Degradation of Glyphosate in Soil Photocatalyzed by Fe3O4/SiO2/TiO2 under Solar Light
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of the Photocatalyst
2.2.1. Preparation of Fe3O4/SiO2
2.2.2. Preparation of Fe3O4/SiO2/TiO2
2.2.3. Photocatalyst Characterization
2.2.4. Degradation Experiment
3. Results and Discussion
3.1. Photocatalysts Characterization
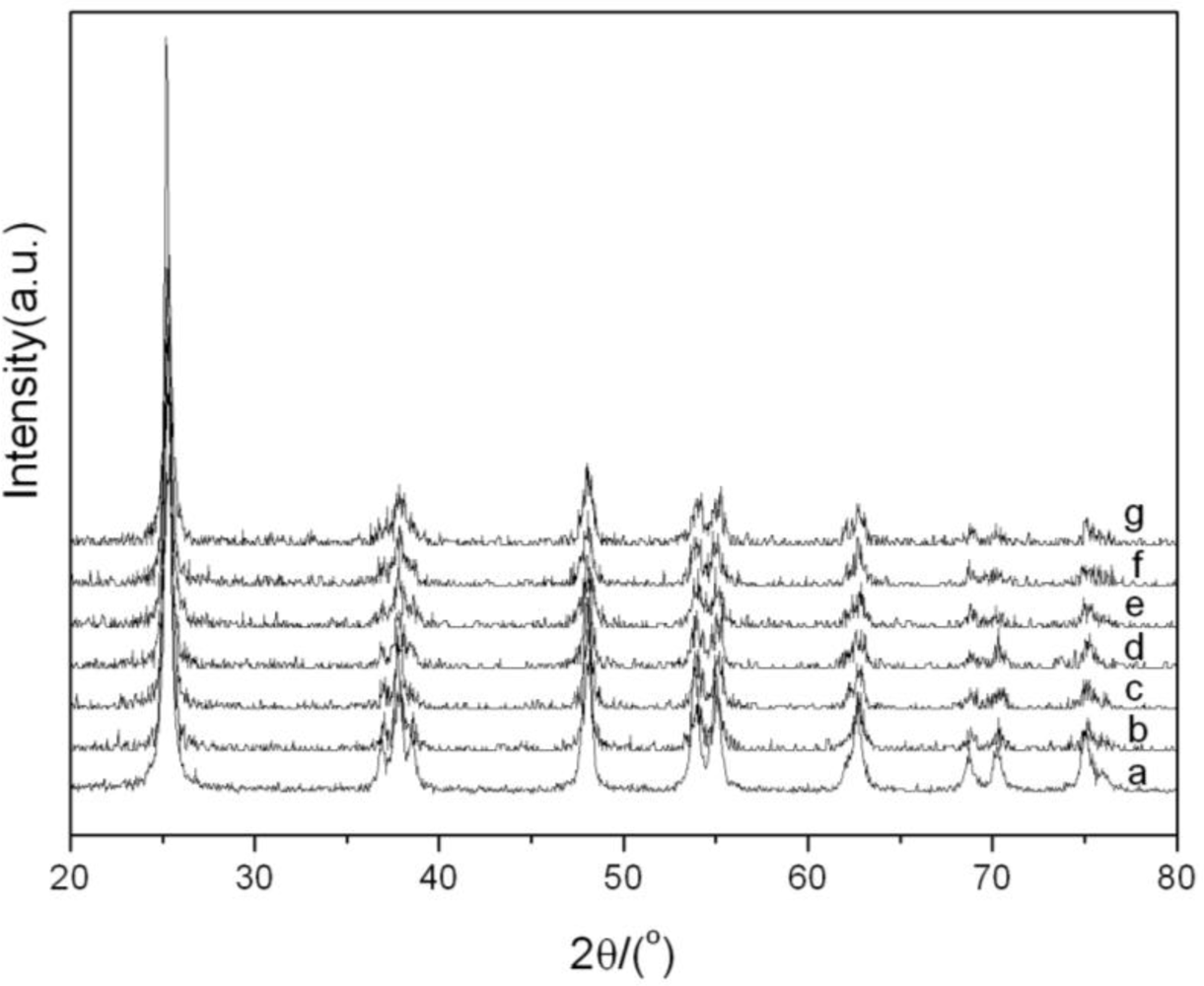
3.1.1. XRD Characterization
3.1.2. SEM Characterization
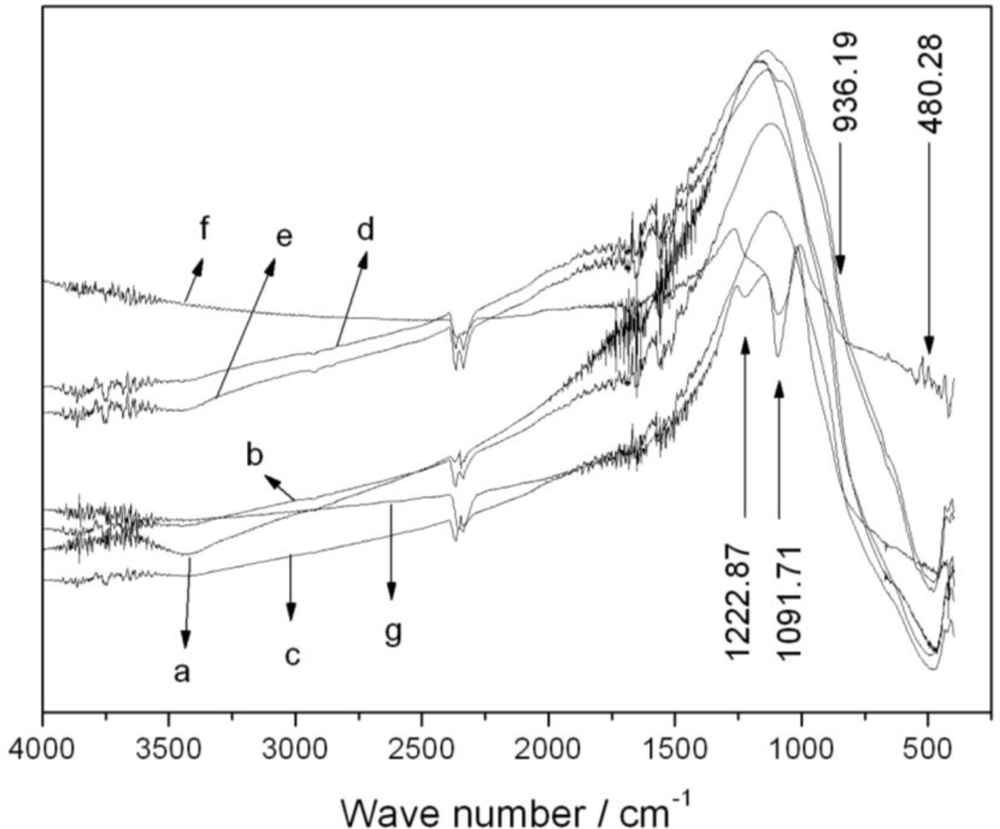
3.1.3. FTIR Characterization
3.1.4. UV-Vis Characterization
3.2. Factors Affecting Glyphosate Degradation
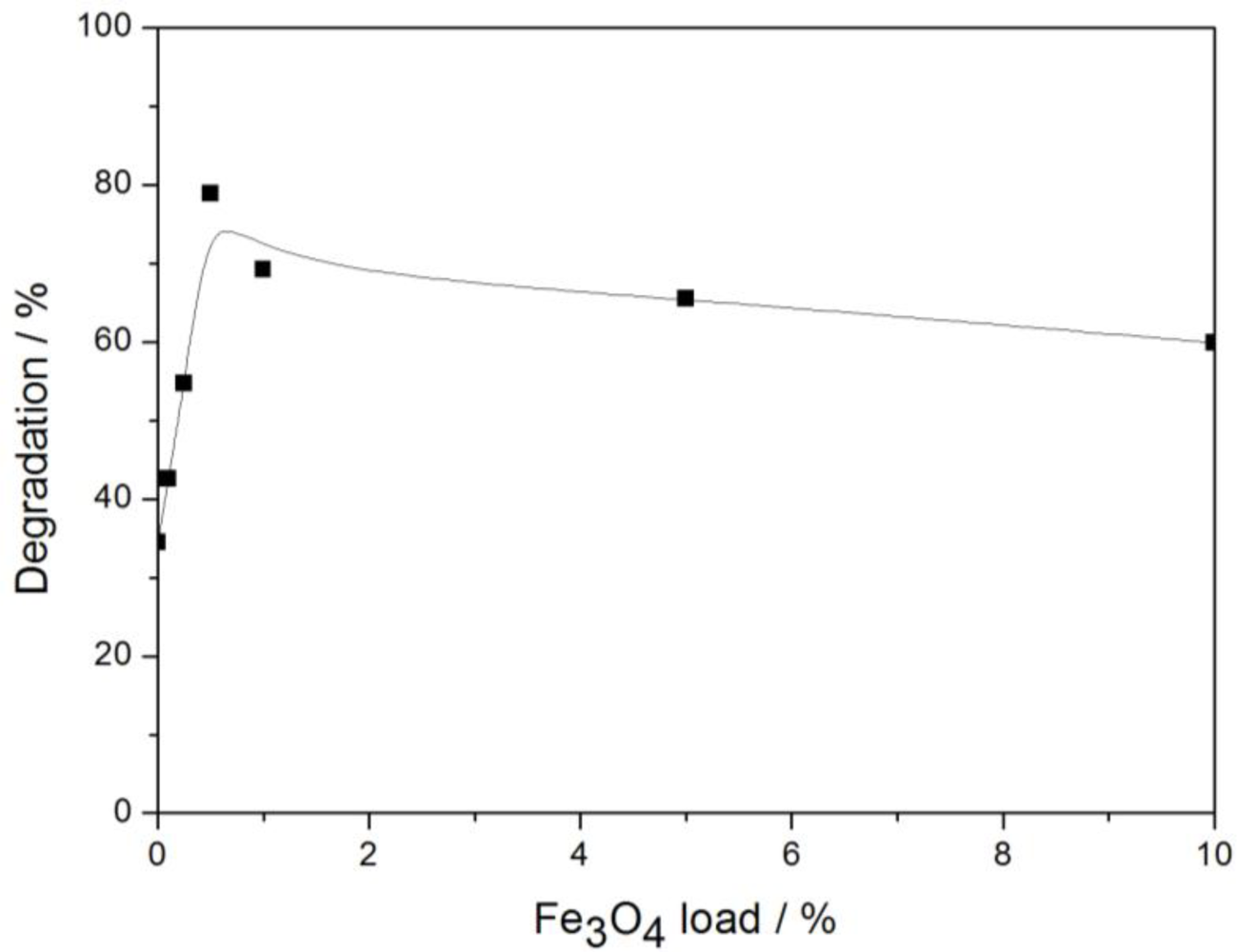
3.2.1. Fe3O4 Loading
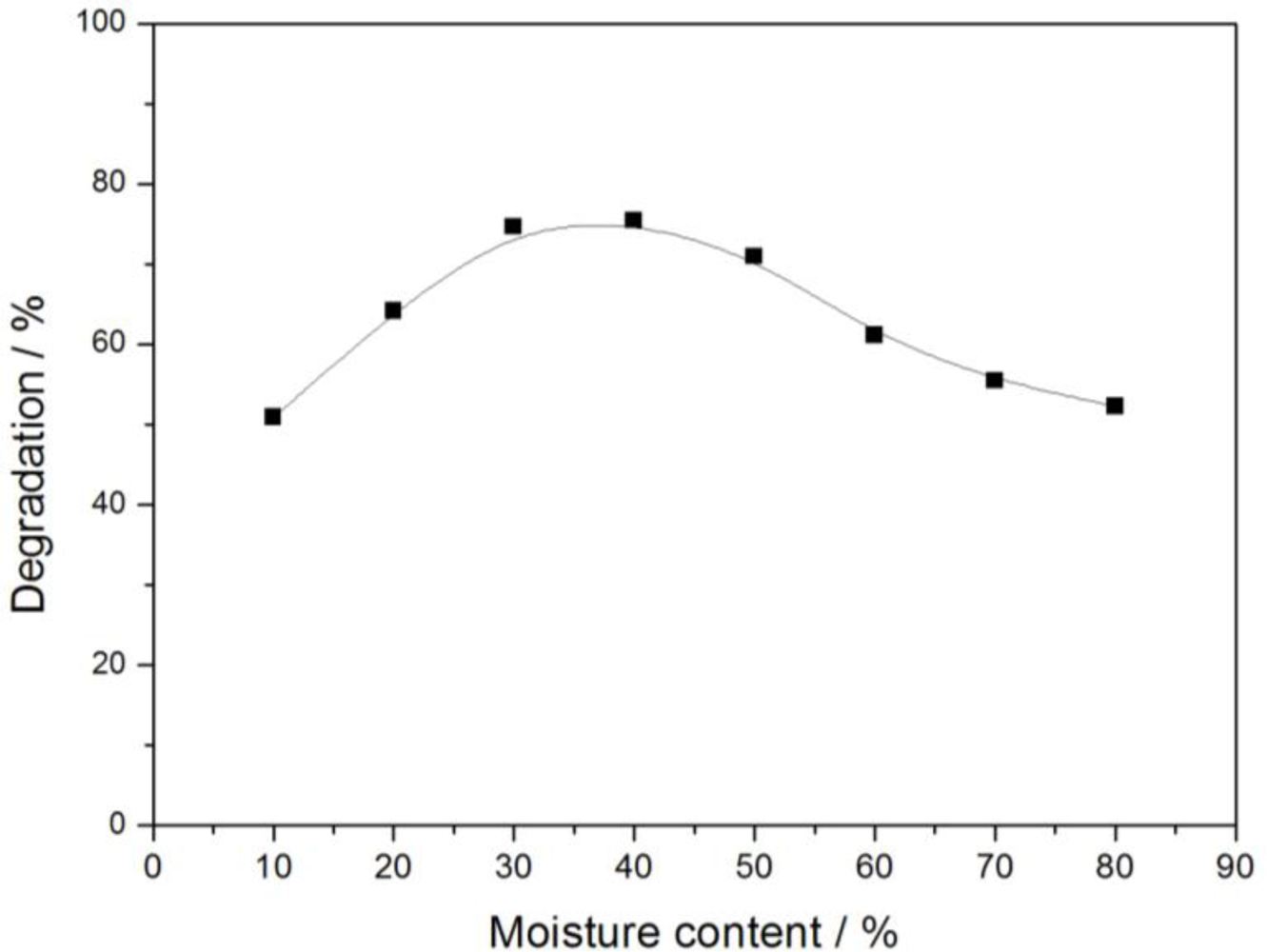
3.2.2. Moisture Content
3.2.3. Photocatalyst Dosage
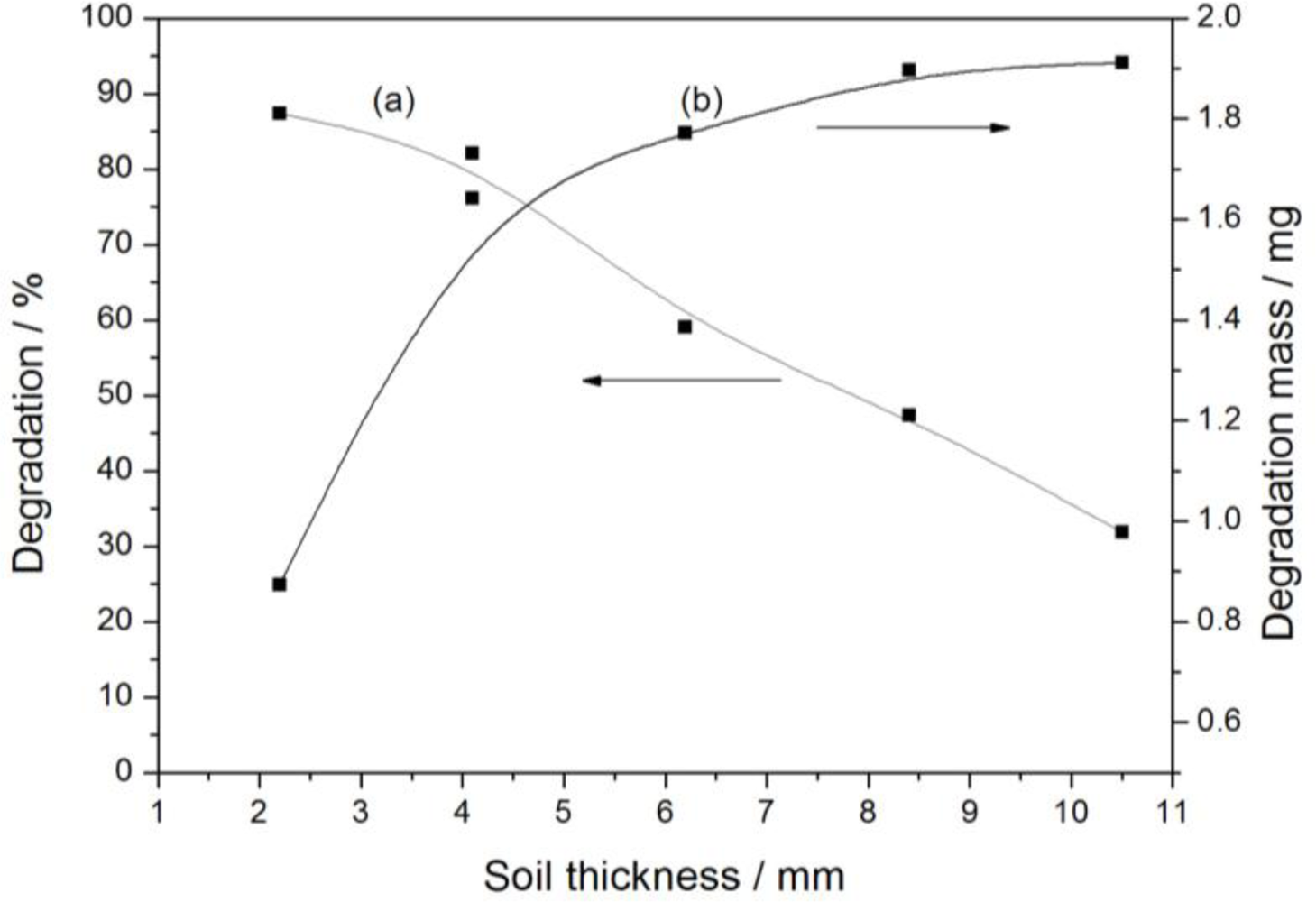
3.2.4. Soil Thickness
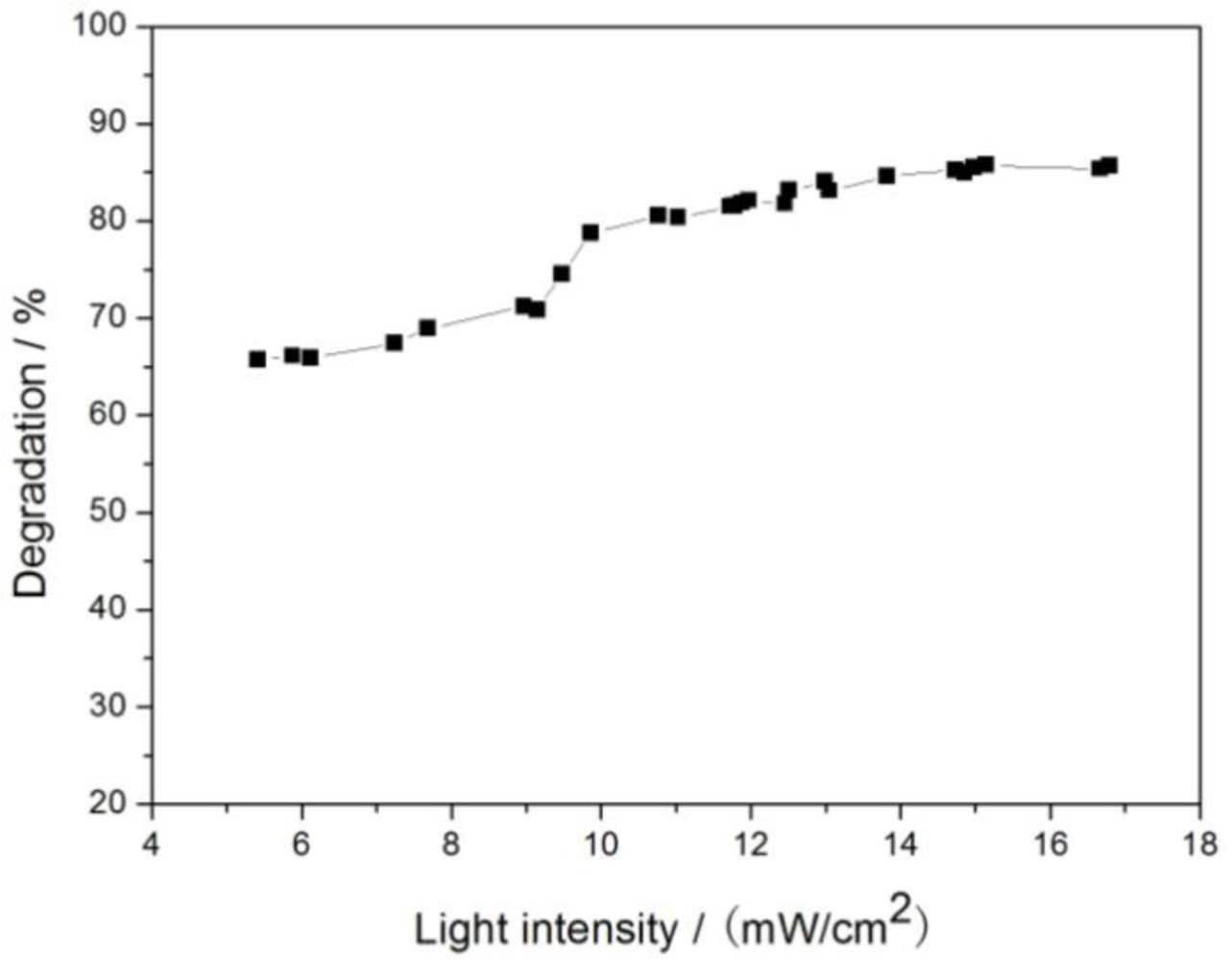
3.2.5. Light Intensity
