Supraphysiological Levels of Quercetin Glycosides are Required to Alter Mineralization in Saos2 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mineral Content
2.2. ALP Activity
2.3. Cell Activity
2.4. Cell Toxicity
2.5. Osteoblast Regulatory Proteins
2.6. Statistics
3. Results
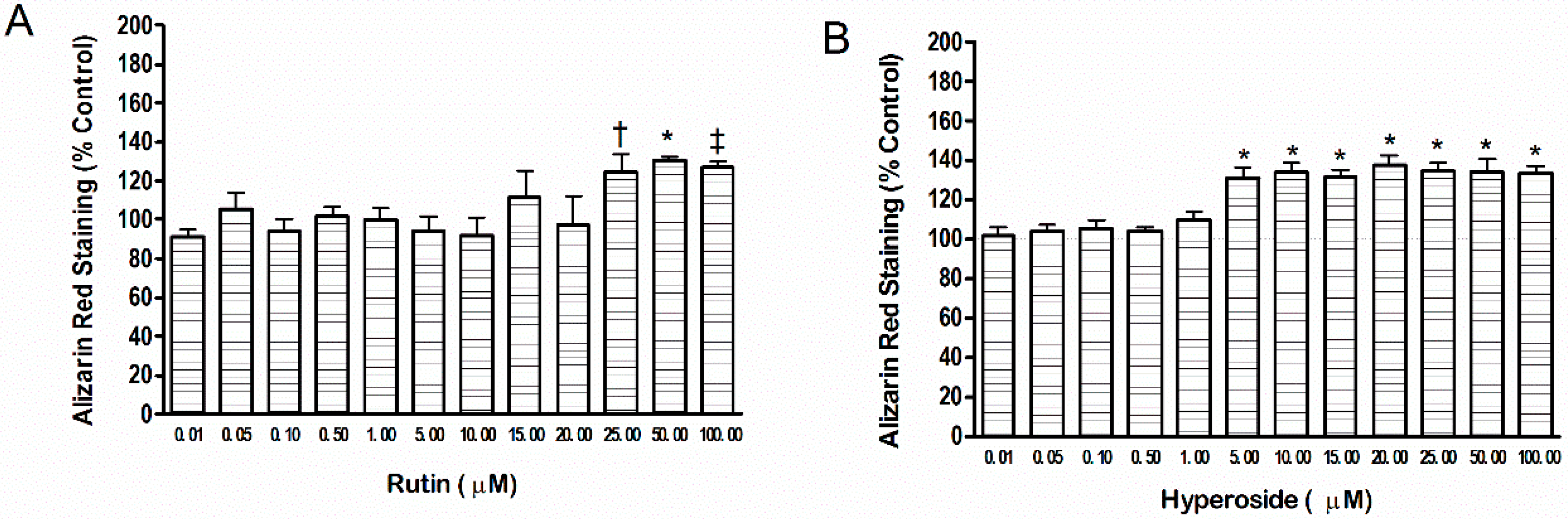
3.1. Mineral Content
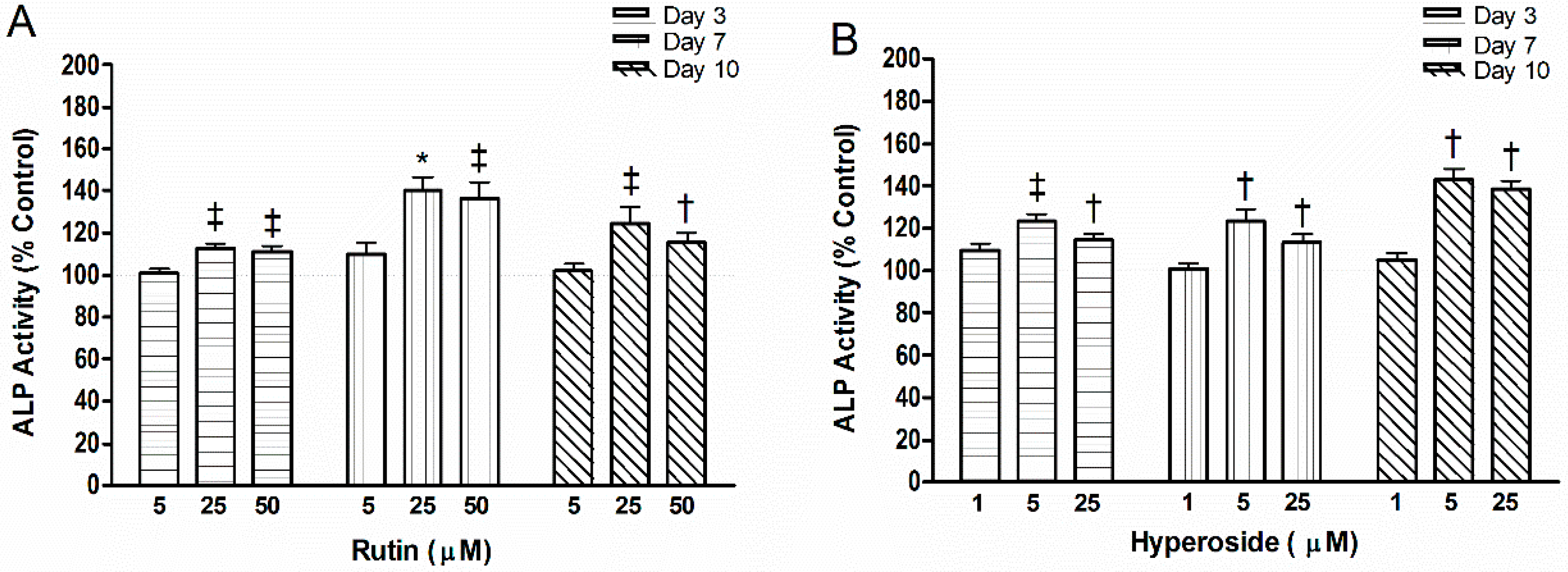
