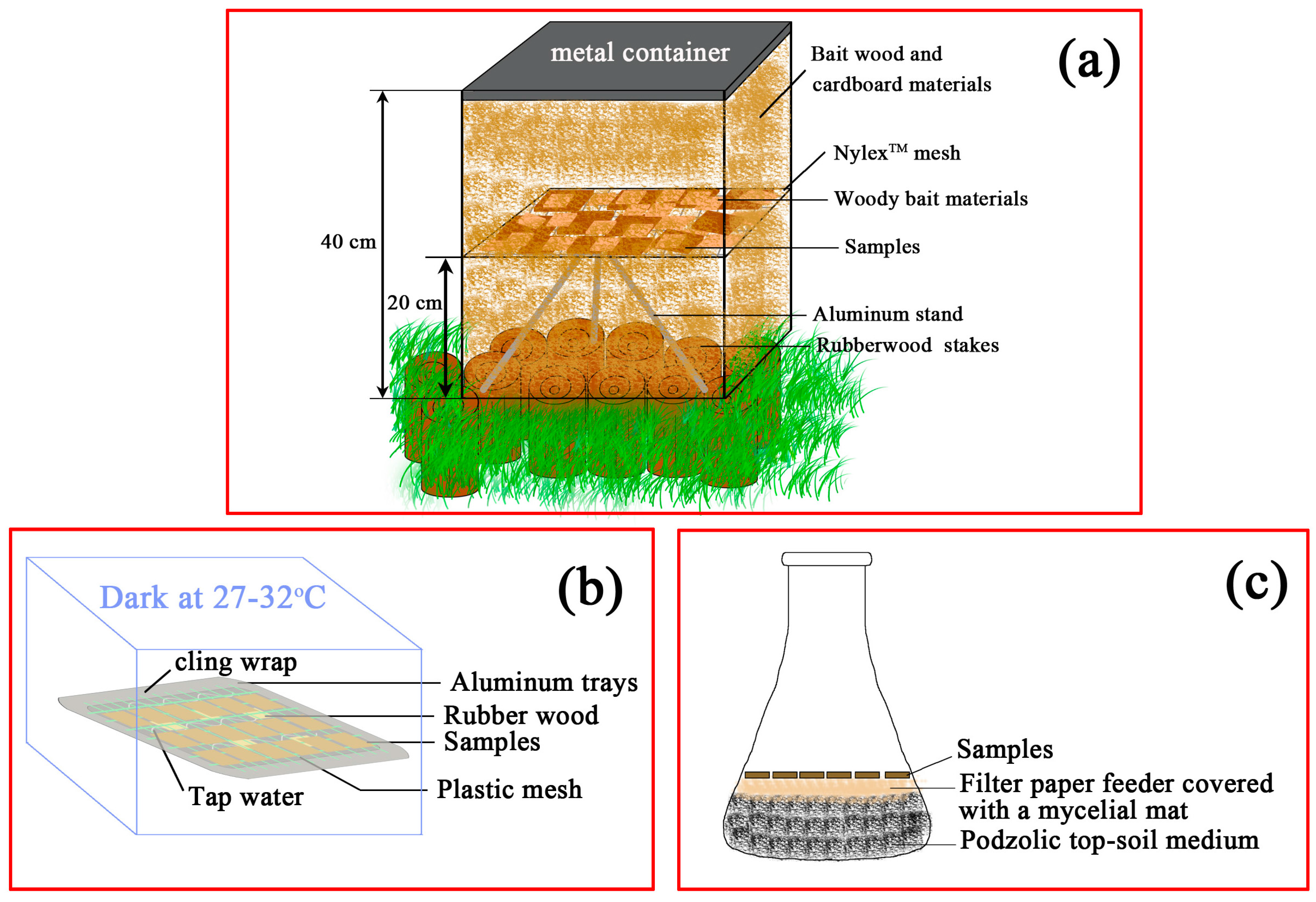
2.3.1. Subterranean Termite Field Test
2.3.2. Laboratory Mold Test
2.3.3. Decay Resistance Test
2.4. Scanning Electron Microscope
3. Results and Discussion