4. Conclusions
Acknowledgments
References
- Sprankle, P; Meggit, WF; Penner, D. Rapid inactivation of glyphosate in the soils. Weed Sci 1976, 3, 224–228. [Google Scholar]
- Lu, X; Zhao, BZ; Zhang, JB; Deng, JC; Li, P; Xin, XL. Property and environmental behavior of herbicide glyphosate. Chin. J. Soil Sci 2005, 5, 785–790. [Google Scholar]
- Dinehart, SK; Smith, LM; Mcmurry, ST; Anderson, TA; Smith, PN; Haukos, DA. Toxicity of a glufosinate-and several glyphosate-based herbicides to juvenile amphibians from the Southern High Plains, USA. Sci. Total Environ 2009, 3, 1065–1071. [Google Scholar]
- Langiano, VDC; Martinez, CBR. Toxicity and effects of a glyphosate-based herbicide on the Neotropical fish Prochilodus lineatus. Comp. Biochem. Physiol. C 2008, 2, 222–231. [Google Scholar]
- Manassero, A; Passalia, C; Negro, AC; Cassano, AE; Zalazar, CS. Glyphosate degradation in water employing the H2O2/UVC process. Water Res 2010, 13, 3875–3882. [Google Scholar]
- Chen, SF; Liu, YZ. Study on the photocatalytic degradation of glyphosate by TiO2 photocatalyst. Chemosphere 2007, 5, 1010–1017. [Google Scholar]
- Leticia, P; Pilar, CMD; John, S. Degradation of Glyphosate and other pesticides by ligninolytic enzymes. Biodegradation 2008, 2, 195–199. [Google Scholar]
- Valente, JS; Tzompantzi, F; Prince, J; Cortez, JGH; Gomez, R. Adsorption and photocatalytic degradation of phenol and 2,4 dichlorophenoxiacetic acid by Mg-Zn-Al layered double hydroxides. Appl. Catal. B 2009, 90, 330–338. [Google Scholar]
- Devi, LG; Murthy, BN. Structural characterization of Th-doped TiO2 photocatalyst and its extension of response to solar light for photocatalytic oxidation of oryzalin pesticide: A comparative study. Cent. Eur. J. Chem 2009, 1, 118–129. [Google Scholar]
