Perturbation of the Developmental Potential of Preimplantation Mouse Embryos by Hydroxyurea
Abstract
:1. Introduction
2. Materials and Methods
Animals
Experiment 1
Ovulation Induction, Embryo Recovery and in vitro Culture
Radioimmunoassay
Experiment 2
In vitro Culture of Embryos in Bioavailable HU
Experiment 3
Intermittent Culture in vitro, of Embryos in Bioavailable HU
Statistical Analyses
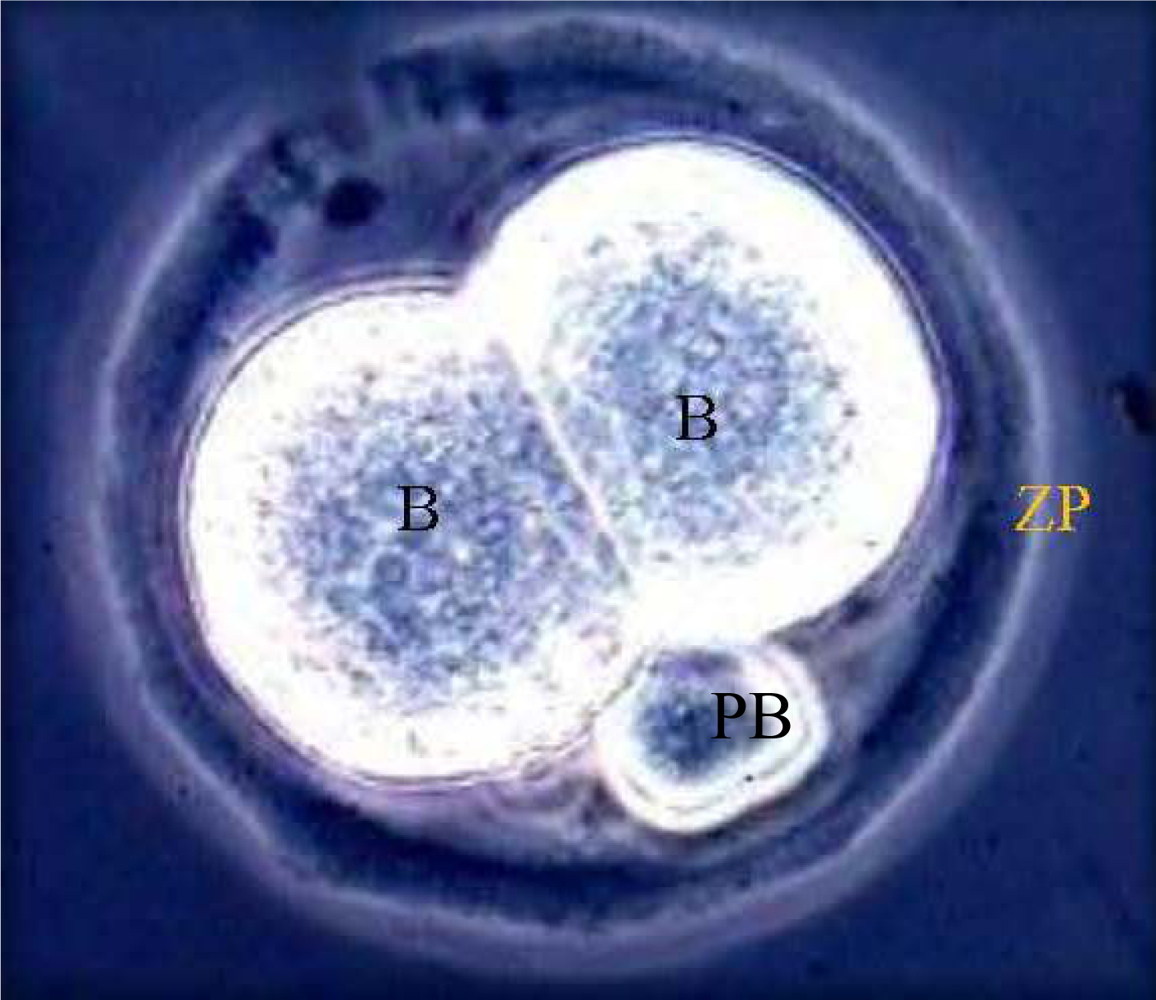
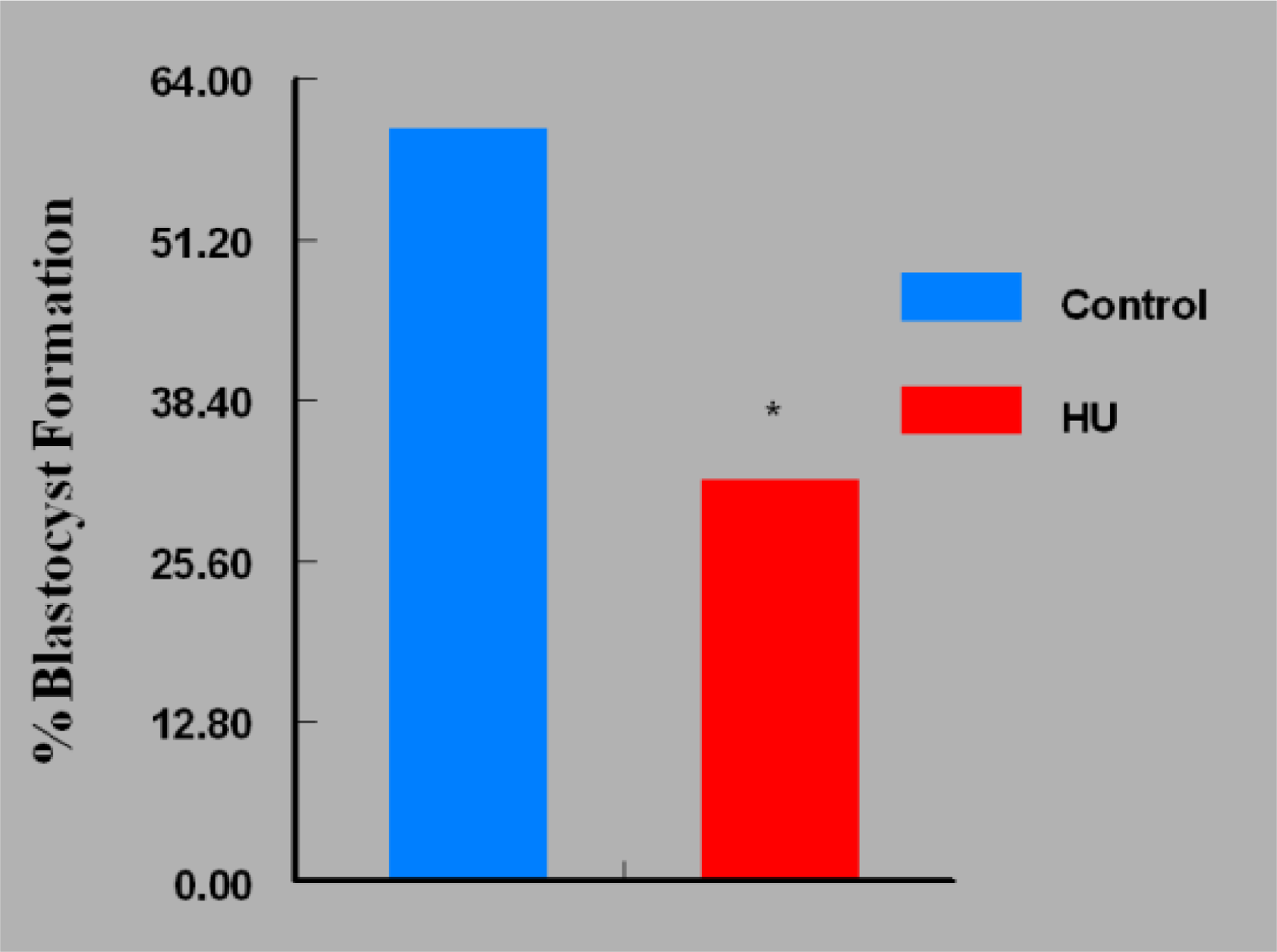
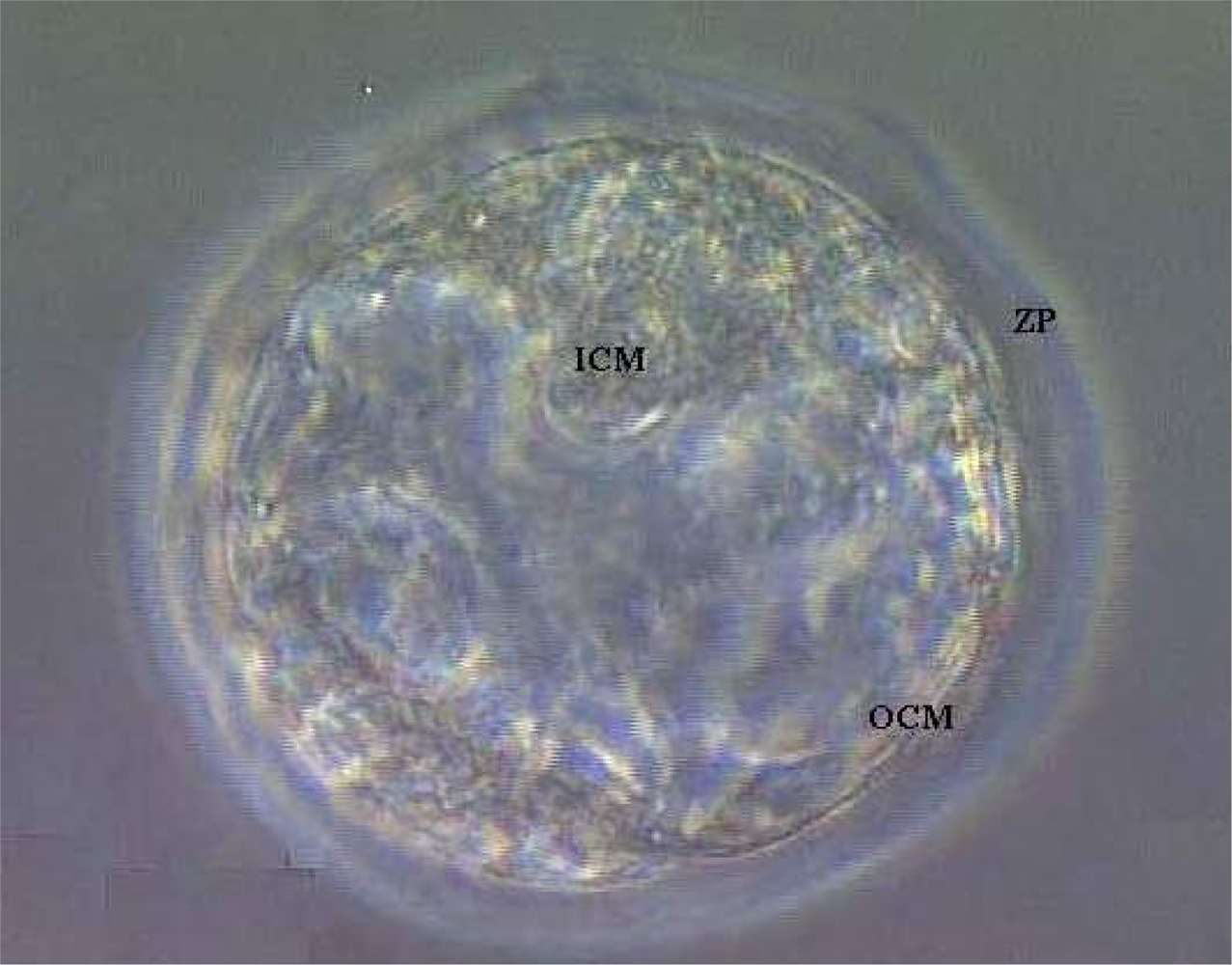
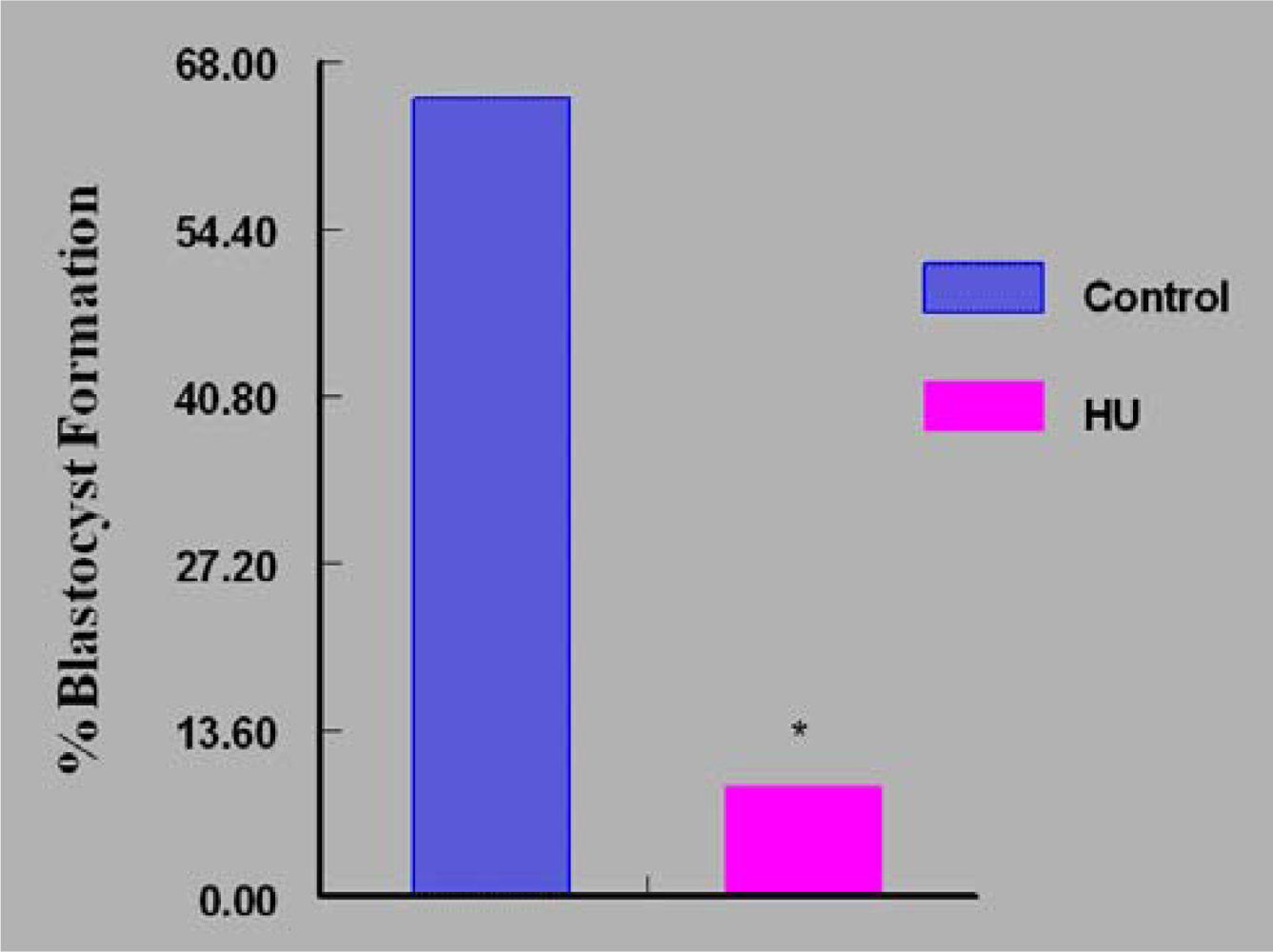
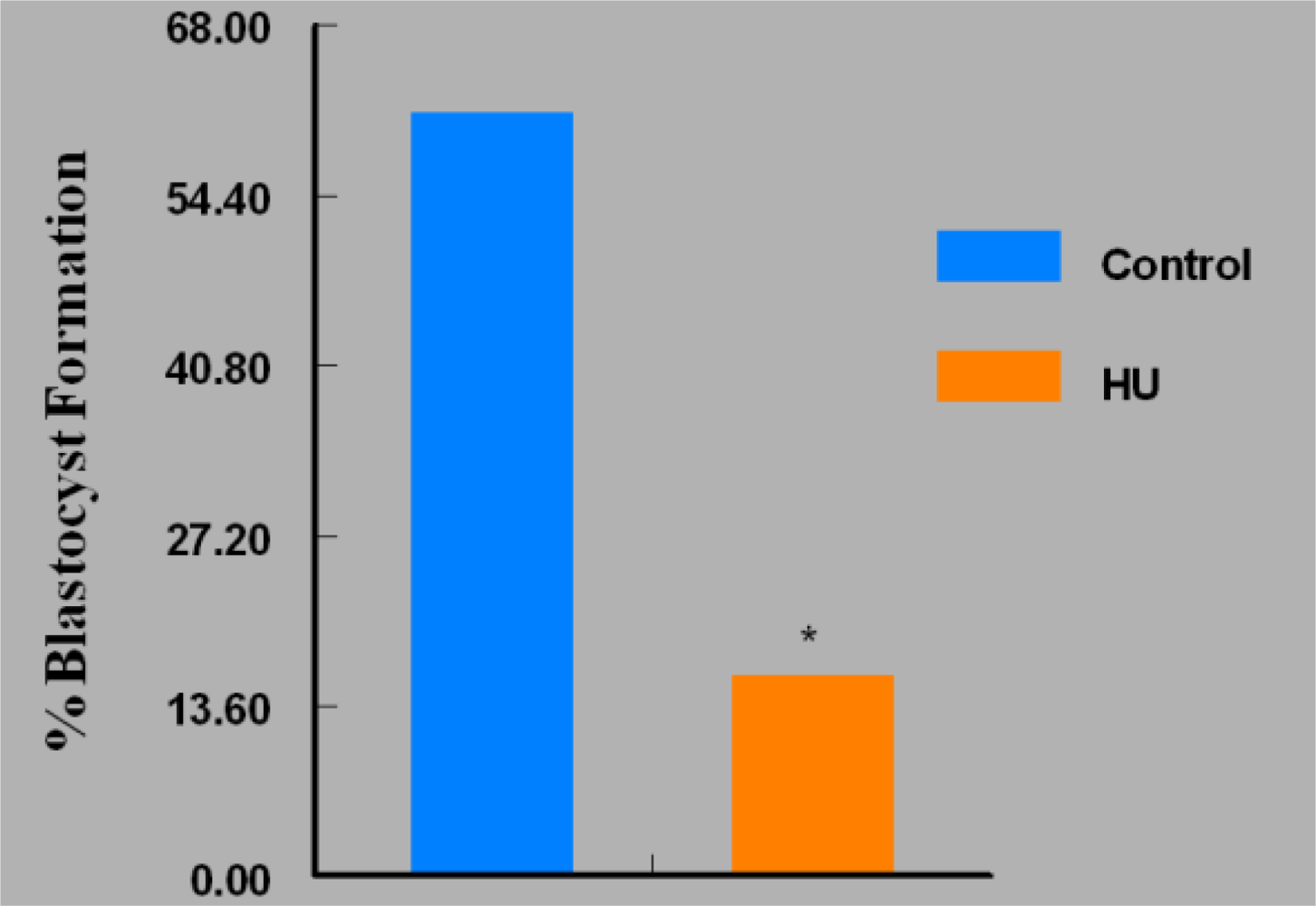
3. Results
4. Discussion
Acknowledgments
References
- Yarbro, JW. Mechanism of action of hydroxyurea. Semin. Oncol 1992, 19, 1–10. [Google Scholar]
- Caramazza, D; Caracciolo, C; Barone, R; Malato, A; Saccullo, G; Cigna, V; Berretta, S; Schinocca, L; Quintini, G; Abbadessa, V; Di Raimondo, F; Siragusa, S. Correlation between leukocytosis and thrombosis in Philadelphia-negative chronic myeloprolipherative neoplasm. Ann. Hematol 2009, 88, 967–971. [Google Scholar]