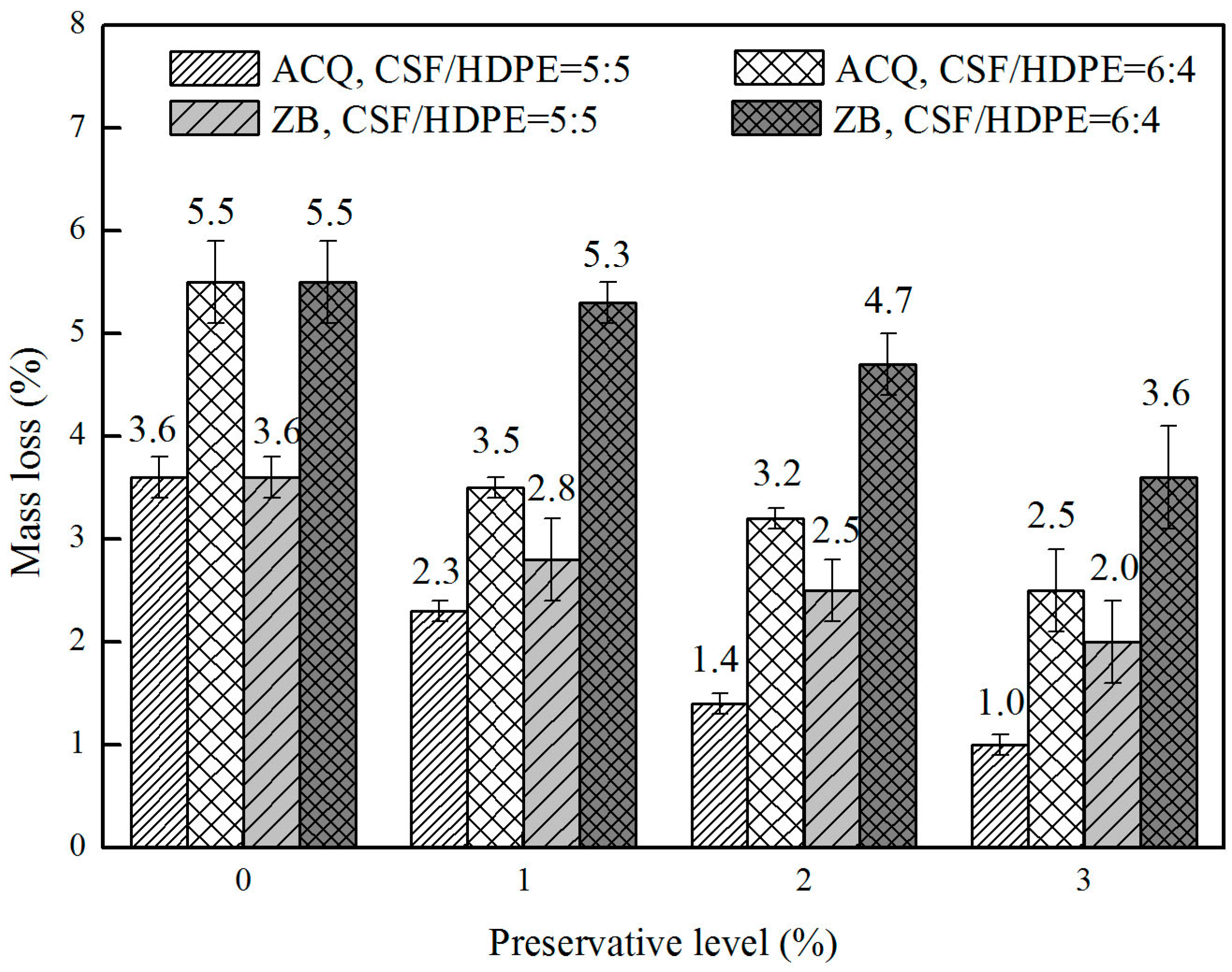
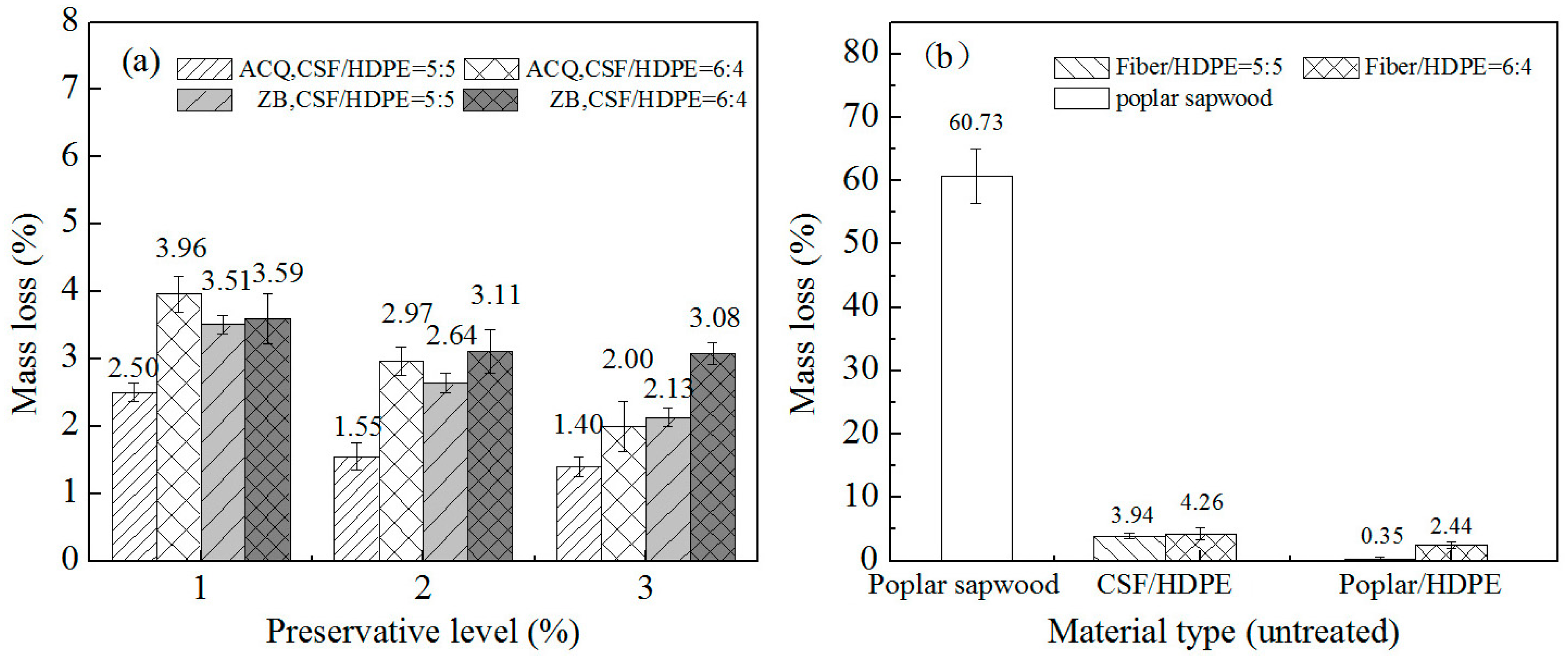
3.1. Termite Resistance
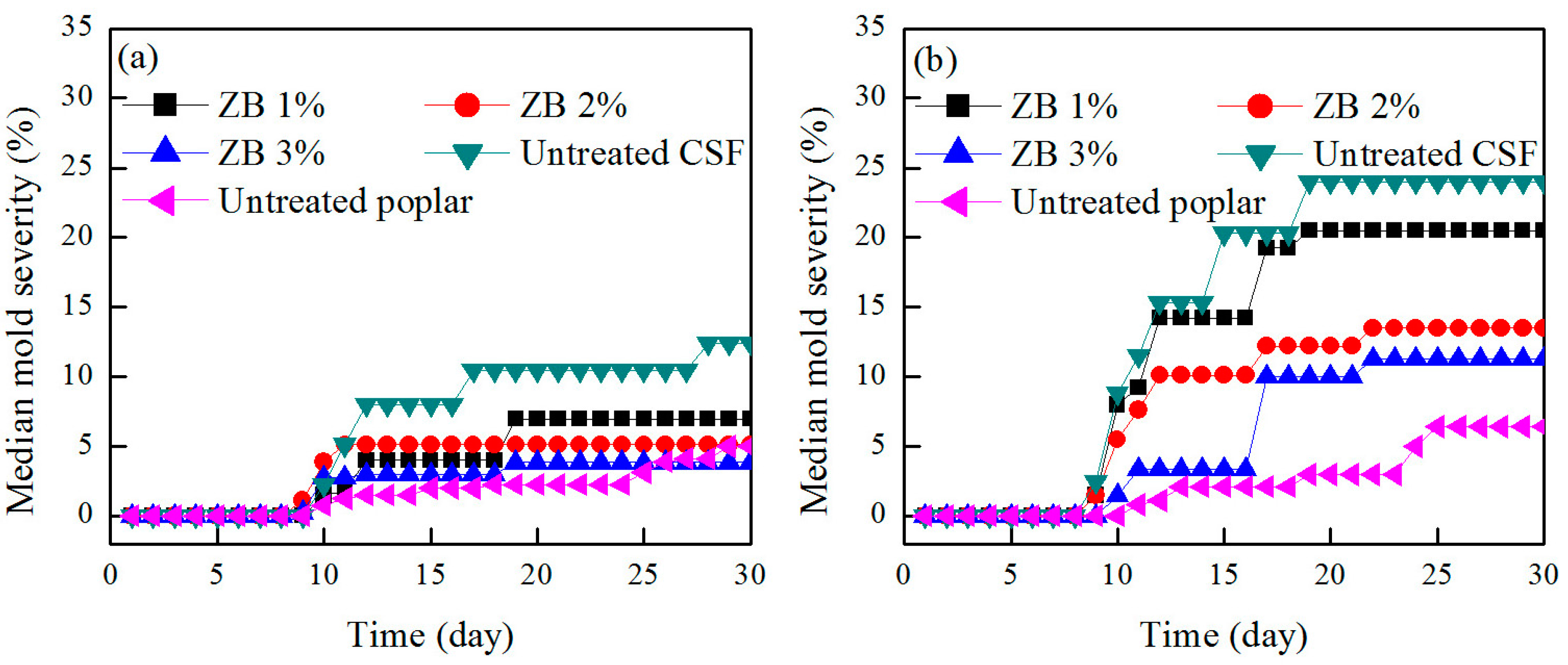
3.2. Mold Resistance
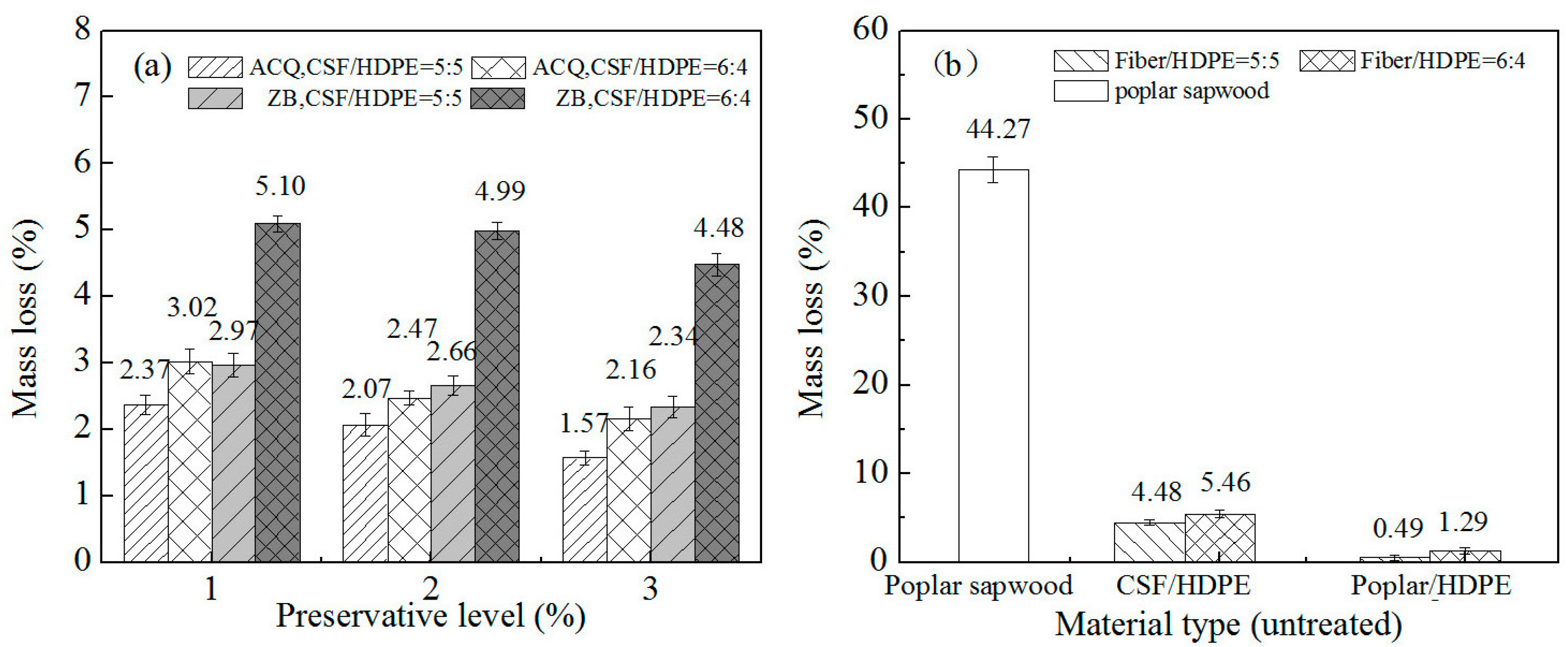
