Chronic Traffic-Induced PM Exposure and Self-Reported Respiratory and Cardiovascular Health in the RHINE Tartu Cohort
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Population and Site
2.2. Questionnaire on Respiratory Health and Cardiac Diseases
- Wheezing “Have you had wheezing or whistling in your chest at any time in the last 12 months?”, and if so, “Have you had this wheezing or whistling when you did not have a cold?”
- Cough “Have you been woken by an attack of coughing at any time in the last 12 months?”
- Chronic bronchitis symptoms “Do you cough up phlegm in this way almost every day for at least three months every year?”, and if so, “Have you had periods of this kind for at least two years in a row?”
- Non-allergic rhinitis “Have you ever experienced nasal symptoms such as nasal congestion, rhinorrhoea (runny nose) and/or sneezing attacks without having a cold?”, and if “Yes”; “No” to question “Do you have any nasal allergies including hay fever?”
- Chest tightness “Have you woken up with a feeling of tightness in your chest at any time in the last 12 months?”
- Breath shortness “Have you been woken by an attack of shortness of breath at any time in the last 12 months?”
- Cardiac diseases “Do you have any cardiac disease?”
- Hypertension “Do you have high blood pressure?”
2.3. Air Pollution Exposure
2.4. Statistical Analyses
3. Results
3.1. Exposure
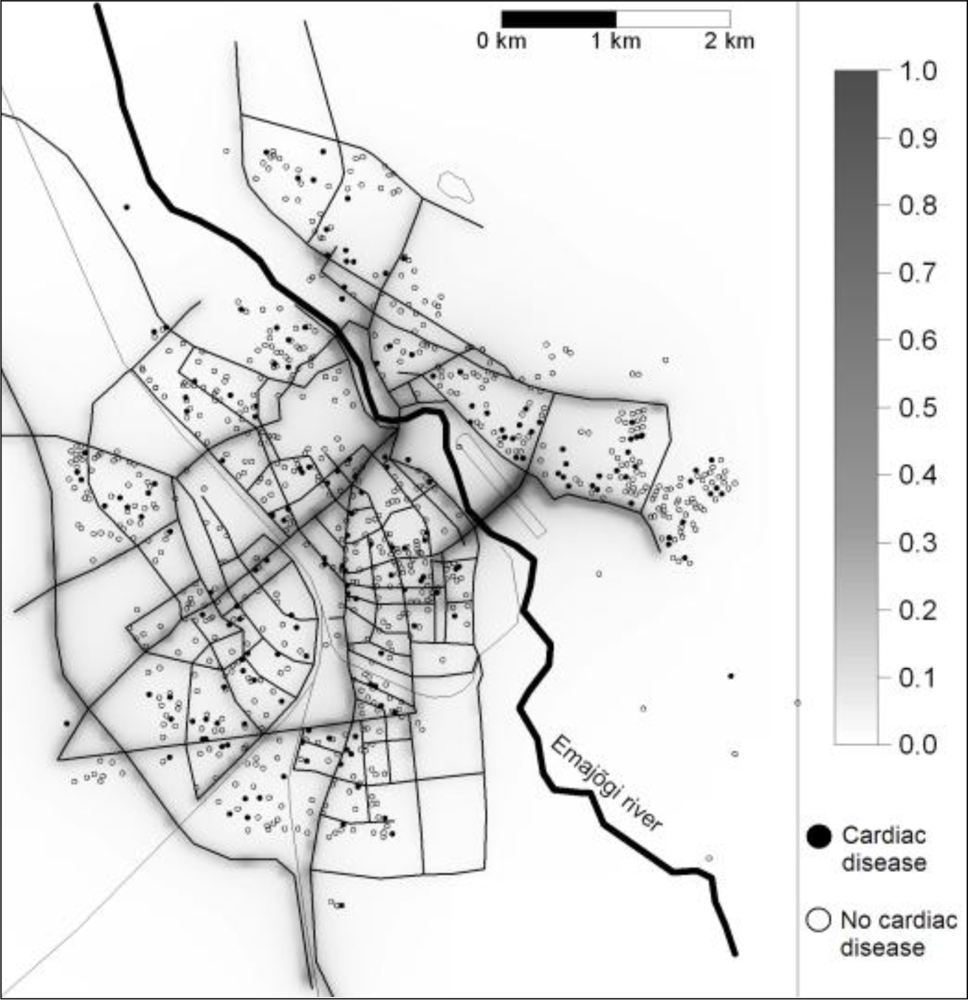
3.2. Associations with PM at Home and Prevalences
4. Discussion
5. Conclusions
Acknowledgments
References
- Pope, C; Dockery, D. Health effects of fine particulate air pollution: lines that connect. J. Air Waste Manage Assoc 2006, 56, 709–742. [Google Scholar]
- Thorpe, A; Harrison, RM. Sources and properties of non-exhaust particulate matter from road traffic: A review. Sci. Total Environ 2008, 400, 270–282. [Google Scholar]
- Brunekreef, B; Forsberg, B. Epidemiological evidence of effects of coarse airborne particles on health. Eur. Resp. J 2005, 26, 309–318. [Google Scholar]
- Brook, RD. Cardiovascular effects of air pollution. Clin. Sci 2008, 115, 175–187. [Google Scholar]
- Laden, F; Schwartz, J; Speizer, FE; Dockery, DW. Reduction in fine particulate air pollution and mortality - Extended follow-up of the Harvard six cities study. Am. J. Respir. Crit. Care Med 2006, 173, 667–672. [Google Scholar]
- Pope, CA; Burnett, RT; Thurston, GD; Thun, MJ; Calle, EE; Krewski, D; Godleski, JJ. Cardiovascular mortality and long-term exposure to particulate air pollution—Epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004, 109, 71–77. [Google Scholar]
- Jacquemin, B; Sunyer, J; Forsberg, B; Aguilera, I; Briggs, D; Garcia-Esteban, R; Gotschi, T; Heinrich, J; Jarvholm, B; Jarvis, D; Vienneau, D; Kunzli, N. Home outdoor NO2 and new onset of self-reported asthma in adults. Epidemiology 2009, 20, 119–126. [Google Scholar]