- Cortelazzo, S; Finazzi, G; Ruggeri, M; Vestri, O; Galli, M; Rodeghiero, F; Barbui, T. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N. Engl. J. Med 1995, 332, 1132–1136. [Google Scholar]
- Silver, RT; Woolf, SH; Hehlmann, R; Appelbaum, FR; Anderson, J; Bennett, C; Goldman, JM; Guilhot, F; Kantarjian, HM; Lichtin, AE; Talpaz, M; Tura, S. An evidence-based analysis of the effect of busulfan,hydroxyurea, interferon, and allogeneic bone marrow transplantation in treating the chronic phase of chronic myeloid leukemia: developed for the American Society of Hematology. Blood 1999, 94, 1517–1536. [Google Scholar]
- Steinberg, MH. Management of sickle cell disease. N. Engl. J. Med 1999, 340, 1021–1030. [Google Scholar]
- Gao, W; Cara, A; Gallo, R; Lori, F. Low levels of deoxynucleotides in peripheral blood lymphocytes: a strategy to inhibit HIV type 1 replication. Proc. Natl. Acad. Sci. USA 1993, 90, 8925–8928. [Google Scholar]
- Bakshi, RP; Hamzeh, F; Frank, I; Eron, JJ, Jr; Bosch, RJ; Rosenkranz, SL; Cramer, YS; Ussery, M; Flexner, C. Effect of hydroxyurea and dideoxyinosine on intracellular 3′-deoxyadenosine-5′-triphosphate concentrations in HIV-infected patients. AIDS Res. Hum. Retroviruses 2007, 11, 1360–1365. [Google Scholar]
- Chahoud, I; Paumgartten, FJ. Dose-response relationships of rat fetal skeleton variations: Relevance for risk assessment. Environ. Res 2009, 109, 922–929. [Google Scholar]
- Woo, GH; Katayama, K; Bak, EJ; Ueno, M; Yamauchi, H; Uetsuka, K; Nakayama, H; Doi, K. Effects of prenatal hydroxyurea-treatment on mouse offspring. Exp. Toxicol. Pathol 2004, 56, 1–7. [Google Scholar]
- Yan, J; Hales, BF. Activator protein-1 (AP-1) DNA binding activity is induced by hydroxyurea in organogenesis stage mouse embryos. Toxicol. Sci 2005, 85, 1013–1023. [Google Scholar]
- Byrd, DC; Pitts, SR; Alexander, CK. Hydroxyurea in two pregnant women with sickle cell anemia. Pharmacotherapy 1999, 12, 1459–1462. [Google Scholar]