3.2. ALP Activity
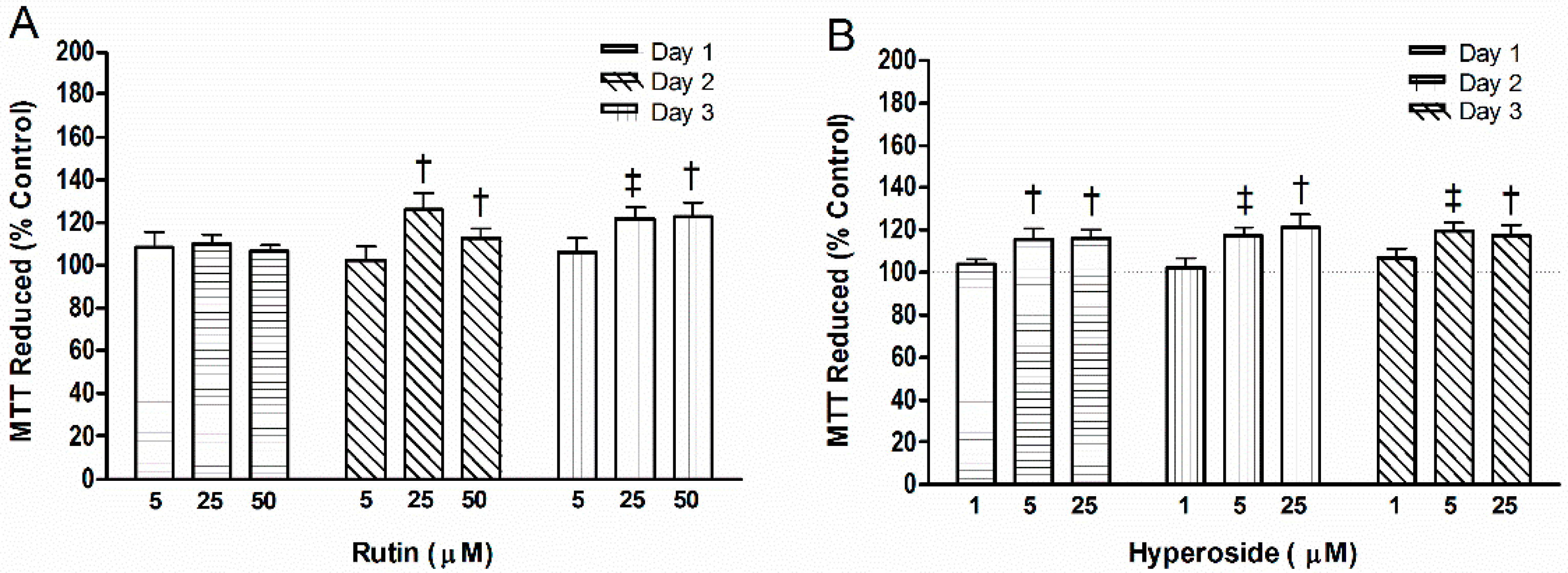
3.3. Cell Mitochondrial Activity
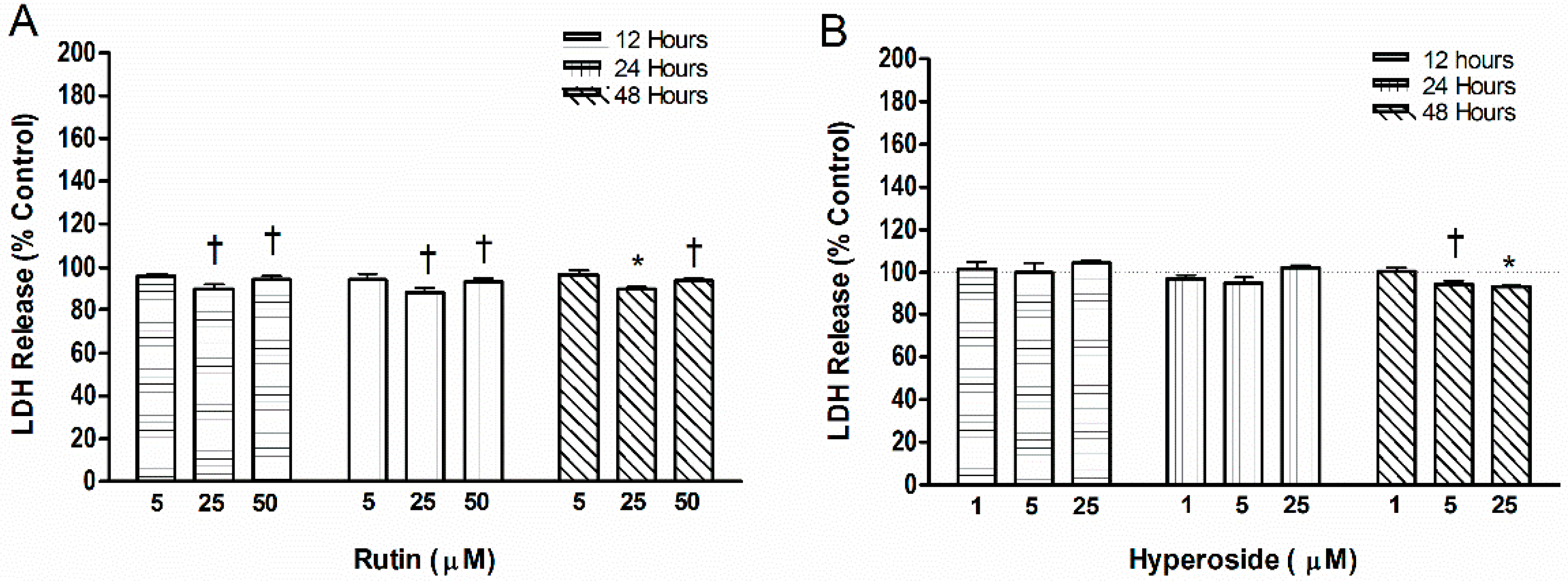
3.4. Cell Toxicity
3.5. Quantification of Regulatory Proteins in Osteoblasts
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase activity |
| BMD | bone mineral density |
| DMSO | dimethylsulfoxide |
| IL6 | Interleukin 6 |
| LDH | lactate dehydrogenase |
| MTT | 2-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| OD | Optical density |
| OPG | osteoprotegerin |
| OPN | osteopontin |
| SOST | sclerostin |
| TNFα | tumor necrosis factor alpha |
References
- National Osteoporosis Foundation (2014) Clinician’s Guide to Prevention and Treatment of Osteoporosis; National Osteoporosis Foundation: Washington, DC, USA.