3.3. Resistance to Wood-Decay Fungi
3.3.1. Resistance to White Rot Fungus
3.3.2. Resistance to Brown Rot Fungus
3.3.3. Resistance to Soft Rot Fungus
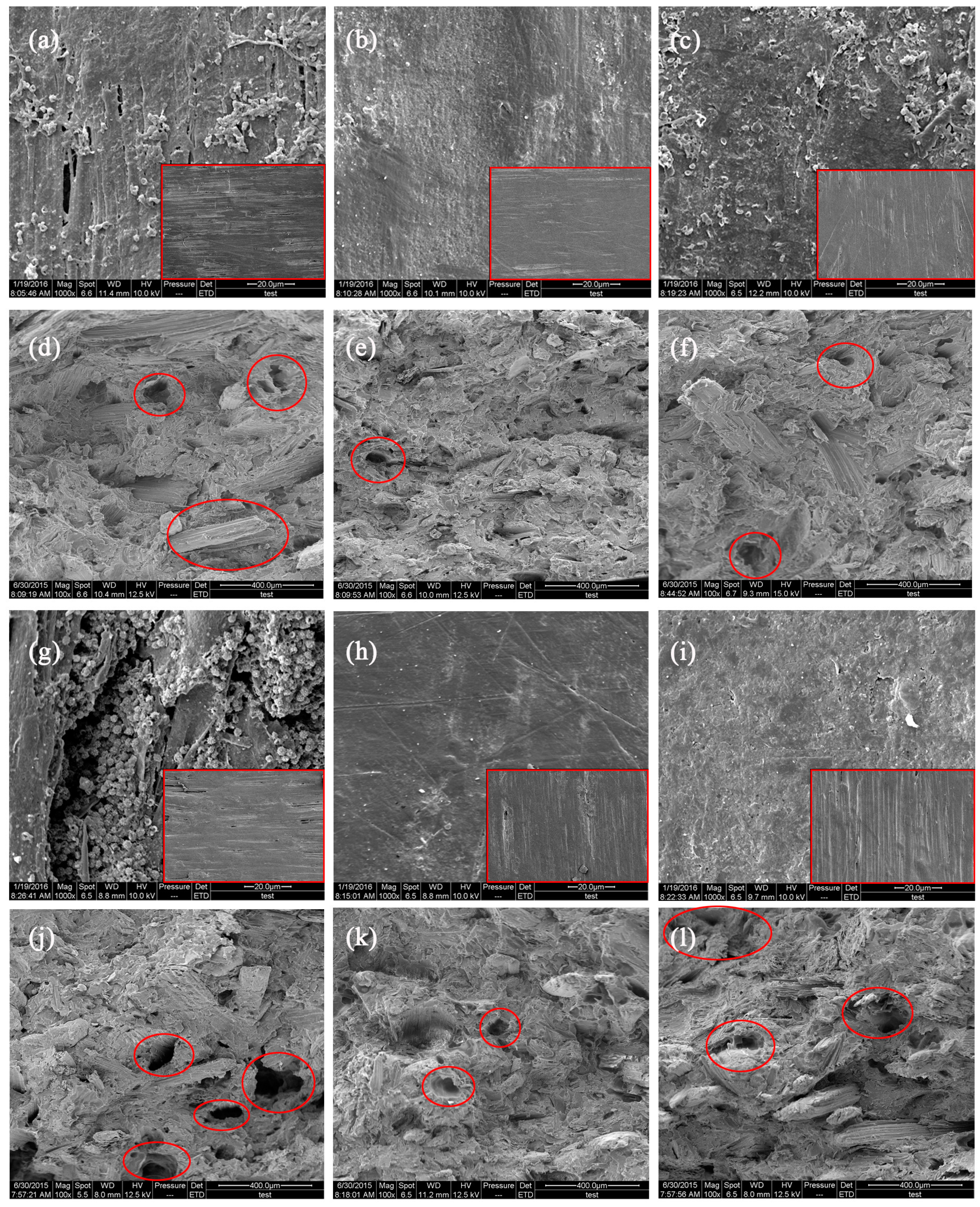