- Jones, KM; Niaz, MS; Brooks, CM; Roberson, SI; Aguinaga, MP; Hills, ER; Rice, VM; Bourne, P; Bruce, D; Archibong, AE. Adverse effects of a clinically relevant dose of hydroxyurea used for the treatment of sickle cell disease on male fertility endpoints. Int. J. Environ. Res. Public Health 2009, 3, 124–144. [Google Scholar]
- Whiten, WK. Nutrient requirements for the culture of preimplantation mouse embryos in vitro. Adv. Biosci 1971, 6, 129–139. [Google Scholar]
- Archibong, AE; Inyang, F; Ramesh, A; Greenwood, M; Nayyar, T; Kopsombut, P; Hood, DB; Nyanda, AM. Alteration of pregnancy related hormones and fetal survival in F-344 rats exposed by inhalation to benzo(a)pyrene. Reprod. Toxicol 2002, 16, 801–808. [Google Scholar]
- Iyamu, WE; Lian, L; Asakura, T. Pharmacokinetic profile of the anti-sickling hydroxyurea in wild-type and transgenic sickle cell mice. Chemotherapy 2001, 47, 270–278. [Google Scholar]
- Zeleznik, AJ; Benyo, DF. Control of follicular development, corpus luteum function, and recognition of pregnancy in higher primates. In Physiology of Reproduction, 2nd ed; Knobil, Neill, Ed.; Raven Press: New York, NY, USA, 1994; pp. 751–782. [Google Scholar]
- Lu, C; Yang, W; Hu, Z; Liu, Y. Granulosa cell proliferation differentiation and its role in follicular development. Chinese Sci. Bull 2005, 50, 2665–2671. [Google Scholar]
- Linke, SP; Clarkin, KC; DiLeonardo, A; Tsou, A; Wahl, GM. A reversible p53-dependent Go/G1 cell cycle arrest induced by ribonucleotide depletion in the absence of detectable DNA damage. Genes Dev 1996, 10, 937–947. [Google Scholar]
- Ireland, JJ; Roche, JF. Development of antral follicles in cattle after prostaglandin-induced luteolysis: changes in serum hormones, steroids in follicular fluid, and gonadotropin receptors. Endocrinology 1982, 111, 2077–2086. [Google Scholar]
- McNatty, KP; Heath, DA; Henderson, KM; Lun, S; Hurst, PR; Ellis, LM; Montgomery, GW; Morrison, L; Thurley, DC. Some aspects of thecal and granulosa cell function during follicular development in the bovine ovary. J. Reprod. Fertil 1984, 72, 39–53. [Google Scholar]