- Bliuc, D.; Nguyen, N.D.; Nguyen, T.V.; Eisman, J.A.; Center, J.R. Compound risk of high mortality following osteoporotic fracture and refracture in elderly women and men. J. Bone Miner Res. 2013, 28, 2317–2324. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W. Osteoporosis and burden of osteoporosis-related fractures. Am. J. Manag. Care 2011, 17, S164–S169. [Google Scholar] [PubMed]
- Lehtonen-Veromaa, M.; Mottonen, T.T.; Nuotio, I.; Irjala, K.M.; Leino, A.E.; Viikari, J.S. Vitamin D and attainment of peak bone mass among peripubertal Finnish girls: A 3-y prospective study. Am. J. Clin. Nutr. 2002, 76, 1446–1453. [Google Scholar] [PubMed]
- Matkovic, V. Calcium and peak bone mass. J. Intern. Med. 1992, 231, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Ward, W.E. Prevention of bone fragility: The role of diet. Int. J. Clin. Rheumatol. 2009, 4, 311–319. [Google Scholar] [CrossRef]
- Hardcastle, A.C.; Aucott, L.; Reid, D.M.; Macdonald, H.M. Associations between dietary flavonoid intakes and bone health in a Scottish population. J. Bone Miner. Res. 2011, 26, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Nash, L.A.; Ward, W.E. Tea and bone health: Findings from human studies, potential mechanisms, and identification of knowledge gaps. Crit. Rev. Food Sci. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.; MacGregor, A.; Jennings, A.; Fairweather-Tait, S.; Spector, T.; Cassidy, A. Habitual flavonoid intakes are positively associated with bone mineral density in women. J. Bone Miner Res. 2012, 27, 1872–1878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; He, L.P.; Liu, Y.H.; Liu, J.; Su, Y.X.; Chen, Y.M. Association between dietary intake of flavonoid and bone mineral density in middle aged and elderly Chinese women and men. Osteoporosis Int. 2014, 25, 2417–2425. [Google Scholar] [CrossRef] [PubMed]
- Devine, A.; Hodgson, J.A.; Dick, A.M.; Prince, R.L. Tea drinking is associated with benefits on bone density in older women. Am. J. Clin. Nutr. 2007, 86, 1243–1247. [Google Scholar] [PubMed]
- Hoover, P.A.; Webber, C.E.; Beaumont, L.F.; Blake, J.M. Postmenopausal bone mineral density: Relationship to calcium intake, calcium absorption, residual estrogen, body composition and physical activity. Can. J. Physiol. Pharmacol. 1996, 74, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Pettinger, M.B.; Ritenbaugh, C.; LaCroix, A.Z.; Robbins, J.; Caan, B.J.; Barad, D.H.; Hakim, I.A. Habitual tea consumption and risk of osteoporosis: A prospective study in the Women’s Health Initiative observational cohort. Am. J. Epidemiol. 2003, 158, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Myers, G.; Prince, R.L.; Kerr, D.A.; Devine, A.; Woodman, R.J.; Lewis, J.R.; Hodgson, J.M. Tea and flavonoid intake predict osteoporotic fracture risk in elderly Australian women: A prospective study. Am. J. Clin. Nutr. 2015, 102, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Lee, H.J.; Lee, C.Y. Antioxidant activity of black tea vs. green tea. J. Nutr. 2002, 132, 785. [Google Scholar] [PubMed]
- Friedman, M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 2007, 51, 116–134. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, D.R.; Nedwin, G.E.; Bringman, T.S.; Smith, D.D.; Mundy, G.R. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumor necrosis factors. Nature 1986, 319, 516–518. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. Role of cytokines in bone resorption. Bone 1995, 17, S63–S67. [Google Scholar] [CrossRef]
