Environmental Impact of Flame Retardants (Persistence and Biodegradability)
Abstract
:1. Introduction
2. Brominated flame retardants
3. Environmental Fate of Brominated Flame Retardants
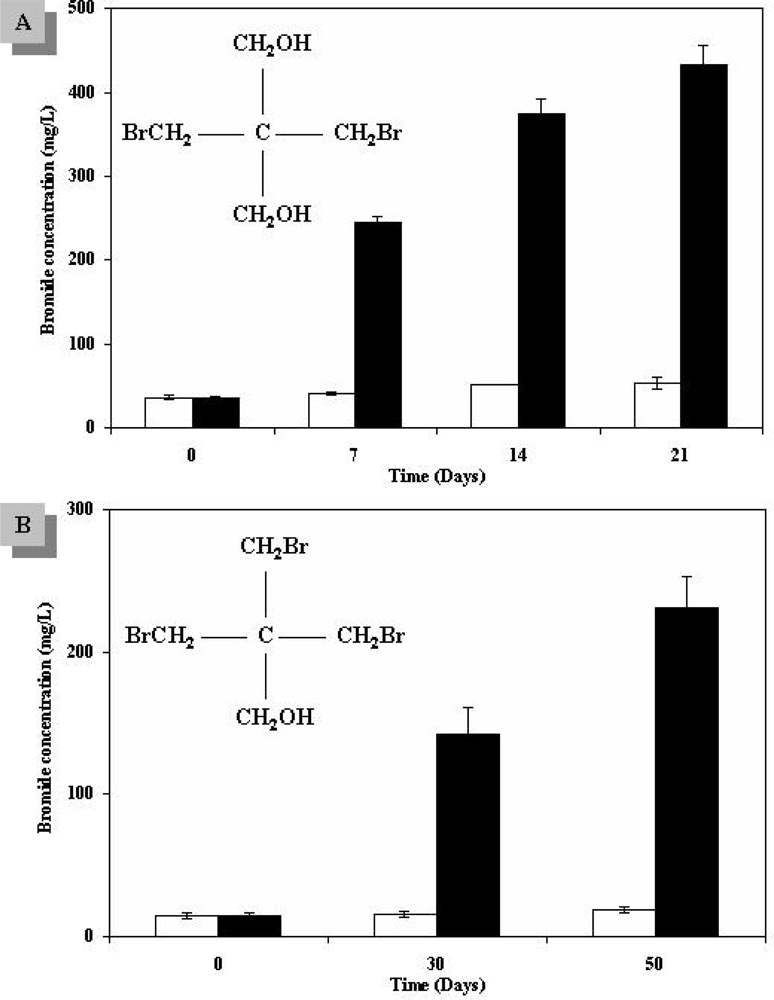
3.1. Biodegradation and Bioremediation
4. Summary
Acknowledgments
References
- Alaee, M; Wenning, RJ. The significance of brominated flame retardants in the environment: current understanding. Issues and challenges. Chemosphere 2002, 46, 579–582. [Google Scholar]
- Market Study Flame Retardants, Ceresana Research: Konstanz Germany, 2008. www.ceresana.com/en/market-studies/additives/flame-retardants(accessed February 4, 2009).
- Levchik, SV. Introduction to flame retardancy and polymer flammability. In Flame retardant polymer nanocomposites; Morgan, AB, Wilkie, CA, Eds.; John Wiley & Sons: NY, USA, 2007; pp. 1–29. [Google Scholar]
- Birnbaum, LS; Staskal, DF. Brominated flame retardants: cause for concern? Environ. Health Perspect 2004, 112, 9–17. [Google Scholar]
- Murphy, J. Modifying specific properties: flammability-flame retardants. In Additives for plastics handbooks; Elsevier Science Ltd: New York, NY, USA, 2001; pp. 115–140. [Google Scholar]
- Alaee, M; Arias, P; Sjödin, A; Bergman, Å. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ. Int 2003, 29, 683–689. [Google Scholar]
- Hartmann, PC; Burgi, D; Giger, W. Organophosphate flame retardants and plasticizers in indoor air. Chemosphere 2004, 57, 781–787. [Google Scholar]
- Janssen, S. Brominated flame retardants rising levels of concern, Health Care With Out Harm (HCWH): Arlington, VA, USA, 2005.