- Modig, L; Toren, K; Janson, C; Jarvholm, B; Forsberg, B. Vehicle exhaust outside the home and onset of asthma among adults. Eur. Respir. J 2009, 33, 1261–1267. [Google Scholar]
- Riikjarv, MA; Annus, T; Braback, L; Rahu, K; Bjorksten, B. Similar prevalence of respiratory symptoms and atopy in Estonian schoolchildren with changing lifestyle over 4 yrs. Eur. Resp. J 2000, 16, 86–90. [Google Scholar]
- Jogi, R; Janson, C; Bjornsson, E; Boman, G; Bjorksten, B. The prevalence of asthmatic respiratory symptoms among adults in Estonian and Swedish university cities. Allergy 1996, 51, 331–336. [Google Scholar]
- Vasar, M; Braback, L; Julge, K; Knutsson, A; Riikjarv, MA; Bjorksten, B. Prevalence of bronchial hyperreactivity as determined by several methods among Estonian schoolchildren. Pediatr. Allergy Immunol 1996, 7, 141–146. [Google Scholar]
- Pallasaho, P; Meren, M; Raukas-Kivioja, A; Ronmark, E. Different labelling of obstructive airway diseases in Estonia, Finland, and Sweden. Eur. J. Epidemiol 2005, 20, 975–983. [Google Scholar]
- Annus, T; Riikjarv, MA; Rahu, K; Bjorksten, B. Modest increase in seasonal allergic rhinitis and eczema over 8 years among Estonian schoolchildren. In Congress of the Asia-Pacific-Association-of-Pediatric-Allergy-Respirology-and-Immunology; Seoul, South Korea, 2004, 2005; Blackwell Publishing: Seoul, South Korea, 2005; pp. 315–320. [Google Scholar]
- Toren, K; Gislason, T; Omenaas, E; Jogi, R; Forsberg, B; Nystrom, L; Olin, AC; Svanes, C; Janson, C; RHINE Group. A prospective study of asthma incidence and its predictors: the RHINE study. Eur. Resp. J 2004, 24, 942–946. [Google Scholar]
- Lindgren, A; Stroh, E; Montnémery, P; Nihlén, U; Jakobsson, K; Axmon, A. Traffic-related air pollution associated with prevalence of asthma and COPD/chronic bronchitis. A cross-sectional study in Southern Sweden. Int. J. Health Geogr 2009, 8, 2. [Google Scholar]
- Sunyer, J; Jarvis, D; Gotschi, T; Garcia-Esteban, R; Jacquemin, B; Aguilera, I; Ackerman, U; de Marco, R; Forsberg, B; Gislason, T; Heinrich, J; Norback, D; Villani, S; Kunzli, N. Chronic bronchitis and urban air pollution in an international study. Occup. Environ. Med 2006, 63, 836–843. [Google Scholar]
- Heinrich, J; Topp, R; Gehring, U; Thefeld, W. Traffic at residential address, respiratory health, and atopy in adults: the National German Health Survey 1998. Environ. Res 2005, 98, 240–249. [Google Scholar]
- Cesaroni, G; Badaloni, C; Porta, D; Forastiere, F; Perucci, CA. Comparison between various indices of exposure to traffic-related air pollution and their impact on respiratory health in adults. Occup. Environ. Med 2008, 65, 683–690. [Google Scholar]