- Stewart, A.J.; Mullen, W.; Crozier, A. On-line high-performance liquid chromatography analysis of the antioxidant activity of phenolic compounds in green and black tea. Mol. Nutr. Food Res. 2005, 49, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Bramati, L.; Minoggio, M.; Gardana, C.; Simonetti, P.; Mauri, P.; Pietta, P. Quantitative characterization of flavonoid compounds in rooibos tea (Aspalathus linearis) by LC-UV/DAD. J. Agric. Food Chem. 2002, 50, 5513–5519. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Feng, L.; Bin, Y.; Li, P.; Duan, C. Screening non-colored phenolics in red wines using liquid chromatography/ultraviolet and mass spectrometry/mass spectrometry libraries. Molecules 2007, 12, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.P.; Rossiter, J.T. Domestic processing of onion bulbs (Allium cepa) and asparagus spears (Asparagus officinalis): Effect on flavonol content and antioxidant status. J. Agric. Food Chem. 2001, 49, 3216–3222. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of polyphenols in blueberries, cranberries, chokeberries and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Formica, J.V.; Regelson, W. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Wong, R.W.K.; Rabie, A.B.M. Effect of quercetin on preosteoblasts and bone defects. Open Orthop. J. 2008, 2, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Yamamoto, H.; Sato, T.; Mizuha, Y.; Kawai, Y.; Taketani, Y.; Kato, S.; Terao, J.; Inakuma, T.; Takeda, E. Dietary quercetin inhibits bone loss without effect on the uterus in ovariectomized mice. J. Bone Miner. Metab. 2009, 27, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Swarnkar, G.; Sharan, K.; Chakravarti, B.; Gautam, A.K.; Rawat, P.; Kumar, M.; Gupta, V.; Manickavasagam, L.; Dwivedi, A.K.; et al. A naturally occurring rare analog of quercetin promotes peak bone mass achievement and exerts anabolic effect on osteoporotic bone. Osteoporosis Int. 2011, 22, 3013–3027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ying, M.D.; Wu, Y.P.; Zhou, Z.H.; Ye, Z.M.; Li, H.; Lin, D.S. Hyperoside, a flavonoid compound, inhibits proliferation and stimulates osteogenic differentiation of human osteosarcoma cells. PLoS ONE 2014, 9, e98973. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Bankar, R.; Roy, P. Assessment of the role of flavonoids for inducing osteoblast differentiation in isolated bone marrow derived mesenchymal stem cells. Phytomedicine 2013, 20, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Fogh, J.; Trempe, G. New human tumor cell lines. In Human Tumor Cells in Vitro; Plenum Press: New York, NY, USA; London, UK, 1975; pp. 115–159. [Google Scholar]
- Nash, L.A.; Sullivan, P.J.; Peters, S.J.; Ward, W.E. Rooibos flavonoids, orientin and luteolin, stimulate mineralization in human osteoblasts through the Wnt pathway. Mol. Nutr. Food. Res. 2015, 59, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.G.; Kung-Sutherland, M.S.; Muzaffar, S.A.; Wylie, J.N.; McBroom, R.J.; Murray, T.M. Positive interaction between 17 beta-estradiol and parathyroid hormone in normal human osteoblasts cultured long term in the presence of dexamethasone. Osteoporosis Int. 1996, 6, 111–119. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Q.; Wang, F.; Zhang, G. Antiosteoporotic compounds from seeds of Cuscuta chinensis. J. Ethnopharmacol. 2011, 135, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.C.; Lu, D.; Bai, J.; Zheng, H.; Ke, Z.Y.; Li, X.M.; Luo, S.Q. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappa-b. Biochem. Biophys. Res. Commun. 2004, 314, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Grassi, F.; Tell, G.; Robbie-Ryan, M.; Gao, Y.; Terauchi, M.; Yang, X.; Romanello, M.; Jones, D.P.; Weitzmann, M.N.; Pacifici, R. Oxidative stress causes bone loss in estrogen-deficient mice through enhanced bone marrow dendritic cell activation. Proc. Natl. Acad. Sci. USA 2007, 104, 15087–15092. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, L.; Wang, Y.; Xu, J.; Nie, Y.; Guo, Y.; Tong, Y.; Qin, L.; Zhang, Q. Curculigoside isolated from Curculigo orchioides prevents hydrogen peroxide-induced dysfunction and oxidative damage in calvarial osteoblasts. Acta Biochim. Biophys. Sin. 2012, 44, 431–441. [Google Scholar] [PubMed]