- Carlsson, H; Nilsson, U. Stman, C. Video display units: an emmition source of the contact allergenic flame retardant triphenyl phosphate in the indoor environment. Environ. Sci. Technol 2000, 34, 3885–3889. [Google Scholar]
- Andresen, JA; Grundmann, A; Bester, K. Organophosphorus flame retardants and plasticisers in surface waters. Sci. Total Environ 2004, 332, 55–166. [Google Scholar]
- Marklund, A; Andersson, B; Haglund, P. Screening of organophosphorus compounds and their distribution in various indoor environments. Chemosphere 2003, 53, 1137–1146. [Google Scholar]
- De Wit, CA; Alaee, M; Muir, DCG. Levels and trends of brominated flame retardants in the Arctic. Chemosphere 2006, 64, 209–233. [Google Scholar]
- Law, RJ; Allchin, CR; De Boer, J; Covaci, A; Herzke, D; Lepom, P; Morris, S; Tronczynski, J; De Wit, CA. Levels and trends of brominated flame retardants in the European environment. Chemosphere 2006, 64, 187–208. [Google Scholar]
- Law, RJ; Herzke, D; Harrad, S; Morris, S; Bersuder, P; Allchin, CR. Levels and trends of HBCD and BDEs in the European and Asian environments with some information for other BFRs. Chemosphere 2008, 73, 223–241. [Google Scholar]
- Quintana, JB; Rodil, R; Reemtsma, T; Garcia-Lopez, M; Rodrigues, I. Organophosphorus flame retardants and plasticizers in water and air II. Analytical methology. Trends Analyt. Chem 2008, 27, 904–915. [Google Scholar]
- Tohka, A; Zevenhoven, R. Brominated flame retardants – a nuisance in thermal waste processing. Presented at TMS Fall 2002 Extraction and Processing Division Meeting on Recycling and Waste treatment in Mineral and Metal Processing. Technical and Economics Aspects, Lulea, Sweden, June 16–20, 2002.
- Troitzsch, JH. Overview of flame retardant. Chemistry Today 1998, 16. [Google Scholar]
- Häggblom, MM; Bossert, ID. Dehalogenation – Microbial processes and environmental applications; Kluwer Academic Publishers: Norwell, MA, USA, 2003. [Google Scholar]
- Sjödin, A; Carlsson, H; Thuresson, K; Sjölin, S; Bergman, Å; Östman, C. Flame retardants in indoor air at an electronics recycling plant and at other work environment. Environ. Sci. Technol 2001, 35, 448–454. [Google Scholar]
- Sjödin, A; Patterson, DG, Jr; Bergman, A. A review on human exposure to brominated flame retardants – particularly polybrominated diphenyl ethers. Environ. Int 2003, 29, 829–839. [Google Scholar]
- Stapleton, HM; Dodder, NG; Offenberg, JH; Schntz, MM; Wise, SA. Polybrominated diphenyl ethers in house dust and clothes dryer lint. Environ. Sci. Technol 2005, 39, 925–931. [Google Scholar]
- Hale, RC; La Guardia, MJ; Harvey, E; Gaylor, MO; Mainor, TM. Brominated flame retardant concentration and trends in abiotic media. Chemosphere 2006, 64, 181–186. [Google Scholar]
- Tollbäck, J; Crescenzi, C; Dyremar, E. Determination of the flame retardant tetrabromobisphenol A in air samples by liquid chromatography-mass spectrometry. J. Chromatogr A 2006, 1104, 106–112. [Google Scholar]
- Watanabe, I; Sakai, SI. Environmental release and behavior of brominated flame retardants. Environ. Int 2003, 29, 665–682. [Google Scholar]