- Ko, FWS; Lai, CKW; Woo, J; Ho, SC; Ho, CWM; Goggins, W; Hui, DSC. 12-year change in prevalence of respiratory symptoms in elderly Chinese living in Hong Kong. Resp. Med 2006, 100, 1598–1607. [Google Scholar]
- Goldberg, MS; Giannetti, N; Burnett, RT; Mayo, NE; Valois, M-F; Brophy, JM. Shortness of breath at night and health status in congestive heart failure: Effects of environmental conditions and health-related and dietary factors. Environ. Res 2009, 109, 166–174. [Google Scholar]
- Dvonch, JT; Kannan, S; Schulz, AJ; Keeler, GJ; Mentz, G; House, J; Benjamin, A; Max, P; Bard, RL; Brook, RD. Acute effects of ambient particulate matter on blood pressure. Differential effects across urban communities. Hypertension 2009, 53, 853–859. [Google Scholar]
- Lipfert, FW; Perry, HM; Miller, JP; Baty, JD; Wyzga, RE; Carmody, SE. Air pollution, blood pressure, and their long-term associations with mortality. Inhal. Toxicol 2003, 15, 493–512. [Google Scholar]
- Madsen, C; Nafstad, P. Associations between environmental exposure and blood pressure among participants in the Oslo Health Study (HUBRO). Eur. J. Epidemiol 2006, 21, 485–491. [Google Scholar]
- Kunzli, N; Jerrett, M; Mack, WJ; Beckerman, B; LaBree, L; Gilliland, F; Thomas, D; Peters, J; Hodis, HN. Ambient air pollution and atherosclerosis in Los Angeles. Environ. Health Perspect 2005, 113, 201–206. [Google Scholar]
- Forbes, L; Patel, M; Rudnicka, A; Cook, D; Bush, T; Stedman, J; Whincup, P; Strachan, D; Anderson, H. Chronic exposure to outdoor air pollution and diagnosed cardiovascular disease: meta-analysis of three large cross-sectional surveys. Environ. Health 2009, 8, 30. [Google Scholar]
- Grazuleviciene, R; Maroziene, L; Dulskiene, V; Malinauskiene, V; Azaraviciene, A; Laurinaviciene, D; Jankauskiene, K. Exposure to urban nitrogen dioxide pollution and the risk of myocardial infarction. Scand. J. Work Environ. Health 2004, 30, 293–298. [Google Scholar]
- Orru, H; Kaasik, M; Antov, D; Forsberg, B. Evolution of traffic flows and traffic-induced air pollution due to structural changes and development during 1993–2006 in Tartu (Estonia). Balt. J. Road. Bridge. Eng 2008, 3, 206–212. [Google Scholar]
- Hazenkamp-Von Arx, ME; Gotschi, T; Ackermann-Liebrich, U; Bono, R; Burney, P; Cyrys, J; Jarvis, D; Lillienberg, L; Luczynska, C; Maldonado, JA; Jaen, A; de Marco, R; Mi, YH; Modig, L; Bayer-Oglesby, L; Payo, F; Soon, A; Sunyer, J; Villani, S; Weyler, J; Kunzli, N. PM2.5 and NO2 assessment in 21 European study centres of ECRHS II: annual means and seasonal differences. Atmos. Environ 2004, 38, 1943–1953. [Google Scholar]