- Fujita, K.; Janz, S. Attenuation of WNT signaling by DKK-1 and -2 regulates BMP2-induced osteoblast differentiation and expression of OPG, RANKL and M-CSF. Mol. Cancer. 2007, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; El-Deiry, W.S. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene 2003, 22, 8628–8633. [Google Scholar] [CrossRef] [PubMed]
- Brandstrom, H.; Jonsson, K.B.; Vidal, O.; Ljunghall, S.; Ohlsson, C.; Vidal, O.; Ljunghall, S.; Ljunggren, O. Regulation of osteoprotegerin mRNA levels by prostaglandin E2 in human bone marrow stroma cells. Biochem. Biophys. Res. Commun. 1998, 248, 454–457. [Google Scholar] [PubMed]
- Hunter, G.K.; Kyle, C.L.; Goldberg, H.A. Modulation of crystal growth formation by bone phosphoproteins: Structural specificity of the osteopontin-mediated inhibition of hydroxyapatite formation. Biochem. J. 1994, 300, 723–728. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, J.R.; Goiko, M.; Mozaffari, M.; Bator, D.; Dauphinee, R.L.; Liao, Y.; Flemming, R.L.; Bramble, M.S.; Hunter, G.K.; Goldberg, H.A. Dynamic light scattering study of inhibition of nucleation and growth of hydroxyapatite crystals by osteopontin. PLoS ONE 2013, 8, e56764. [Google Scholar]
- Hunter, G.K. Role of osteopontin in modulation of hydroxyapatite formation. Calcif. Tissue Int. 2013, 93, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Chellaiah, M.A.; Kizer, N.; Biswas, R.; Alvarez, U.; Strauss-Schoenberger, J.; Rifas, L.; Rittling, S.R.; Denhardt, D.T.; Hruska, K.A. Osteopontin deficiency produces osteoclasts dysfunction due to reduced CD44 surface expression. Mol. Biol. Cell 2003, 14, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C.; Almeida, M. Gone with the Wnts: Beta-catenin, T-cell factor, forkhead box o, and oxidative stress in age-dependent disease of bone, lipid, and glucose metabolism. Mol. Endocrinol. 2007, 21, 2605–2614. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, M.N.; Sanchez, C.; Scalfo, F.; Ameye, L.; Henrotin, Y.; Offord, E. Effect of rosemary extract and related flavonoid carnosol on chondro-protection and on the bone-cartilage crosstalk. Osteoarthr. Cartil. 2010, 18, S38–S39. [Google Scholar] [CrossRef]
- Prideaux, M.; Wijenayaka, A.R.; Kumarasinghe, D.D.; Ormsby, R.T.; Evdokiou, A.; Findlay, D.M.; Atkins, G.J. Saos2 osteosarcoma cells as an in vitro model for studying the transition of human osteoblasts to osteocytes. Calif. Tissue Int. 2014, 95, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. In search of an osteoblast cell model for in vitro research. Eur. Cell. Mater. 2012, 24, 1–17. [Google Scholar] [PubMed]
- Horcajada-Molteni, M.N.; Crespy, V.; Coxam, V.; Davicco, M.J.; Remesy, C.; Barlet, J.P. Rutin inhibits ovariectomy-induced osteopenia in rats. J. Bone Miner Res. 2000, 15, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Breiter, T.; Laue, C.; Kressel, G.; Groll, S.; Engelhardt, U.H.; Hahn, A. Bioavailability and antioxidant potential of rooibos flavonoids in humans following the consumption of different rooibos formulations. Food Chem. 2011, 128, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Furuta, T.; Kasuya, Y. Determination of rutin in human plasma by high performance liquid chromatography utilizing solid-phase extraction and ultraviolet detection. J. Chromatogr. B.-Biomed. Sci. Appl. 2001, 759, 161–168. [Google Scholar] [CrossRef]