- Lhomme, L; Brosillon, S; Wolbert, D. Photocatalytic degradation of a triazole pesticide, cyproconazole, in water. J. Photochem. Photobio. A 2007, 1, 34–42. [Google Scholar]
- Tamimi, M; Qourzal, S; Assabbane, A; Chovelon, JM; Ferronato, C; Ait-Ichou, Y. Photocatalytic degradation of pesticide methomyl: Determination of the reaction pathway and identification of intermediate products. Photochem. Photobiol. Sci 2006, 5, 477–482. [Google Scholar]
- Higarashi, MM; Jardim, WF. Remediation of pesticide contaminated soil using TiO2 mediated solar light. Catal. Today 2002, 76, 201–207. [Google Scholar]
- Villaverde, J; Maqueda, C; Undabeytia, T; Morillo, E. Effect of various cyclodextrins on photodegradation of a hydrophobic herbicide in aqueous suspensions of different soil colloidal components. Chemosphere 2007, 69, 575–584. [Google Scholar]
- Qamar, M; Muneer, M. A comparative photocatalytic activity of titanium dioxide and zinc oxide by investigating the degradation of vanillin. Desalination 2009, 249, 535–540. [Google Scholar]
- Zhang, LL; Lv, FJ; Zhang, WG; Li, RQ; Zhong, H; Zhao, YJ; Wang, X. Photo degradation of methyl orange by attapulgite-SnO2-TiO2 nanocomposites. J. Hazard. Mater 2009, 171, 294–300. [Google Scholar]
- Lliev, V; Tomova, D; Rakovsky, S; Eliyas, A; Puma, GL. Enhancement of photocatalytic oxidation of oxalic acid by gold modified WO3/TiO2 photocatalysts under UV and visible light irradiation. J. Mol. Catal. A Chem 2010, 327, 51–57. [Google Scholar]
- Zhao, WX; Bai, ZP; Ren, AL; Wu, C. Sunlight photocatalytic activity of CdS modified TiO2 loaded on activated carbon fibers. Appl. Surf. Sci 2010, 256, 3493–3498. [Google Scholar]