- Orru, H; Kimmel, V; Forsberg, B; Soon, A. Elemental Composition and Oxidative Properties of PM2.5 in Relation of Origin of Air Masses in ERCHS II City Tartu. In 20th Annual Conference of the International-Society-for-Environmental-Epidemiology; , Pasadena, CA, USA, Oct, 12–16, 2008; Lippincott Williams & Wilkins: Pasadena, CA, USA, 2008; pp. S226–S226. [Google Scholar]
- Turner, DB. A diffusion model for an urban area. J. Appl. Meteorol 1964, 3, 83–91. [Google Scholar]
- Kaasik, M; Kimmel, V. Validation of the improved AEROPOL model against the Copenhagen data set. Int. J. Environ. Pollut 2003, 20, 114–120. [Google Scholar]
- Carruthers, DJ; Holroyd, RJ; Gunt, JCR; Weng, W-S; Robins, AG; Apsley, DD; Thomson, DJ; Smith, FB. UK-ADMS: a new approach to modelling dispersion in the Earth’s atmospheric boundary layer. J. Wind Eng. Ind. Aerodyn 1994, 52, 139–153. [Google Scholar]
- Cimorelli, AJ; Perry, SG; Venkatram, A; Weil, JC; Paine, RJ; Wilson, RB; Lee, RF; Peters, WD; Brode, RW. AERMOD: A dispersion model for industrial source applications. Part I: General model formulation and boundary layer characterization. J. Appl. Meteorol 2005, 44, 682–693. [Google Scholar]
- Kaasik, M; Lukk, T; Kartau, K; Dovnar, T. Nowcasting and forecasting the street pollution dispersion for Tallinn metropolitan area 2007. In Developments in Environmental Science; Borrego, C, Renner, E, Eds.; Elsevier: Oxford, UK, 2007; Volume 6, pp. 744–746. [Google Scholar]
- Kimmel, V; Kaasik, M. Assessment of Urban Air Quality in South Estonia by Simple Measures. Environ. Model. Assess 2003, 8, 47–53. [Google Scholar]
- Sofiev, M; Kaasik, M; Hongisto, M. Model Simulations of the Alkaline Dust Distribution from Estonian Sources over the Baltic Sea Basin. Water Air Soil Pollut 2003, 146, 211–223. [Google Scholar]
- Härkönen, J; Nikmo, J; Karppinen, A; Kukkonen, J. A refined modelling system for estimating the emissions, dispersion, chemical transformation and dry deposition of traffic-originated pollution from a road. In A Refined Modelling System for Estimating the Emissions, Dispersion, Chemical Transformation and Dry Deposition of Traffic-Originated Pollution from a Road; Cuvelier, C, Ed.; European Joint Research Centre: Belgirate, Italy, 2001; pp. 311–313. [Google Scholar]
- Eneroth, K; Johansson, C; Bellander, T. Exposure Comparison between Measurements and Calculations Based on Dispersion Modelling; Stockholms och Uppsala Läns Luftvårsförbund: Stockholm, Sweden, 2006; p. 23. [Google Scholar]
- Gerlofs-Nijland, ME; Dormans, J; Bloemen, HJT; Leseman, D; Boere, AJF; Kelly, FJ; Mudway, IS; Jimenez, AA; Donaldson, K; Guastadisegni, C; Janssen, NAH; Brunekreef, B; Sandstrom, T; Cassee, FR. Toxicity of coarse and fine particulate matter from sites with contrasting traffic profiles. Inhal. Toxicol 2007, 19, 1055–1069. [Google Scholar]