- Ying, X.; Meng, X.; Wang, S.; Wang, D.; Li, H.; Wang, B.; Du, Y.; Liu, X.; Zhang, W.; Kang, T. Simultaneous determination of three polyphenols in rat plasma after orally administering hawthorn leaves extract by the HPLC method. Nat. Prod. Res. 2012, 26, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Miyake, K.; Arima, H.; Hirayama, F.; Yamamoto, M.; Horikawa, T.; Sumiyoshi, H.; Noda, S.; Uekama, K. Improvement of solubility and oral bioavailability of rutin by complexation with 2-hydroxypropyl-beta-cyclodextrin. Pharm. Dev. Technol. 2000, 5, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Butterweck, V.; Lieflander-Wulf, U.; Winterhoff, H.; Nahrsedt, A. Plasma levels of hypericin in presence of procyanidin b2 and hyperoside: A pharmacokinetic study in rats. Planta Medica 2003, 69, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Ioku, K.; Tsushida, T.; Takei, Y.; Nakatani, N.; Terao, J. Antioxidant activity of quercetin and quercetin monoglucosides in solution and phospholipid bilayers. Biochim. Biophys. Acta 1995, 1234, 99–104. [Google Scholar] [CrossRef]
- Jo, S.; Ka, E.; Lee, H.; Apostolidis, E.; Jang, H.; Kwon, Y. Comparison of antioxidant potential and rat intestinal a-glucosides inhibitory activities of quercetin, rutin and isoquercetin. Int. J. Appl. Res. Nat. Prod. 2010, 2, 52–60. [Google Scholar]
- Silva, M.M.; Santos, M.R.; Caroco, G.; Rocha, R.; Justino, G.; Mira, L. Structure-antioxidant activity relationships of flavonoids: A re-examination. Free Radic. Res. 2002, 36, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.; Ubeaud, G.; Jung, L. Anti- and pro-oxidant activity of rutin and quercetin derivatives. J. Pharm. Pharmacol. 2003, 55, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Schroder, H.C.; Feng, Q.; Diehl-Seifert, B.; Grebenjuk, V.A.; Muller, W.E. Isoquercetrin and polyphosphate co-enhance mineralization of human osteoblast-like SaOs-2 cells via separate activation of two RUNX2 cofactors AFT6 and Ets1. Biochem. Pharmacol. 2014, 89, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.M.; Boersma, M.G.; Boeren, S.; Bladeren, P.J.; Vervoort, J.; Rietjens, I.M.C.M. Structure-activity study of the quinone/quinone methide chemistry of flavonoids. Chem. Res. Toxicol. 2001, 14, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Canada, A.T.; Giannella, E.; Nguyen, T.D.; Mason, R.P. The production of reactive oxygen species by dietary flavonols. Free Radic. Biol. Med. 1990, 9, 441–449. [Google Scholar] [CrossRef]
| Condition | TNFα | IL6 | OPN | OPG | SOST |
|---|---|---|---|---|---|
| Control (pg/mL) | 0.43 ± 0.01 | 3.24 ± 0.08 | 247.50 ± 10.71 | 1659.25 ± 107.78 | 810.00 ± 22.15 |
| 50 µM Rutin (pg/mL) | 0.20 ± 0.02 a,* | 1.36 ± 0.09 a,* | 126.21 ± 10.78 * | 431.25 ± 11.37 a,‡ | 572.00 ± 19.07 * |
| Rutin (pg/mL/mol) # | −46 | −376 | −24,258 | −245,600 | −47,600 |
| 25 µM Hyperoside( pg/mL) | 0.25 ± 0.01 b,* | 1.90 ± 0.06 b,* | 145.75 ± 2.75 * | 709.25 ± 25.28 b,‡ | 542.75 ± 10.01 * |
| Hyperoside pg/mL/mol # | −72 | −536 | −40,700 | −380,000 | −106,900 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nash, L.A.; Peters, S.J.; Sullivan, P.J.; Ward, W.E. Supraphysiological Levels of Quercetin Glycosides are Required to Alter Mineralization in Saos2 Cells. Int. J. Environ. Res. Public Health 2016, 13, 460. https://doi.org/10.3390/ijerph13050460
Nash LA, Peters SJ, Sullivan PJ, Ward WE. Supraphysiological Levels of Quercetin Glycosides are Required to Alter Mineralization in Saos2 Cells. International Journal of Environmental Research and Public Health. 2016; 13(5):460. https://doi.org/10.3390/ijerph13050460
Chicago/Turabian StyleNash, Leslie A., Sandra J. Peters, Philip J. Sullivan, and Wendy E. Ward. 2016. "Supraphysiological Levels of Quercetin Glycosides are Required to Alter Mineralization in Saos2 Cells" International Journal of Environmental Research and Public Health 13, no. 5: 460. https://doi.org/10.3390/ijerph13050460