- Dong, WG; Chen, XC; Lang, ZM; Hu, WH; Rong, R; Lu, B. Fluorescence quenching indirect determination of glyphosate in waste water. Hebei J. Indust. Sci. Technol 1999, 53, 51–53. [Google Scholar]
- Zhang, P; Wang, LF; Chen, YH. In situ diffuse reflectance FTIR spectroscopy characterization of titanium silicalite-1 catalytic oxidization of styrene. Spectrosc. Spect. Anal 2007, 5, 886–888. [Google Scholar]
- Hu, J; Wang, JJ; Zhou, LH; Xie, SH; Liu, HL. The mechanism of mesoporous phase transition of titanic-silica mesoporous materials. Acta Phys.-Chim. Sin 2006, 6, 679–683. [Google Scholar]



| Sample | BET specific surface area (m2/g) | Average pore diameter (nm) | Total pore volume (cm3/g) |
|---|---|---|---|
| TiO2 | 127.38 | 19.56 | 0.2731 |
| 0.1% Fe3O4/ SiO2/ TiO2 | 84.39 | 24.47 | 0.2418 |
| 0.25% Fe3O4/ SiO2/ TiO2 | 76.91 | 28.72 | 0.2273 |
| 0.5% Fe3O4/ SiO2/ TiO2 | 64.27 | 31.67 | 0.2080 |
| 1% Fe3O4/ SiO2/ TiO2 | 50.49 | 37.83 | 0.1843 |
| 5% Fe3O4/ SiO2/ TiO2 | 41.08 | 40.51 | 0.1694 |
| 10% Fe3O4/ SiO2/ TiO2 | 32.82 | 49.17 | 0.1467 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xu, X.; Ji, F.; Fan, Z.; He, L. Degradation of Glyphosate in Soil Photocatalyzed by Fe3O4/SiO2/TiO2 under Solar Light. Int. J. Environ. Res. Public Health 2011, 8, 1258-1270. https://doi.org/10.3390/ijerph8041258
Xu X, Ji F, Fan Z, He L. Degradation of Glyphosate in Soil Photocatalyzed by Fe3O4/SiO2/TiO2 under Solar Light. International Journal of Environmental Research and Public Health. 2011; 8(4):1258-1270. https://doi.org/10.3390/ijerph8041258
Chicago/Turabian StyleXu, Xuan, Fangying Ji, Zihong Fan, and Li He. 2011. "Degradation of Glyphosate in Soil Photocatalyzed by Fe3O4/SiO2/TiO2 under Solar Light" International Journal of Environmental Research and Public Health 8, no. 4: 1258-1270. https://doi.org/10.3390/ijerph8041258