- Remberger, M; Sternbeck, J; Palm, A; Kaj, L; Strömberg, K; Brorström-Luden, E. The environmental occurrence of hexabromocyclododecane in Sweden. Chemosphere 2004, 54, 9–21. [Google Scholar]
- De Wit, CA. An overview of brominated flame retardants in the environment. Chemosphere 2002, 46, 583–624. [Google Scholar]
- De Boer, J; Wester, PG; Van der Horst, A; Leonards, PEG. Polybrominated diphenyl ethers in influents, suspended particulate matter, sediments, sewage treatment plant and effluents and biota from Netherlands. Environ. Pollut 2003, 122, 63–74. [Google Scholar]
- Kupper, T; De Alencastro, LF; Gatsigazi, R; Furrer, R; Grandjean, D; Tarradellas, J. Concentrations and specific loads of brominated flame retardants in sewage sludge. Chemosphere 2008, 71, 1173–1180. [Google Scholar]
- Sjödin, A; Hagmar, L; Klasson-Wehler, E; Kronholm-Diab, K; Jakobsson, E; Bergman, A. Flame retardant exposure: Polybrominated diphenyl ethers in blood from Swedish workers. Environ. Health Perspect 1999, 107, 643–648. [Google Scholar]
- Blanck, HM; Marcus, M; Hertzberg, V; Tolbert, PE; Rubin, C; Henderson, AK. Determinants of polybrominated biphenyl serum decay among women in the Michigan PBB cohort. Environ. Health Perspect 2000, 108, 147–152. [Google Scholar]
- Jakobsson, K; Thuresson, K; Rylander, L; Sjodin, A; Hagmar, L; Bergman, A. Exposure to polybrominated diphenyl ethers and tetrabromobisphenol A among computer technicians. Chemosphere 2002, 46, 709–716. [Google Scholar]
- Lind, Y; Darnerud, PO; Atuma, S; Aune, M; Becker, W; Bjerseliius, R; Canattingius, S; Glynn, A. Polybrominated diphenyl ethers in breast milk from Uppsala County, Sweden. Environ. Res 2003, 93, 186–194. [Google Scholar]
- Weiss, J; Meijer, L; Sauer, P; Linderholm, L; Bergman, A; Athanassiadis, I. PBDE and HBCD levels in blood from Dutch mothers and infants – analysis of a Dutch Groningen infant cohort. Organohalogen Compound 2004, 66, 2677–2682. [Google Scholar]
- Herzke, D; Berger, U; Kallenborn, R; Nygard, T; Vetter, W. Brominated flame retardants and other organobromines in Norwegian bird eggs. Chemosphere 2005, 61, 441–449. [Google Scholar]
- Janak, K; Covaci, A; Voorspoels, S; Becher, G. Hexabromocyclododecane in marine species from the Western Scheldt Estuary: diastereoisomer- and enantiomer- specific accumulation. Environ. Sci. Technol 2005, 39, 1987–1994. [Google Scholar]
- Muir, DCG; Backus, S; Derocher, AE; Dietz, R; Evans, TJ; Gabrielsen, GW; Nagy, J; Norstrom, RJ; Sonne, C; Stirling, I; Taylor, MK; Letcher, RJ. Brominated flame retardant in Polar bear (Ursus maritimus) from Alaska, the Canadian Arctic, East Greenland and Svalbard. Environ. Sci. Technol 2006, 40, 449–455. [Google Scholar]
- Jenssen, BM; Sǿrmo, EG; Baek, K; Bytingsvik, J; Gaustad, H; Ruus, A; Skaare, JU. Brominated flame retardants in North-East Atlantic Marine Ecosystems. Environ. Health Perspect 2007, 115, 35–41. [Google Scholar]