- Fortune, JE; Hansel, W. Concentration of steroids and gonadotropins in follicular fluid from normal heifers and heifers primed for superovulation. Biol. Reprod 1985, 32, 1069–1079. [Google Scholar]
- Spicer, LJ; Matton, P; Echternkamp, SE; Convey, EM; Tucker, HA. Relationship between histological signs of atresia, steroids in follicular fluid, and gonadotropin binding in individual bovine antral follicles during postpartum anovulation. Biol. Reprod 1987, 36, 890–898. [Google Scholar]
- Akca, H; Ozes, ON. Hydroxyurea induces p53 accumulation and apoptosis in human cervical carcinoma cells. Turk. J. Biol 2002, 26, 145–150. [Google Scholar]
- Yeo, EJ; Hwang, YC; Kang, CM; Kim, IH; Kim, DI; Parka, JS; Choy, HE; Park, WY; Park, SC. Senescence-like changes induced by hydroxyurea in human diploid fibroblasts. Exp. Gerontol 2000, 35, 553–571. [Google Scholar]



© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sampson, M.; Archibong, A.E.; Powell, A.; Strange, B.; Roberson, S.; Hills, E.R.; Bourne, P. Perturbation of the Developmental Potential of Preimplantation Mouse Embryos by Hydroxyurea. Int. J. Environ. Res. Public Health 2010, 7, 2033-2044. https://doi.org/10.3390/ijerph7052033
Sampson M, Archibong AE, Powell A, Strange B, Roberson S, Hills ER, Bourne P. Perturbation of the Developmental Potential of Preimplantation Mouse Embryos by Hydroxyurea. International Journal of Environmental Research and Public Health. 2010; 7(5):2033-2044. https://doi.org/10.3390/ijerph7052033
Chicago/Turabian StyleSampson, Mariam, Anthony E. Archibong, Adriane Powell, Brandon Strange, Shannon Roberson, Edward R. Hills, and Phillip Bourne. 2010. "Perturbation of the Developmental Potential of Preimplantation Mouse Embryos by Hydroxyurea" International Journal of Environmental Research and Public Health 7, no. 5: 2033-2044. https://doi.org/10.3390/ijerph7052033