- Schwarze, PE; Ovrevik, J; Lag, M; Refsnes, M; Nafstad, P; Hetland, RB; Dybing, E. Particulate matter properties and health effects: consistency of epidemiological and toxicological studies. Human Exp. Toxicol 2006, 25, 559–579. [Google Scholar]
- Valavanidis, A; Fiotakis, K; Vlachogianni, T. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev 2008, 26, 339–362. [Google Scholar]
- Wichmann, HE. Diesel exhaust particles. Inhal. Toxicol 2007, 1, 241–244. [Google Scholar]
- Kafoury, R; Madden, M. Diesel exhaust particles induce the over expression of tumor necrosis factor-α (TNF-α) gene in alveolar macrophages and failed to induce apoptosis through activation of nuclear factor-κB (NF-κB). IJERPH 2005, 2, 107–113. [Google Scholar]
- de Marco, R; Cerveri, I; Bugiani, M; Ferrari, M; Verlato, G. An undetected burden of asthma in Italy: the relationship between clinical and epidemiological diagnosis of asthma. Eur. Resp. J 1998, 11, 599–605. [Google Scholar]
- Wilhelmsen, L; Rosengren, A; Hagman, M; Lappas, G. “Nonspecific” chest pain associated with high long-term mortality: results from the primary prevention study in Goteborg, Sweden. Clin. Cardiol 1998, 21, 477–482. [Google Scholar]
- Lampe, FC; Whincup, PH; Wannamethee, SG; Ebrahim, S; Walker, M; Shaper, AG. Chest pain on questionnaire and prediction of major ischaemic heart disease events in men. Eur. Heart J 1998, 19, 63–73. [Google Scholar]
- Walker, MK; Whincup, PH; Shaper, AG; Lennon, LT; Thomson, AG. Validation of patient recall of doctor-diagnosed heart attack and stroke: A postal questionnaire and record review comparison. Am. J. Epidemiol 1998, 148, 355–361. [Google Scholar]
- Meisinger, C; Schuler, A; Lowel, H; Grp, MK. Postal questionnaires identified hospitalizations for self-reported acute myocardial infarction. J. Clin. Epidemiol 2004, 57, 989–992. [Google Scholar]
- Psaty, BM; Kuller, LH; Bild, D; Burke, GL; Kittner, SJ; Mittelmark, M; Price, TR; Rautaharju, PM; Robbins, J. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann. Epidemiol 1995, 5, 270–277. [Google Scholar]
- Olsson, L; Svardsudd, K; Nilsson, G; Ringqvist, I; Tibblin, G. Validity of a postal questionnaire with regard to the prevalence of myocardial-infarction in a general-population sample. Eur. Heart J 1989, 10, 1011–1016. [Google Scholar]
- Rosenlund, M; Bellander, T; Nordquist, T; Alfredsson, L. Traffic-generated air pollution and myocardial infarction. Epidemiology 2009, 20, 265–271. [Google Scholar]
- Hoffmann, B; Moebus, S; Mohlenkamp, S; Stang, A; Lehmann, N; Dragano, N; Schmermund, A; Memmesheimer, M; Mann, K; Erbel, R; Jockel, KH; Heinz Nixdorf Recall, S. Residential exposure to traffic is associated with coronary atherosclerosis. Circulation 2007, 116, 489–496. [Google Scholar]