- Thomsen, K; Molander, P; Daae, HL; Janak, K; Froshaug, M; Liane, VH; Thorud, S; Becher, G; Dybing, E. Occupational exposure to Hexabromocyclododecane at an Industrial Plant. Environ. Sci. Technol 2007, 41, 5210–5216. [Google Scholar]
- Johnson-Restrepo, B; Adams, DH; Kannan, K. Tetrabromobisphenol A (TBBPA) and hexabromocyclododecanes (HBCDs) in tissues of humans, dolphins, and sharks from the United States. Chemosphere 2008, 70, 1935–1944. [Google Scholar]
- Kakimoto, K; Akutsuk, K; Konishi, Y; Tanaka, Y. Time trend of hexabromocyclododecane in the breast milk of Japanese women. Chemosphere 2008, 71, 1110–1114. [Google Scholar]
- Polder, A; Venter, B; Skaare, JU; Bouwman, H. Polybrominated diphenyl ethers and HBCD in bird eggs of South Africa. Chemosphere 2008, 73, 148–154. [Google Scholar]
- Roosens, L; Dirtu, AC; Goemans, G; Belpaire, C; Gheorghe, A; Neels, H; Blust, R; Covaci, A. Brominated flame retardants and polychlorinated biphenyls in fish from the river Scheldt, Belgium. Environ. Int 2008, 34, 976–983. [Google Scholar]
- Simonsen, FA; Stavnsbjerg, M; Møller, LM; Madsen, T. Brominated flame retardants: Toxicity and ecotoxicity; Environment project No. 568. Danish Environmental Protection Agency: København, K Denmark, 2000. [Google Scholar]
- Darnerud, PO. Toxic effects of brominated flame retardants in man and in wildlife. Environ. Int 2003, 29, 841–853. [Google Scholar]
- Mariussen, E; Fonnum, F. The effect of brominated flame retardants on neurotransmitter uptake into rat brain synaptosomes and vesicles. Neurochem. Int 2003, 43, 533–542. [Google Scholar]
- Canesi, L; Lorusso, LC; Ciacci, C; Betti, M; Gallo, G. Effects of the brominated flame retardant tetrabromobisphenol-A (TBBPA) on cell signaling and function of Mytilus hemocytes: involvement of MAP kinases and protein kinase C. Aquat. Toxicol 2005, 75, 277–287. [Google Scholar]
- Legler, J. New insight into the endocrine disrupting effects of brominated flame retardants. Chemosphere 2008, 73, 216–222. [Google Scholar]
- Rayne, S; Ikonomou, MG; MacMurray, DW. Anaerobic microbial and photochemical degradation of 4,4′-dibromodiphenyl ether. Water Res 2003, 37, 551–560. [Google Scholar]
- Eriksson, J; Green, N; Marsh, G; Bergman, A. Photochemical decomposition of 15 polybrominated diphenyl ether congeners in methanol/water. Environ. Sci. Technol 2004, 38, 3119–3125. [Google Scholar]
- Raff, J; Hites, R. Deposition versus photochemical removal of PBDEs from Lake Superior air. Environ. Sci. Technol 2007, 41, 6725–6731. [Google Scholar]
- Hakk, H; Letcher, RJ. Metabolism in the toxicokinetics and fate of brominated flame retardants – a review. Environ. Int 2003, 29, 801–828. [Google Scholar]
- Boon, JP; Lewis, WE; Tjoen-A-Choy, MR; Allchin, CR; Law, RJ; De Boer, J; Hallers-Tjabbes, CCT; Zegers, BN. Levels of Polybrominated diphenyl ether (PBDE) flame retardants in animals representing different trophic levels of the North Sea food web. Environ. Sci. Technol 2002, 36, 4025–4032. [Google Scholar]
- Bayen, S; Wurl, O; Karuppiah, S; Sivasothi, N; Lee, HK; Obbard, JP. Persistent organic pollutants in mangrove food webs in Singapore. Chemosphere 2005, 61, 303–313. [Google Scholar]