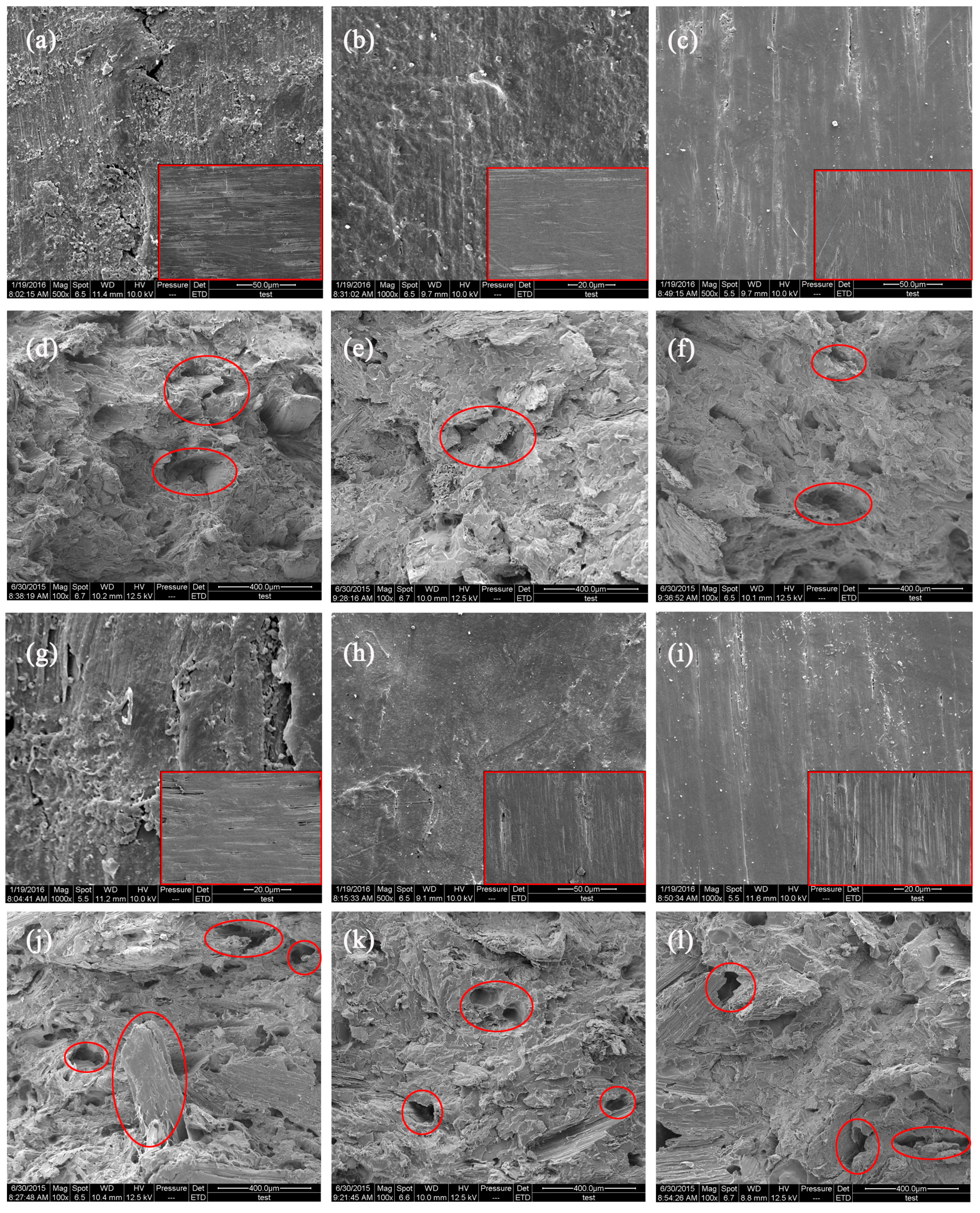
3.3.4. Scanning Electron Microscopy Analysis
4. Conclusions
- CSF/HDPE containing 3% ACQ is more resistant to termites, white rot fungi, and brown rot fungi than the untreated control composites, even at high CSF formulations (e.g., 60%). Additionally, ACQ enhances the biotic stress resistance of CSF/HDPE composites more than ZB.