- Babisch, W. Transportation noise and cardiovascular risk: updated review and synthesis of epidemiological studies indicate that the evidence has increased. Noise Health 2006, 8, 1–29. [Google Scholar]
- Schwela, D; Kephalopoulos, S; Prasher, D. Confounding or aggravating factors in noise-induced health effects: air pollutants and other stressors. Noise Health 2005, 7, 41–50. [Google Scholar]
- Allen, RW; Davies, H; Cohen, MA; Mallach, G; Kaufman, JD; Adar, SD. The spatial relationship between traffic-generated air pollution and noise in 2 US cities. Environ. Res 2009, 109, 334–342. [Google Scholar]
| Age (years) | Men | Smokers | BMI | Prevalence (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | Min-max | (%) | Mean | Min-max | Cardiac disease | Hypertension | Chronic bronchitis | |
| 35 | 25–50 | 48 | 35.3 | 24.2 | 15.4–54.9 | 12.4 | 12.4 | 5.7 |
| OR3 (95% CI4) | ||
|---|---|---|
| Per 1 μg·m−3 increase in exposure | Per increase in exposure corresponding to the fifth to the 95th percentile | |
| Cough1 | 1.01 (0.28–3.64) | 1.00 (0.75–1.34) |
| Chronic bronchitis1 | 0.78 (0.53–11.44) | 0.95 (0.87–1.73) |
| Non–allergic rhinitis1 | 1.79 (0.45–7.17) | 1.14 (0.83–1.56) |
| Wheezing1 | 1.99 (0.36–11.83) | 1.17 (0.79–1.75) |
| Chest tightness1 | 2.34 (0.48–11.48) | 1.21 (0.85–1.74) |
| Shortness of breath1 | 2.92 (0.46–18.65) | 1.27 (0.84–1.94) |
| Cardiac diseases1 | 7.56 (1.40–40.68) | 1.58 (1.08–2.31) |
| Cardiac diseases2 | 9.04 (1.62–50.42) | 1.64 (1.12–2.43) |
| Hypertension1 | 4.65 (0.76–28.41) | 1.42 (0.94–2.13) |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Orru, H.; Jõgi, R.; Kaasik, M.; Forsberg, B. Chronic Traffic-Induced PM Exposure and Self-Reported Respiratory and Cardiovascular Health in the RHINE Tartu Cohort. Int. J. Environ. Res. Public Health 2009, 6, 2740-2751. https://doi.org/10.3390/ijerph6112740
Orru H, Jõgi R, Kaasik M, Forsberg B. Chronic Traffic-Induced PM Exposure and Self-Reported Respiratory and Cardiovascular Health in the RHINE Tartu Cohort. International Journal of Environmental Research and Public Health. 2009; 6(11):2740-2751. https://doi.org/10.3390/ijerph6112740
Chicago/Turabian StyleOrru, Hans, Rain Jõgi, Marko Kaasik, and Bertil Forsberg. 2009. "Chronic Traffic-Induced PM Exposure and Self-Reported Respiratory and Cardiovascular Health in the RHINE Tartu Cohort" International Journal of Environmental Research and Public Health 6, no. 11: 2740-2751. https://doi.org/10.3390/ijerph6112740
APA StyleOrru, H., Jõgi, R., Kaasik, M., & Forsberg, B. (2009). Chronic Traffic-Induced PM Exposure and Self-Reported Respiratory and Cardiovascular Health in the RHINE Tartu Cohort. International Journal of Environmental Research and Public Health, 6(11), 2740-2751. https://doi.org/10.3390/ijerph6112740