- Sǿrmo, EG; Salmer, MP; Jenssen, BM; Hop, H; Baek, K; Kovacs, KM; Lydersen, C; Falk-Petersen, S; Gabrielsen, GW; Lie, E; Skaare, JU. Biomagnification of polybrominated diphenyl ether and hexabromocyclododecane flame retardants in the polar bear food chain in Svalbard, Norway. Environ. Toxicol. Chem 2006, 25, 2502–2511. [Google Scholar]
- Schecter, A; Päpke, O; Tung, KC; Staskal, D; Birnbaum, L. Polybrominated diphenyl ethers contamination of United States food. Environ. Sci. Technol 2004, 38, 5306–5311. [Google Scholar]
- Camara, B; Herrera, C; Gonzalez, M; Couve, E; Hofer, B; Seeger, M. From PCBs to highly toxic metabolites by the biphenyl pathway. Environ. Microbiol 2004, 6, 842–850. [Google Scholar]
- Ronen, Z; Abeliovich, A. Anaerobic-aerobic process for microbial degradation of Tetrabromobisphenol A. Appl. Environ. Microbiol 2000, 66, 2372–2377. [Google Scholar]
- Chaudhry, GR; Chapalamadugu, S. Biodegradation of Halogenated Organic Compounds. Microbiol. Rev 1991, 55, 59–79. [Google Scholar]
- Fetzner, S; Lingens, F. Bacterial dehalogenases: biochemistry, genetics, and biotechnological applications. Microbiol. Mol. Biol. Rev 1994, 58, 641–685. [Google Scholar]
- Smidt, H; De Vos, WM. Anaerobic microbial dehalogenation. Annu. Rev. Microbiol 2004, 58, 43–73. [Google Scholar]
- Janssen, BD; Oppentocht, JE; Poelarends, GJ. Microbial dehalogenation. Curr. Opin. Biotechnol 2001, 12, 254–258. [Google Scholar]
- Van Pee, KH; Unversucht, S. Biological dehalogenation and halogenation reactions. Chemosphere 2003, 52, 299–312. [Google Scholar]
- Segev, O; Abeliovich, A; Kushmaro, A. Biodegradation of Dibromoneopentyl glycol by bacterial consortium. Chemosphere 2007, 68, 958–964. [Google Scholar]
- Segev, O; Meusel, W; Friedenberger, M; Brenner, A; Kushmaro, A. Aerobic biodegradation of the brominated flame retardants, Dibromoneopentyl Glycol and Tribromoneopentyl Alcohol. Biodegradation 2009. [Google Scholar]
- Arnon, S; Eilon, A; Ronen, Z; Nejidat, A; Yakirevich, A; Nativ, R. Biodegradation of 2,4,6-Tribromophenol during transport in fractured chalk. Environ. Sci. Technol 2005, 39, 748–755. [Google Scholar]
- Brenner, A; Mukmanov, I; Abeliovich, A; Kushmaro, A. Biodegradability of tetrabromobisphenol A and tribromophenol by activated sludge. Ecotoxicology 2006, 15, 399–402. [Google Scholar]
- Boyle, AW; Phelps, CD; Young, LY. Isolation from Estuarine Sediments of a Desulfovibrio strain which can grow on lactate coupled to the reductive dehalogenation of 2,4,6-tribromophenol. Appl. Environ. Microbiol 1999, 65, 1133–1140. [Google Scholar]
- Ronen, Z; Vasiluk, L; Abeliovich, A; Nejidat, A. Activity and survival of tribromophenol degrading bacteria in a contaminated desert soil. Soil Biol. Biochem 2000, 32, 1643–1650. [Google Scholar]
- Takashi, Y; Takahama, Y; Yamada, Y. Biodegradation of 2,4,6-tribromophenol by Ochrobacterium sp. Strain TB01. Biosci. Biotechnol. Biochem 2008, 72, 1264–1271. [Google Scholar]