- Compared with the untreated poplar/HDPE composite, the untreated CSF/HDPE is more susceptible to mold infections and decay.
- The CSF of the CSF/HDPE composites is susceptible to fungal attacks, with the soft rot decay causing the largest mass losses, followed by the white rot fungus, and then the brown rot fungus.
- Manufacturing AFPCs using preservative-treated CSF may be feasible, and represents a potentially viable way to convert an agricultural waste product into value-added composites.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xu, B.T. Analysis report of corn market in Heilongjiang province in the year of 2014. HL Grain. 2015, 5, 31–33. [Google Scholar]
- Xu, Y.G.; Ma, Q.; Zhou, H.; Jiang, C.M.; Yu, W.T. Effect of straw returning and deep loosening on soil physical and chemical properties and maize yields. Chin. J. Soil Sci. 2015, 46, 428–432. [Google Scholar]
- Panthapulakkal, S.; Sain, M. Agro-residue reinforced high-density polyethylene composites: Fiber characterization and analysis of composite properties. Compos. A Appl. Sci. Manuf. 2007, 38, 1445–1454. [Google Scholar] [CrossRef]
- Ashori, A.; Nourbakhsh, A. Bio-based composites from waste agricultural residues. Waste Manag. 2010, 30, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Nourbakhsh, A.; Ashori, A. Wood plastic composites from agro-waste materials: Analysis of mechanical properties. Bioresour. Technol. 2010, 101, 2525–2528. [Google Scholar] [CrossRef] [PubMed]
- Han, G.P.; Cheng, W.L.; Manning, M.; Eloy, P. Performance of zinc borate-treated oriented structural straw board against mold fungi, decay fungi, and termites-A Preliminary Trial. Bioresources 2012, 7, 2986–2995. [Google Scholar]
- Cheng, W.L.; Han, G.P.; Fang, D. Oriented structural boards from split wheat straw: Effects of straw length, panel density, and resin content. Bioresources 2013, 8, 4497–4504. [Google Scholar] [CrossRef]
- Ndiaye, D.; Fanton, E.; Morlat-Therias, S.; Vidal, L.; Tidjani, A.; Gardette, J. Durability of wood polymer composites: Part 1. Influence of wood on the photochemical properties. Compos. Sci. Technol. 2008, 68, 2779–2784. [Google Scholar] [CrossRef]
- Fabiyi, J.S.; McDonald, A.G.; Morrell, J.J.; Freitag, C. Effects of wood species on durability and chemical changes of fungal decayed wood plastic composites. Compos. A Appl. Sci. Manuf. 2011, 42, 501–510. [Google Scholar] [CrossRef]
- Liu, F.H.; Han, G.P.; Cheng, W.L. Preparation and properties of corn stalk fiber-high density polyethylene composites. J. Northeast Univ. 2015, 43, 119–122. [Google Scholar]
- Rowell, R.M.; Young, R.A.; Rowell, J.K. Paper and Composites from Agro-Based Resources; CPC Press: Boca Raton, FL, USA, 1997; pp. 83–131. [Google Scholar]
- Kamdem, D.P.; Jiang, H.; Cui, W.; Freed, J.; Matuana, L.M. Properties of wood plastic composites made of recycled HDPE and wood flour from CCA-treates wood removed from service. Compos. A Appl. Sci. Manuf. 2004, 35, 347–355. [Google Scholar] [CrossRef]
- Shang, L.; Han, G.P.; Zhu, F.Z.; Ding, J.S.; Shupe, T.; Wang, Q.W.; Wu, Q.L. High-density polyethylene-based composites with pressure-treated wood fibers. Bioresources 2012, 7, 5181–5189. [Google Scholar] [CrossRef]
- Tascioglu, C.; Yoshimura, T.; Tsunoda, K. Biological performance of wood-plastic composites containing zinc borate: Laboratory and 3-year filed test results. Compos. B Eng. 2013, 51, 185–190. [Google Scholar] [CrossRef]
- Bolin, C.A.; Smith, S. Life cycle assessment of ACQ-treated lumber with comparison to wood plastic composite decking. J. Clean. Prod. 2011, 19, 620–629. [Google Scholar] [CrossRef]
- Sean, T.; Brunnett, G.; Cote, F. Protection of oriented strand board with borate. For. Prod. J. 1999, 49, 47–51. [Google Scholar]
- Lee, S.Y.; Wu, Q.; Smith, W.R. Formosan subterranean termite resistance of borate-modified strand board manufactured from southern wood species: A laboratory trial. Wood Fiber Sci. 2004, 36, 107–118. [Google Scholar]
- Wong, A.H.H. Performance of two imidaclorprid-treated Malaysian hardwoods in an accelerated aboveground termite test. Presented at the International Research Group on Wood Protection, Document No.: IRG/WP 05-30389, Bangalore, India, 24–28 April 2005. [Google Scholar]
- Creffield, J.W. Afield method for demermining the above-ground resistance of wood and wood products to attack by subterranean termites. Presented at the International Research Group on Wood Preservation, Document No.: IRG/WP 94-20035, Bali, Indonesia, 29 May–3 June 1994. [Google Scholar]
- Wong, A.H.H. A Novel Malaysian Biological Hazard Class Selection Guide; Newsletter of the Malaysian Wood Preserving Association (MWPA): Kuala Lumpur, Malaysian, 2004; Volume 4, Issue 17, pp. 7–8. [Google Scholar]
- Wong, A.H.H.; Eden, D.R.; Chittenden, C.M.; Hedley, M.E.; Wakeling, R.N. Comparison of the FRIM and Forest Research laboratory methods for screening of anti-sapstain formulations. Presented at the International Research Group on Wood Protection, Document No.: IRG/WP 99-20170, Rosenheim, Germany, 6–11 June 1999. [Google Scholar]
- ASTM. Accelerated laboratory test of natural decay resistance of woods. ASTM D 2017-81. In Annual Book of ASTM Standards; American Standards for Testing and Materials: Philadelphia, PA, USA, 1984; Volume 4, pp. 447–452. [Google Scholar]
- Wong, A.H.H. Laboratory evaluation of soft rot resistance of nondurable lesser-known Malaysian hardwoods. Presented at the International Research Group on Wood Protection, Document No.: IRG/WP 06-10582, Tromso, Norway, 18–22 June 2006. [Google Scholar]
- Kartal, S.N.; Aysal, S.; Terzi, E.; Yìlgör, N.; Yoshimura, T.; Tsunoda, K. Wood and bamboo-PP composites: fungal and termite resistance, water absorption, and FT-IR analyses. Bioresources 2013, 8, 1222–1244. [Google Scholar] [CrossRef]
- Schirp, A.; Ibach, R.E.; Pendlenton, D.E.; Wolcott, M.P. Biological degradation of wood-plastic composites (WPC) and strategies for improving the resistance of WPC against biological decay. Development of Commercial Wood Preservatives. In Efficacy, Environmental and Health Issues; Schultz, T.P., Militz, H., Eds.; American Chemical Society Publication: Washington, WA, USA, 2008; pp. 480–507. [Google Scholar]
- Khavkine, M.; Kazayawoko, M.; Law, S.; Balatinecz, J.J. Durability of wood flour-thermoplastic composites under extreme environmental conditions and fungal exposure. Int. J. Polym. Mater. 2000, 46, 255–269. [Google Scholar] [CrossRef]
- Schirp, A.; Wolcott, M.P. Influence of fungal decay and moisture absorption on mechanical properties of extruded wood-plastic composites. Wood Fiber Sci. 2007, 37, 643–652. [Google Scholar]
- Verhey, S.; Lanks, P. Wood particle size affects the decay resistance of wood/thermoplastic composites. For. Prod. J. 2002, 52, 78–81. [Google Scholar]
- Simonsen, J.; Freitag, C.M.; Silva, A.; Morrell, J.J. Wood/plastic ratio: Effect on performance of borate biocides against a brown rot fungus. Holzforschung 2004, 58, 205–208. [Google Scholar] [CrossRef]
- Feng, J.; Shi, Q.S.; Huang, X.M.; Ouyang, Y.S.; Chen, Y.B. Decay effect of Lentinus Lepideus on HDPE based wood-plastic composite. China Plast. Ind. 2013, 41, 78–82. [Google Scholar]

| Code | Fiber (wt %) | HDPE (wt %) | ACQ (phc) | ZB (phc) | MAPE (wt %) | Paraffin (wt %) |
|---|---|---|---|---|---|---|
| A | CSF-50 | 47 | 1 | - | 2 | 1 |
| B | CSF-50 | 47 | 2 | - | 2 | 1 |
| C | CSF-50 | 47 | 3 | - | 2 | 1 |
| D | CSF-60 | 37 | 1 | - | 2 | 1 |
| E | CSF-60 | 37 | 2 | - | 2 | 1 |
| F | CSF-60 | 37 | 3 | - | 2 | 1 |
| G | CSF-50 | 47 | - | 1 | 2 | 1 |