- Gerecke, AC; Giger, W; Hartmann, PC; Heeb, NV; Kohler, HPE; Schmid, P; Zennegg, M; Kohler, M. Anaerobic degradation of brominated flame retardants in sewage sludge. Chemosphere 2006, 64, 311–317. [Google Scholar]
- He, J; Robrock, KR; Alvarez-Cohen, L. Microbial Reductive Debromination of Polybrominated Diphenyl Ethers (PBDEs). Environ. Sci. Technol 2006, 40, 4429–4434. [Google Scholar]
- Zhou, J; Jiang, W; Ding, J; Zhang, X; Gao, S. Effect of Tween 80 and β-cyclodextrin on degradation of decabromodiphenyl ether (BDE-209) by White Rot Fungi. Chemosphere 2007, 70, 172–177. [Google Scholar]
- Robrock, KR; Korytar, P; Alvarez-Cohen, L. Pathways for the anaerobic microbial debromination of polybrominated diphenyl ethers. Environ. Sci. Technol 2008, 42, 2845–2852. [Google Scholar]
- Tokarz, JA, III; Ahn, MY; Leng, J; Filley, TR; Nies, L. Reductive debromination of polybrominated diphenyl ethers in anaerobic sediment and a biomimetic system. Environ. Sci. Technol 2008, 42, 1157–1164. [Google Scholar]
- Davis, JW; Gonsior, S; Marty, G; Ariano, J. The transformation of hexabromocyclododecane in aerobic and anaerobic soils and aquatic sediments. Water Res 2005, 39, 1075–1084. [Google Scholar]
- Davis, JW; Gonsior, S; Markham, DA; Friederich, U; Hunziker, RW; Arianob, J. Biodegradation and product identification of [14C]hexabromocyclododecane in wastewater sludge and freshwater aquatic sediments. Environ. Sci. Technol 2006, 40, 5395–5401. [Google Scholar]
- Steinmetz, R; Mitchner, NA; Grant, A; Allen, DL; Bigsby, RM; Ben-Jonathan, N. The xenoestrogen bisphenol A induces growth, differentiation, and c-fos gene expression in the female reproductive tract. Endocrinology 1998, 139, 2741–2747. [Google Scholar]
- Welshons, WV; Nagel, SC; Vom Saal, FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 2006, 147, S56–S69. [Google Scholar]
- Allard, AS; Remberger, M; Neilson, AH. Bacterial O-Methylation of halogen-substituted phenols. Appl. Environ. Microbiol 1987, 53, 839–845. [Google Scholar]
- Häggblom, MM; Fennel, DE; Ahn, YB; Ravit, B; Kerkhof, LJ. Anaerobic dehalogenation of halogenated organic compound: Novel strategies for bioremediation of contaminated sediments. In Soil and Water Pollution Monitoring, Protection and Remediation; Twardowska, I, Allen, HE, Häggblom, M, Stefaniak, S, Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 505–521. [Google Scholar]


Share and Cite
Segev, O.; Kushmaro, A.; Brenner, A. Environmental Impact of Flame Retardants (Persistence and Biodegradability). Int. J. Environ. Res. Public Health 2009, 6, 478-491. https://doi.org/10.3390/ijerph6020478
Segev O, Kushmaro A, Brenner A. Environmental Impact of Flame Retardants (Persistence and Biodegradability). International Journal of Environmental Research and Public Health. 2009; 6(2):478-491. https://doi.org/10.3390/ijerph6020478
Chicago/Turabian StyleSegev, Osnat, Ariel Kushmaro, and Asher Brenner. 2009. "Environmental Impact of Flame Retardants (Persistence and Biodegradability)" International Journal of Environmental Research and Public Health 6, no. 2: 478-491. https://doi.org/10.3390/ijerph6020478