| H | CSF-50 | 47 | - | 2 | 2 | 1 |
| I | CSF-50 | 47 | - | 3 | 2 | 1 |
| J | CSF-60 | 37 | - | 1 | 2 | 1 |
| K | CSF-60 | 37 | - | 2 | 2 | 1 |
| L | CSF-60 | 37 | - | 3 | 2 | 1 |
| M | CSF-50 | 47 | - | - | 2 | 1 |
| N | CSF-60 | 37 | - | - | 2 | 1 |
| O | Poplar-50 | 47 | - | - | 2 | 1 |
| P | Poplar-60 | 37 | - | - | 2 | 1 |
| Rating | Mold Coverage (%) over Materials Surface |
|---|---|
| 0 | No mold coverage/growth (sound specimen) |
| 1 | Range: 1–5% mold coverage (median: 3%) |
| 2 | Range: 6–20% mold coverage (median: 13%) |
| 3 | Range: 21–35% mold coverage (median: 28%) |
| 4 | Range: 36–50% mold coverage (median: 43%) |
| 5 | Range: 51–75% mold coverage (median: 63%) |
| 6 | Range: 76–100% mold coverage (median: 88%) |
| Sample Group | Type of CSF | Preservative Level (wt %) | Air Dry Moisture Content (%) | Mass Loss (%) | Visual Rating |
|---|---|---|---|---|---|
| CSF/HDPE = 50/50 | untreated | 0 | 0.58(0.04) ab | 3.6(0.5) ef | 8.8(0.8) bcd |
| ACQ treated | 1 | 0.97(0.27) d | 2.3(0.1) bc | 9.6(0.4) cde | |
| 2 | 0.88(0.08) bc | 1.4(0.1) cd | 9.5(0.0) bcde | ||
| 3 | 0.83(0.11) cd | 1.0(0.1) bc | 9.6(0.1) bc | ||
| ZB treated | 1 | 0.48(0.01) a | 2.8(0.4) ab | 9.5(0.3) bcde | |
| 2 | 0.49(0.11) a | 2.5(0.3) b | 9.5(0.0) bcde | ||
| 3 | 0.40(0.03) a | 2.0(0.4) c | 9.3(0.7) bcd | ||
| CSF/HDPE = 60/40 | untreated | 0 | 0.58(0.06) ab | 5.5(0.4) h | 9.0(0.8) bc |
| ACQ treated | 1 | 0.94(0.11) cd | 3.5(0.1) cd | 9.8(0.3) de | |
| 2 | 0.86(0.12) bc | 3.2(0.1) de | 9.6(0.2) cde | ||
| 3 | 1.65(0.56) e | 2.5(0.4) cd | 9.8(0.3) de | ||
| ZB treated | 1 | 0.58(0.02) ab | 5.3(0.2) ef | 9.4(0.6) bcde | |
| 2 | 0.63(0.02) abc | 4.7(0.3) gh | 9.4(0.2) b | ||
| 3 | 0.63(0.05) abc | 3.6(0.5) h | 9.3(0.8) bcd | ||
| Wood control | Poplar * | - | 11.12(0.38) f | 100.0(0.0) i | 0.0(0.0) a |
| Sample Group | Type of Material | Preservative Level (wt %) | Time for the First Occurrence of Mold Attack | Median Mold Growth after the Test Completed (%) |
|---|---|---|---|---|
| CSF/HDPE = 50/50 | ACQ-treated CSF | 1 | No mold growth | 0 |
| 2 | No mold growth | 0 | ||
| 3 | No mold growth | 0 | ||
| ZB-treated CSF | 1 | Day 9 | 7 | |
| 2 | Day 9 | 5.13 | ||
| 3 | Day 10 | 3.85 | ||
| Untreated CSF | - | Day 9 | 12.4 | |
| Wood/HDPE = 50/50 | Untreated poplar | - | Day 10 | 5 |
| CSF/HDPE = 60/40 | ACQ-treated CSF | 1 | No mold growth | 0 |
| 2 | No mold growth | 0 | ||
| 3 | No mold growth | 0 | ||
| ZB-treated CSF | 1 | Day 9 | 20.5 | |
| 2 | Day 9 | 13.5 | ||
| 3 | Day 10 | 11.25 | ||
| Untreated CSF | - | Day 9 | 24 | |
| Wood/HDPE = 60/40 | Untreated poplar | - | Day 11 | 6.4 |
| Wood control 1 | Pure poplar a | - | Day 9 | 69.25 |
| Wood control 2 | Pure rubber b | - | Day 2 | 88 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xuan, L.; Hui, D.; Cheng, W.; Wong, A.H.H.; Han, G.; Tan, W.K.; Tawi, C.A.D. Effect of Preservative Pretreatment on the Biological Durability of Corn Straw Fiber/HDPE Composites. Materials 2017, 10, 789. https://doi.org/10.3390/ma10070789
Xuan L, Hui D, Cheng W, Wong AHH, Han G, Tan WK, Tawi CAD. Effect of Preservative Pretreatment on the Biological Durability of Corn Straw Fiber/HDPE Composites. Materials. 2017; 10(7):789. https://doi.org/10.3390/ma10070789
Chicago/Turabian StyleXuan, Lihui, Dongxue Hui, Wanli Cheng, Andrew H. H. Wong, Guangping Han, Wei Khong Tan, and Carlson A. D. Tawi. 2017. "Effect of Preservative Pretreatment on the Biological Durability of Corn Straw Fiber/HDPE Composites" Materials 10, no. 7: 789. https://doi.org/10.3390/ma10070789
APA StyleXuan, L., Hui, D., Cheng, W., Wong, A. H. H., Han, G., Tan, W. K., & Tawi, C. A. D. (2017). Effect of Preservative Pretreatment on the Biological Durability of Corn Straw Fiber/HDPE Composites. Materials, 10(7), 789. https://doi.org/10.3390/ma10070789





